Abstract
The nuclear envelope, composed of two lipid bilayers and numerous accessory proteins, has evolved to house the genetic material of all eukaryotic cells. In so doing, the nuclear envelope provides a physical barrier between chromosomes and the cytoplasm. Once believed to be highly stable, recent studies demonstrate that the nuclear envelope is prone to rupture. These rupture events expose chromosomal DNA to the cytoplasmic environment and have the capacity to promote DNA damage. Thus nuclear rupture may be an unappreciated mechanism of mutagenesis.
INTRODUCTION
Loss of genome stability is an enabling hallmark of tumor progression, in which elevated rates of mutation and numerical/structural chromosomal aberrations combine to drive unrestrained cellular proliferation and resistance to cell death (Hanahan and Weinberg, 2011). Mechanisms that drive mutagenesis and copy-number changes in cancer represent the molecular underpinnings of tumor initiation and progression, and many of these mechanisms have been well described (Gordon et al., 2012; Helleday et al., 2014). However, the causes of genomic instability are not yet comprehensively understood, and identifying new mechanisms remains a critically important focus in cancer cell biology.
A new mechanism promoting genomic instability in cancer cells, in which the nuclear envelope ruptures and exposes chromosomal DNA to the cytoplasmic environment, has been described (Vargas et al., 2012). Depending on the cellular context, the phenomenon of nuclear rupture inflicts various degrees of DNA damage to cells. Rupture of primary nuclei, although transient due to quick repair of the nuclear envelope, promotes a low level of DNA damage that is localized to small chromosomal regions adjacent to rupture sites (Denais et al., 2016; Raab et al., 2016). By contrast, rupture of micronuclei, which are commonly generated by abnormal chromosome segregation, is permanent and leads to catastrophic chromosomal damage (Hatch et al., 2013; Zhang et al., 2015).
Nuclear envelope rupture is significantly more common in cancer cells than normal cells, although the basis for this difference remains unresolved (Vargas et al., 2012). Understanding how defects in this foundational nuclear fortification contribute to genome instability represents a burgeoning field in cancer research. Here we summarize the known causes and consequences of nuclear rupture and speculate on the cell biological factors that may regulate this phenomenon.
THE NUCLEAR ENVELOPE
Selective pressure to shield DNA from damage in the face of increasing cellular complexity helped drive the evolution of the nuclear envelope (Cavalier-Smith, 2010). This radical innovation in cellular organization marked the beginning of eukaryotic life and enabled rampant evolutionary diversification. Reflecting its role of chaperoning access to critical genetic information, the metazoan nuclear envelope is complex and dynamic, engaging in multiple interactions with components of the cytoplasm and the cytoskeleton at any given time (Chow et al., 2012; Hatch and Hetzer, 2014).
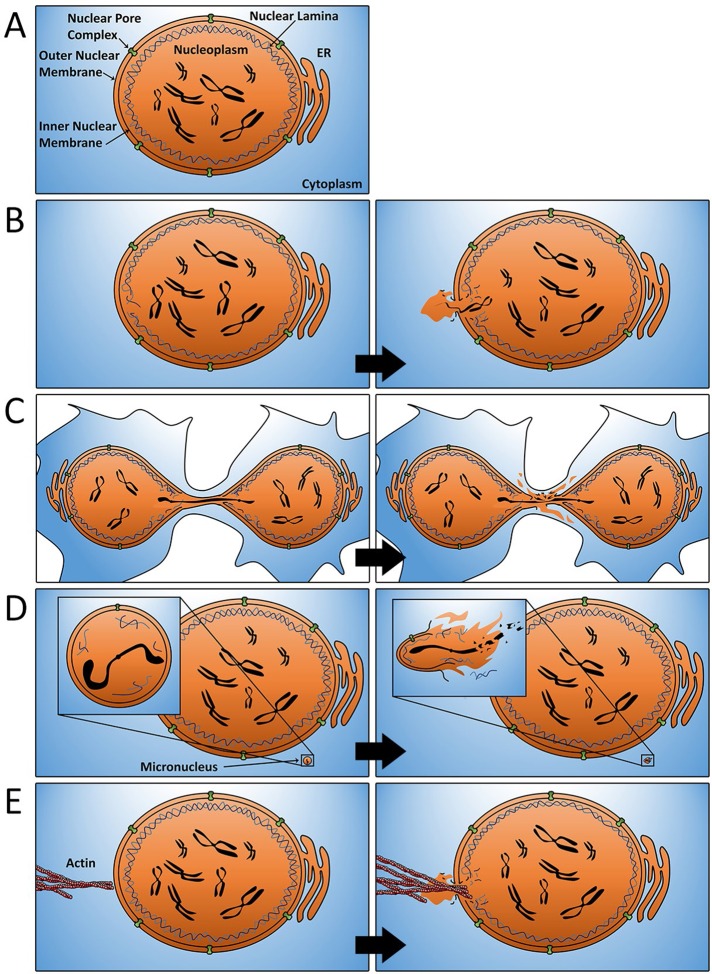
The nuclear envelope is composed of three distinct components: the lamina, the inner and outer nuclear membranes, and nuclear pore complexes (Hetzer, 2010). The lamina plays a central role in maintaining the structural integrity of the nuclear envelope and is composed of lamins A/C, B1, and B2 (Dechat et al., 2010). On this bedrock of lamina meshwork lies the inner nuclear membrane, which runs contiguous with both the outer nuclear membrane and the endoplasmic reticulum (Figure 1A). Finally, dotted throughout the nuclear envelope and extending through both the inner and outer membranes and lamina are nuclear pore complexes (Wente and Rout, 2010). Nuclear pore complexes regulate the active transport of molecules greater than ∼40 kDa in size in and out of the nucleus (Wente and Rout, 2010). The various components of the nuclear envelope work in concert to promote cross-talk between the nucleus and the remainder of the cell while simultaneously protecting the genetic contents within from the potentially damaging environment of the cytoplasm.
FIGURE 1:
Mechanisms of nuclear envelope rupture. (A) The structure of the nuclear envelope in normal cells. (B) Transient rupture of the nuclear envelope occurs at sites exhibiting structural deficiencies in the underlying lamina. Although quickly repaired, these rupture events promote the localized mixing of nucleoplasmic and cytosolic contents. (C) Chromosome bridges that form as a consequence of defective mitosis result in elongated “teardrop”-shaped nuclei in daughter cells. Defective lamin deposition around these chromosome bridges creates a localized region of the nuclear envelope that is particularly susceptible to rupture. (D) Micronuclei (insets) are defective in nuclear lamina assembly and undergo complete and irreversible nuclear envelope rupture. Rupture of micronuclei during S-phase promotes chromothripsis. (E) Under conditions of hyperactivated Rho GTPases, increased actomyosin contractility may puncture the nuclear envelope and promote rupture.
NUCLEAR ENVELOPE RUPTURE
In physiologically normal cells, breakdown of the nuclear envelope occurs only during mitosis to enable condensed chromosomes to freely segregate to daughter cells (Smoyer and Jaspersen, 2014). This highly regulated process of mitotic nuclear envelope disassembly is governed in large part by activation of the mitotic kinase Cdk1, which phosphorylates key components of the lamina and nuclear pore complexes to promote their disassembly (Heald and McKeon, 1990; Peter et al., 1990; Guttinger et al., 2009). After the completion of mitosis, the nuclear envelope rapidly reassembles around the decondensing chromosomes to once again shield them from the surrounding cytoplasm.
By contrast, nuclear envelope breakdown during interphase is anomalous and strongly indicative of an underlying cellular defect (Hatch and Hetzer, 2014). Transient nuclear envelope rupture has been best studied with regard to laminopathies, in which expression of mutant lamin proteins leads to gaps in the nuclear lamina structure and predisposes the nuclear envelope to intermittent nonlethal loss of integrity during interphase (De Vos et al., 2011; Figure 1B). Viruses also promote nuclear envelope rupture upon infection, perhaps as a means to gain access to the nucleus (de Noronha et al., 2001; Cohen et al., 2011). It has been demonstrated that cancer cells are predisposed to nuclear envelope rupture (Vargas et al., 2012). This is perhaps unsurprising, considering that the nuclear envelope of cancer cells has long been known to be commonly structurally impaired (Bernhard and Granboulan, 1963; Bell and Lammerding, 2016). Indeed, as far back as 1860, abnormalities in nuclear size and shape were used to identify cells that had become cancerous (Beale, 1860).
A key unresolved question regarding nuclear envelope rupture is why the phenomenon commonly occurs in cancer cells yet is exceedingly rare in normal cells. One obvious explanation lies in the fact that cancer cells commonly exhibit aberrant expression of lamin proteins, which impairs the structural integrity of the nuclear lamina (Chow et al., 2012; Ho and Lammerding, 2012; Bell and Lammerding, 2016; Vargas et al., 2012). Confirming this notion, studies have revealed that depletion of B-type lamins increases the frequency of nuclear envelope rupture, whereas, conversely, overexpression of B-type lamins mitigates rupture rates in cancer cells (Vargas et al., 2012; Hatch et al., 2013).
An alternative, non–mutually exclusive explanation for the elevated rate of nuclear envelope rupture in cancer cells is that many cellular defects commonly associated with cancer have been shown to promote nuclear rupture. For example, abnormalities in chromosome segregation during mitosis that produce chromosome bridges and/or micronuclei are frequent in transformed cells yet rare in nontransformed cells (Ganem and Pellman, 2012; Gordon et al., 2012). Chromosome bridges typically arise from dicentric chromosomes, which originate from inappropriately repaired double-strand breaks or from fusions of critically shortened telomere regions, which are then pulled to opposing poles during anaphase and persist well into the next cell cycle. By physically connecting the two nuclei, chromosome bridges induce abnormal “teardrop” morphologies to both daughter nuclei. A direct or indirect consequence of this defect is that nuclear lamins fail to efficiently assembly around chromatin bridges, and this predisposes the envelope around the bridge to rupture (Maciejowski et al., 2015; Figure 1C). Similarly, micronuclei, which form when anaphase lagging chromosomes reassemble nuclear envelopes independently of the spatially separated primary nucleus during telophase, are also highly prone to rupture (Hatch et al., 2013; Zhang et al., 2015; Figure 1D). Research suggests strongly that compositional defects, including insufficient lamin deposition, are also responsible for this effect (Hatch et al., 2013). It is tempting to speculate that other defects arising in mitosis may similarly disrupt normal nuclear lamina assembly and promote rupture. For example, asynchronously segregating chromosomes may delay and/or disrupt the efficient rebuilding of the nuclear lamina. Similarly, mitotic or cytokinetic failures that produce tetraploid cells with abnormally large nuclei may also impede efficient nuclear lamina assembly and predispose such large cells to rupture.
In addition to defects in lamina structure, it is now clear that mechanical forces from the actin cytoskeleton can also promote nuclear rupture. Two studies recently demonstrated that cells forced to migrate through narrow microfabricated constriction channels are prone to rupture due to increased internal pressure in the nucleus (Denais et al., 2016; Raab et al., 2016). This rupture occurred at the leading edge of the nucleus and was preceded by the appearance of a nuclear protrusion lacking nuclear pore complexes and lamina. Of importance, rupture was driven, at least in part, by forces from actomyosin contraction, as inhibition of myosin II activity significantly reduced the occurrence of rupture (Denais et al., 2016). This raises the possibility that deregulation of actomysosin contractility may play a significant role in promoting rupture even in nonmigrating cells. In this sense, stimulation of actin contractility due to hyperactivation or overexpression of Rho GTPases, which is common in many cancer cells, may offer an additional explanation for increased rupture events (Mark Petronczki, personal communication; Vega and Ridley, 2008). One intriguing possibility is that abnormal actin fibers may directly puncture fragile nuclear envelopes that are already weakened by a structurally defective lamina (Figure 1E). Alterations in the microtubule cytoskeleton and/or increases in centrosome number may also play a role in promoting nuclear envelope rupture. During mitosis, centrosomes and microtubules play important roles in facilitating nuclear envelope breakdown, and depletion of centrosomes has been shown to delay nuclear envelope disassembly (Smoyer and Jaspersen, 2014). Thus it is plausible that extra centrosomes, which are a hallmark of cancer cells, facilitate nuclear envelope rupture. Indeed, a convergence of multiple abnormalities in cancer cells, such as abnormal nuclear lamina structure, extra centrosomes, and/or hyperactive Rho, may be required to promote rupture.
NUCLEAR RUPTURE AS A SOURCE OF DNA DAMAGE
Having established that nuclear envelope rupture is a defect commonly observed in human cancer cells, we are left to ask what functional consequences result from such a phenomenon. Indeed, the dramatic disruption of cellular architecture after rupture is not a benign event; nuclear rupture is now known to induce significant, and sometimes catastrophic, genome instability (Chow et al., 2012; Crasta et al., 2012; Hatch et al., 2013; Maciejowski et al., 2015; Zhang et al., 2015). Recent studies have shown that DNA damage appears minutes after nuclear envelope rupture and at a chromosomal location immediately adjacent to the rupture site (Denais et al., 2016; Raab et al., 2016). However, the mechanism underlying the generation of this damage has yet to be fully resolved. One possibility is that the chromosomal DNA becomes exposed to harmful cytoplasmic factors from which it otherwise would have been shielded. For example, evidence suggests that a cytoplasmic exonuclease, TREX1, may cleave chromosomal DNA outside the nucleus (Maciejowski et al., 2015). Whether additional nucleases exist to induce similar damage remains to be determined. Cytoplasmic DNA may also be subject to the mechanical stress generated by the actin and microtubule cytoskeletons, which could cause shearing, particularly since the chromosomal DNA is not condensed and may be more susceptible to damage. It is also possible that regions of single-stranded DNA, either from actively transcribing regions or regions undergoing replication, are more prone to acquiring damage in the cytoplasm.
In addition, it has been proposed that the temporary loss of compartmentalization, including the integration of organelles and other cytosolic elements into the nucleus, may indirectly promote DNA damage (Bernhard and Granboulan, 1963; de Noronha et al., 2001; Vargas et al., 2012). Reactive oxygen species generated by mitochondria that have infiltrated the nucleus represent one potential source of such damage (Sieprath et al., 2012; Vargas et al., 2012).
Whereas transient rupture of the primary nucleus represents a source of DNA damage, expeditious repair and resealing of the nuclear envelope driven by endosomal sorting complexes required for transport III (ESCRT III) machinery tempers its catastrophic potential (Denais et al., 2016; Raab et al., 2016). This is not the case in the context of micronuclei, where nuclear envelope rupture is permanent, leaving the chromosomal contents therein completely exposed to the surrounding environment (Figure 1D; Hatch et al., 2013). It has been directly demonstrated that such chromosomes undergo a catastrophic shattering and rearrangement event called chromothripsis in which thousands of clustered chromosomal rearrangements occur in a single event (Stephens et al., 2011; Crasta et al., 2012; Zhang et al., 2015). Of interest, micronuclear rupture promotes chromosome damage only in S-phase cells, as no major damage results after rupture of micronuclei in G0 or G1 cells (Crasta et al., 2012; Hatch et al., 2013; Zhang et al., 2015). One simple explanation for this result is that rupture may lead to the rapid dilution of DNA replication factors from actively replicating chromatin, thus generating stalled or collapsed replication forks that are prone to cytoplasmic nucleases (Leibowitz et al., 2015). Similar to micronuclei, rupture of the nuclear envelope surrounding chromosome bridges also promotes massive DNA damage (Maciejowski et al., 2015). Thus nuclear rupture may represent a major source of the complex genetic variability found in chromosomally unstable cancers.
Of importance, it is interesting to consider whether exploiting the phenomenon of nuclear envelope rupture may have therapeutic value, especially given that the phenomenon predominantly occurs in cancer cells. Supporting this proposition, inhibition of nuclear envelope repair in cancer cells is lethal in conjunction with inhibition of DNA repair mechanisms (Raab et al., 2016). Only ESCRT III is known to be involved in nuclear envelope resealing after rupture, and its contribution is relatively minor compared with its integral role in nuclear envelope reformation after mitosis (Olmos et al., 2015; Vietri et al., 2015; Denais et al., 2016; Raab et al., 2016). Therefore identifying additional factors that are required for nuclear envelope repair after rupture but are dispensable for nuclear envelope reformation after mitosis may represent more ideal therapeutic targets. Other vulnerabilities that arise in cancer cells as a result of the need to accommodate this “fragile-nucleus” phenotype may similarly represent additional novel therapeutic opportunities.
CONCLUSION
Cancer cells have been characterized by their abnormal nuclear structure for well more than a century, despite the functional consequences of this defect being largely unknown (Beale, 1860). Thus the recent finding that this widely appreciated abnormality is indicative of elevated rates of nuclear rupture and resultant DNA damage is a discovery that reaches across decades to integrate early observations with contemporary molecular understanding. The demonstration that the weakened cancer cell nucleus, with its propensity for rupture, serves as a previously unappreciated source of mutagenic stimuli and genome instability has brought the field of nuclear envelope dynamics into sharp focus. Elucidating why cancer cells are more prone to rupture than their nontransformed counterparts and uncovering the causes and consequences of nuclear envelope rupture in the context of tumor progression are inquiries rife with biological significance. Indeed, the exploitation of altered nuclear envelope dynamics in tumor cells presents an entirely untested therapeutic arena to explore. Investigating nuclear envelope rupture as an important driver of genome instability and tumor progression is likely to be a rewarding line of scientific inquiry for years to come, with significant implications in both basic and translational cancer biology.
Acknowledgments
We apologize to those authors whose work could not be cited due to space constraints. We thank Alex Spektor for comments on the manuscript. S.L., R.J.Q., and N.J.G. are members of the Shamim and Ashraf Dahod Breast Cancer Research Laboratories. S.L. is supported by grants from the Skin Cancer Foundation and the American Skin Association. N.J.G. is supported by the Searles Foundation, the Smith Family Foundation, the Melanoma Research Alliance, and National Institutes of Health Grants CA154531-05 and GM117150-01.
Abbreviations used:
- Cdk1
cyclin-dependent kinase-1
- ESCRT
endosomal sorting complexes required for transport
- GTP
guanosine triphosphate
- TREX1
three prime repair exonuclease 1.
Footnotes
REFERENCES
- Beale LS. Examination of sputum from a case of cancer of the pharynx and the adjacent parts. Arch Med Lond. 1860;2:44. [Google Scholar]
- Bell ES, Lammerding J. Causes and consequences of nuclear envelope alterations in tumour progression. Eur J Cell Biol. 2016 doi: 10.1016/j.ejcb.2016.06.007. doi: j.ejcb.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard W, Granboulan N. The fine structure of the cancer cell nucleus. Exp Cell Res. 1963;24(Suppl 9):19–53. doi: 10.1016/0014-4827(63)90243-x. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution. Biol Direct. 2010;5:7. doi: 10.1186/1745-6150-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KH, Factor RE, Ullman KS. The nuclear envelope environment and its cancer connections. Nat Rev Cancer. 2012;12:196–209. doi: 10.1038/nrc3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Marr AK, Garcin P, Pante N. Nuclear envelope disruption involving host caspases plays a role in the parvovirus replication cycle. J Virol. 2011;85:4863–4874. doi: 10.1128/JVI.01999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 2012;482:53–58. doi: 10.1038/nature10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. Nuclear lamins. Cold Spring Harb Perspect Biol. 2010;2:a000547. doi: 10.1101/cshperspect.a000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352:353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Noronha CM, Sherman MP, Lin HW, Cavrois MV, Moir RD, Goldman RD, Greene WC. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science. 2001;294:1105–1108. doi: 10.1126/science.1063957. [DOI] [PubMed] [Google Scholar]
- De Vos WH, Houben F, Kamps M, Malhas A, Verheyen F, Cox J, Manders EM, Verstraeten VL, van Steensel MA, Marcelis CL, van den Wijngaard A, et al. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum Mol Genet. 2011;20:4175–4186. doi: 10.1093/hmg/ddr344. [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Pellman D. Linking abnormal mitosis to the acquisition of DNA damage. J Cell Biol. 2012;199:871–881. doi: 10.1083/jcb.201210040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet. 2012;13:189–203. doi: 10.1038/nrg3123. [DOI] [PubMed] [Google Scholar]
- Guttinger S, Laurell E, Kutay U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol. 2009;10:178–191. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 2013;154:47–60. doi: 10.1016/j.cell.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch E, Hetzer M. Breaching the nuclear envelope in development and disease. J Cell Biol. 2014;205:133–141. doi: 10.1083/jcb.201402003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61:579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Helleday T, Eshtad S, Nik-Zainal S. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet. 2014;15:585–598. doi: 10.1038/nrg3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer MW. The nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000539. doi: 10.1101/cshperspect.a000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Lammerding J. Lamins at a glance. J Cell Sci. 2012;125:2087–2093. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz ML, Zhang CZ, Pellman D. Chromothripsis: a new mechanism for rapid karyotype evolution. Annu Rev Genet. 2015;49:183–211. doi: 10.1146/annurev-genet-120213-092228. [DOI] [PubMed] [Google Scholar]
- Maciejowski J, Li Y, Bosco N, Campbell PJ, de Lange T. Chromothripsis and kataegis induced by telomere crisis. Cell. 2015;163:1641–1654. doi: 10.1016/j.cell.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos Y, Hodgson L, Mantell J, Verkade P, Carlton JG. ESCRT-III controls nuclear envelope reformation. Nature. 2015;522:236–239. doi: 10.1038/nature14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Nakagawa J, Doree M, Labbe JC, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990;61:591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Raab M, Gentili M, de Belly H, Thiam HR, Vargas P, Jimenez AJ, Lautenschlaeger F, Voituriez R, Lennon-Dumenil AM, Manel N, Piel M. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science. 2016;352:359–362. doi: 10.1126/science.aad7611. [DOI] [PubMed] [Google Scholar]
- Sieprath T, Darwiche R, De Vos WH. Lamins as mediators of oxidative stress. Biochem Biophys Res Commun. 2012;421:635–639. doi: 10.1016/j.bbrc.2012.04.058. [DOI] [PubMed] [Google Scholar]
- Smoyer CJ, Jaspersen SL. Breaking down the wall: the nuclear envelope during mitosis. Curr Opin Cell Biol. 2014;26:1–9. doi: 10.1016/j.ceb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, Pleasance ED, Lau KW, Beare D, Stebbings LA, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas JD, Hatch EM, Anderson DJ, Hetzer MW. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus. 2012;3:88–100. doi: 10.4161/nucl.18954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Vietri M, Schink KO, Campsteijn C, Wegner CS, Schultz SW, Christ L, Thoresen SB, Brech A, Raiborg C, Stenmark H. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature. 2015;522:231–235. doi: 10.1038/nature14408. [DOI] [PubMed] [Google Scholar]
- Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol. 2010;2:a000562. doi: 10.1101/cshperspect.a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, Meyerson M, Pellman D. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–184. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]