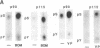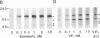Abstract
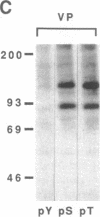
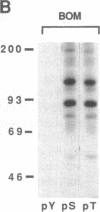
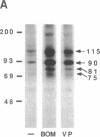
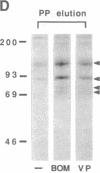
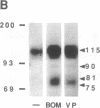
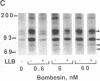
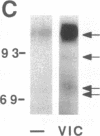
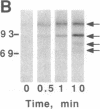
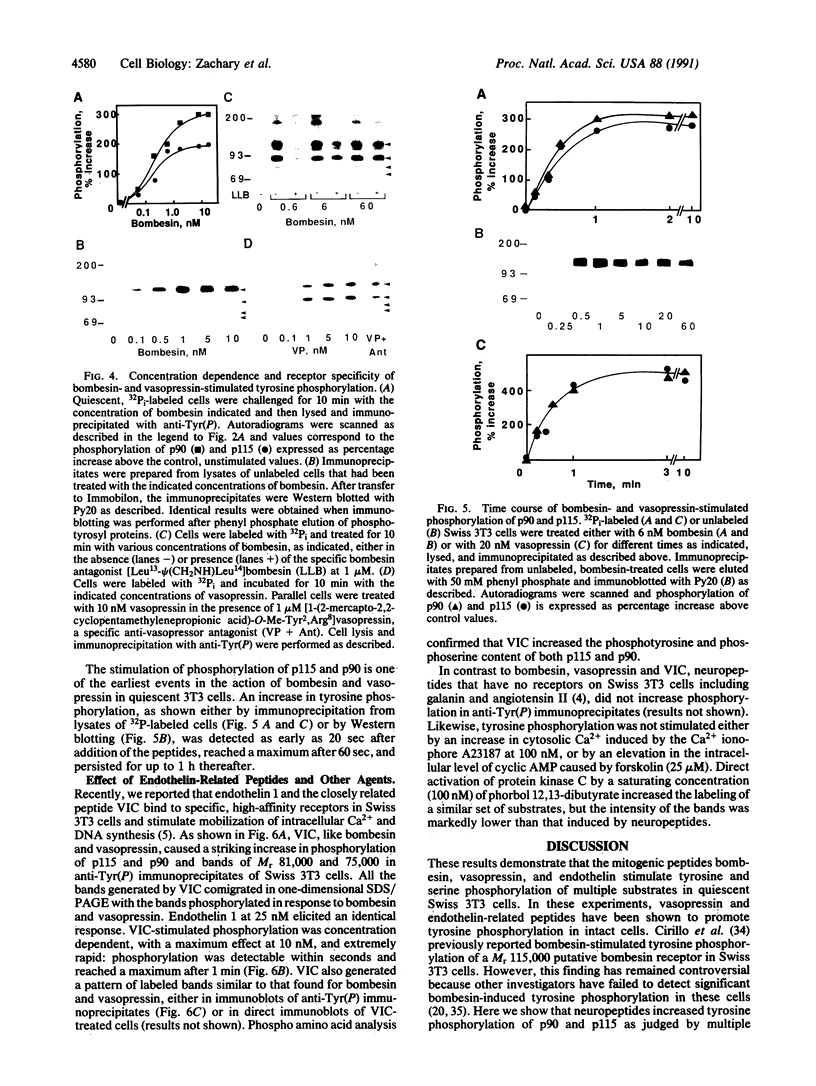
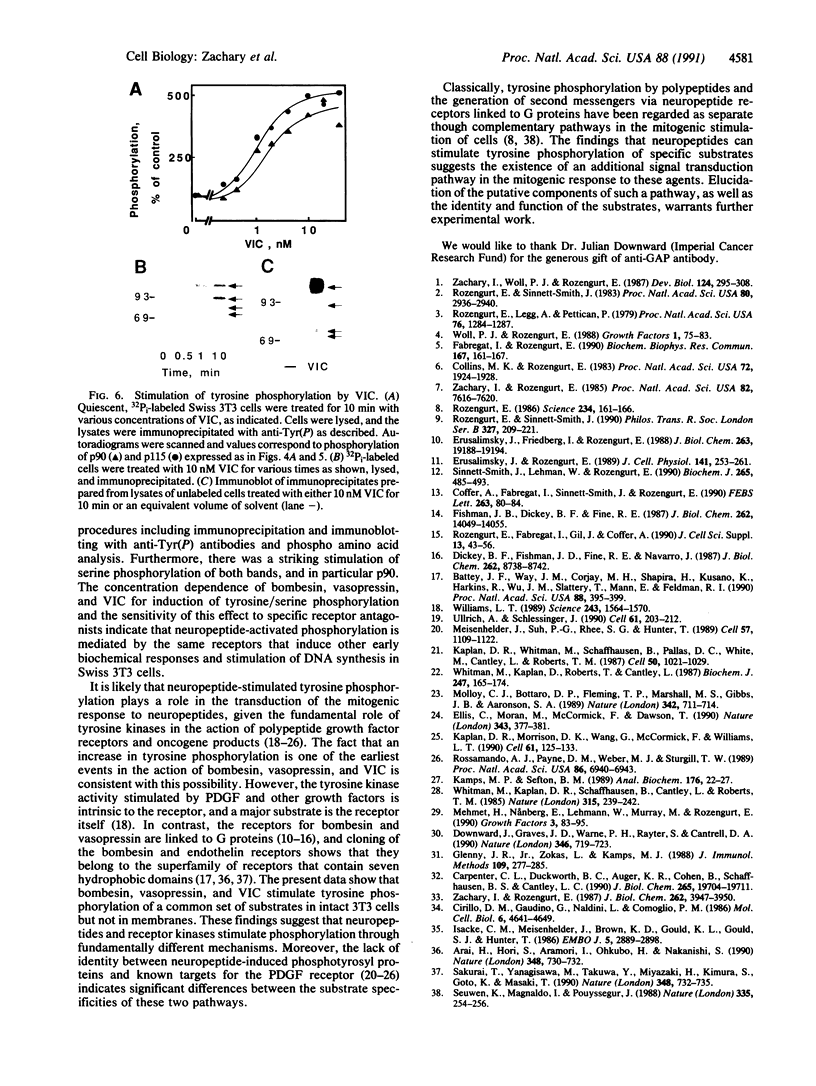
The mitogenic neuropeptides bombesin and vasopressin markedly increased tyrosine and serine phosphorylation of multiple substrates in quiescent Swiss 3T3 fibroblasts, including two major bands of Mr 90,000 and 115,000. Tyrosine phosphorylation of these proteins was increased as judged by immunoprecipitation of 32Pi-labeled cells and immunoblotting of unlabeled cells with monoclonal antiphosphotyrosine antibodies, elution with phenyl phosphate, and phospho amino acid analysis. Phosphotyrosyl proteins generated by bombesin and vasopressin did not correspond either by apparent molecular weight or by immunological and biochemical criteria to several known tyrosine kinase substrates, including phospholipase C gamma, the microtubule-associated protein 2 kinase, GTPase-activating protein, or phosphatidylinositol kinase. The effect was rapid (within seconds), concentration dependent, and inhibited by specific receptor antagonists for both bombesin and vasopressin. The endothelin-related peptide, vasoactive intestinal contractor, also elicited a rapid and concentration-dependent tyrosine/serine phosphorylation of a similar set of substrates. These results demonstrate that neuropeptides, acting through receptors linked to GTP-binding proteins, stimulate tyrosine phosphorylation of a common set of substrates in quiescent Swiss 3T3 cells and suggest the existence of an additional signal transduction pathway in neuropeptide-induced mitogenesis.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990 Dec 20;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Battey J. F., Way J. M., Corjay M. H., Shapira H., Kusano K., Harkins R., Wu J. M., Slattery T., Mann E., Feldman R. I. Molecular cloning of the bombesin/gastrin-releasing peptide receptor from Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):395–399. doi: 10.1073/pnas.88.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. L., Duckworth B. C., Auger K. R., Cohen B., Schaffhausen B. S., Cantley L. C. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990 Nov 15;265(32):19704–19711. [PubMed] [Google Scholar]
- Cirillo D. M., Gaudino G., Naldini L., Comoglio P. M. Receptor for bombesin with associated tyrosine kinase activity. Mol Cell Biol. 1986 Dec;6(12):4641–4649. doi: 10.1128/mcb.6.12.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffer A., Fabregat I., Sinnett-Smith J., Rozengurt E. Solubilization of the bombesin receptor from Swiss 3T3 cell membranes. Functional association to a guanine nucleotide regulatory protein. FEBS Lett. 1990 Apr 9;263(1):80–84. doi: 10.1016/0014-5793(90)80710-z. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Vasopressin induces selective desensitization of its mitogenic response in Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1924–1928. doi: 10.1073/pnas.80.7.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey B. F., Fishman J. B., Fine R. E., Navarro J. Reconstitution of the rat liver vasopressin receptor coupled to guanine nucleotide-binding proteins. J Biol Chem. 1987 Jun 25;262(18):8738–8742. [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990 Jan 25;343(6256):377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- Erusalimsky J. D., Friedberg I., Rozengurt E. Bombesin, diacylglycerols, and phorbol esters rapidly stimulate the phosphorylation of an Mr = 80,000 protein kinase C substrate in permeabilized 3T3 cells. Effect of guanine nucleotides. J Biol Chem. 1988 Dec 15;263(35):19188–19194. [PubMed] [Google Scholar]
- Erusalimsky J. D., Rozengurt E. Vasopressin rapidly stimulates protein kinase C in digitonin-permeabilized Swiss 3T3 cells: involvement of a pertussis toxin-insensitive guanine nucleotide binding protein. J Cell Physiol. 1989 Nov;141(2):253–261. doi: 10.1002/jcp.1041410204. [DOI] [PubMed] [Google Scholar]
- Fabregat I., Rozengurt E. Vasoactive intestinal contractor, a novel peptide, shares a common receptor with endothelin-1 and stimulates Ca2+ mobilization and DNA synthesis in Swiss 3T3 cells. Biochem Biophys Res Commun. 1990 Feb 28;167(1):161–167. doi: 10.1016/0006-291x(90)91745-e. [DOI] [PubMed] [Google Scholar]
- Fishman J. B., Dickey B. F., Fine R. E. Purification and characterization of the rat liver vasopressin (V1) receptor. J Biol Chem. 1987 Oct 15;262(29):14049–14055. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Isacke C. M., Meisenhelder J., Brown K. D., Gould K. L., Gould S. J., Hunter T. Early phosphorylation events following the treatment of Swiss 3T3 cells with bombesin and the mammalian bombesin-related peptide, gastrin-releasing peptide. EMBO J. 1986 Nov;5(11):2889–2898. doi: 10.1002/j.1460-2075.1986.tb04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Mehmet H., Nånberg E., Lehmann W., Murray M. J., Rozengurt E. Early signals in the mitogenic response of Swiss 3T3 cells: a comparative study of purified PDGF homodimers. Growth Factors. 1990;3(2):83–95. doi: 10.3109/08977199009108271. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Fabregat I., Coffer A., Gil J., Sinnett-Smith J. Mitogenic signalling through the bombesin receptor: role of a guanine nucleotide regulatory protein. J Cell Sci Suppl. 1990;13:43–56. doi: 10.1242/jcs.1990.supplement_13.6. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of fibroblast mitogenesis: specific receptors, signal transduction and early events. Philos Trans R Soc Lond B Biol Sci. 1990 Mar 12;327(1239):209–221. doi: 10.1098/rstb.1990.0055. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990 Dec 20;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Seuwen K., Magnaldo I., Pouysségur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988 Sep 15;335(6187):254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith J., Lehmann W., Rozengurt E. Bombesin receptor in membranes from Swiss 3T3 cells. Binding characteristics, affinity labelling and modulation by guanine nucleotides. Biochem J. 1990 Jan 15;265(2):485–493. doi: 10.1042/bj2650485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987 Oct 1;247(1):165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Woll P. J., Rozengurt E. Two classes of antagonist interact with receptors for the mitogenic neuropeptides bombesin, bradykinin, and vasopressin. Growth Factors. 1988;1(1):75–83. doi: 10.3109/08977198809000249. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. High-affinity receptors for peptides of the bombesin family in Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7616–7620. doi: 10.1073/pnas.82.22.7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Identification of a receptor for peptides of the bombesin family in Swiss 3T3 cells by affinity cross-linking. J Biol Chem. 1987 Mar 25;262(9):3947–3950. [PubMed] [Google Scholar]
- Zachary I., Woll P. J., Rozengurt E. A role for neuropeptides in the control of cell proliferation. Dev Biol. 1987 Dec;124(2):295–308. doi: 10.1016/0012-1606(87)90483-0. [DOI] [PubMed] [Google Scholar]