Abstract
BACKGROUND
Grade 2 gliomas occur most commonly in young adults and cause progressive neurologic deterioration and premature death. Early results of this trial showed that treatment with procarbazine, lomustine (also called CCNU), and vincristine after radiation therapy at the time of initial diagnosis resulted in longer progression-free survival, but not overall survival, than radiation therapy alone. We now report the long-term results.
METHODS
We included patients with grade 2 astrocytoma, oligoastrocytoma, or oligodendroglioma who were younger than 40 years of age and had undergone subtotal resection or biopsy or who were 40 years of age or older and had undergone biopsy or resection of any of the tumor. Patients were stratified according to age, histologic findings, Karnofsky performance-status score, and presence or absence of contrast enhancement on preoperative images. Patients were randomly assigned to radiation therapy alone or to radiation therapy followed by six cycles of combination chemotherapy.
RESULTS
A total of 251 eligible patients were enrolled from 1998 through 2002. The median follow-up was 11.9 years; 55% of the patients died. Patients who received radiation therapy plus chemotherapy had longer median overall survival than did those who received radiation therapy alone (13.3 vs. 7.8 years; hazard ratio for death, 0.59; P=0.003). The rate of progression-free survival at 10 years was 51% in the group that received radiation therapy plus chemotherapy versus 21% in the group that received radiation therapy alone; the corresponding rates of overall survival at 10 years were 60% and 40%. A Cox model identified receipt of radiation therapy plus chemotherapy and histologic findings of oligodendroglioma as favorable prognostic variables for both progression-free and overall survival.
CONCLUSIONS
In a cohort of patients with grade 2 glioma who were younger than 40 years of age and had undergone subtotal tumor resection or who were 40 years of age or older, progression-free survival and overall survival were longer among those who received combination chemotherapy in addition to radiation therapy than among those who received radiation therapy alone. (Funded by the National Cancer Institute and others; ClinicalTrials.gov number, NCT00003375.)
Grade 2 gliomas are relatively uncommon, constituting 5 to 10% of all primary brain tumors in adults. Progressive neurologic symptoms eventually develop in nearly all patients, and nearly all patients die prematurely. At the time of the initiation of our trial, studies had shown that chemotherapy caused tumor regressions in patients with recurrent low-grade gliomas, with regimens that included procarbazine, lomustine (also called CCNU), and vincristine,1 carmustine (also called BCNU) plus interferon,2 and mechlorethamine, vincristine, and procarbazine.3 Similarly, the combination of procarbazine, CCNU, and vincristine, when administered as initial therapy, has been shown to result in tumor regressions.4–6
The initial report that provided the efficacy analysis as specified in the protocol was published in 2012.7 (The protocol is available with the full text of this article at NEJM.org.) At the time of that analysis, 38% of the patients had died, with a median follow-up time of 5.9 years. Here we report the results of a phase 3 trial with long-term follow-up.
METHODS
TRIAL OVERSIGHT
Before the enrollment of the patients, each participating institution obtained approval from an institutional review board, and each patient provided written informed consent. The principal investigators listed in the protocol were responsible for the design and oversight of the trial and for the development of the protocol. The NRG Oncology Statistics and Data Center was responsible for the collection and maintenance of the data. The confidentiality of the data is governed by the policy of the National Institutes of Health. There were no commercial sponsors of this trial. All the authors participated in the analysis of the data, the preparation of the initial draft of the manuscript, and the decision to submit the manuscript for publication. All the authors vouch for the accuracy and completeness of the data and analyses reported and for the adherence of the trial to the protocol.
KEY ELIGIBILITY CRITERIA
Patients were eligible for this trial if they had supratentorial grade 2 astrocytoma, oligodendroglioma, or oligoastrocytoma that was histologically confirmed on pathological review by a central laboratory before randomization. Patients who were 18 to 39 years of age were eligible if they had undergone a subtotal resection or biopsy, and those who were 40 years of age or older were eligible if they had undergone biopsy or resection of any of the tumor. All the patients had to have a Karnofsky performance-status score of 60 or more (on a scale from 0 to 100, with lower numbers indicating greater disability) and a neurologic-function score of 3 or less (on a scale from 0 to 5, with lower numbers indicating better neurologic function) (Table S1 in the Supplementary Appendix, available at NEJM.org). Patients were excluded from trial participation if they had tumor that had spread to noncontiguous leptomeninges, if they had gliomatosis cerebri, if they had had synchronous cancer within the previous 5 years (except for in situ cervical carcinoma or nonmelanoma skin cancer), if they had received prior radiation therapy to the brain or head and neck region, if they had received prior chemotherapy for any reason, if they had chronic lung disease (unless pulmonary-function tests revealed a carbon monoxide diffusion capacity of 60% or more of the predicted value), if they had active infection, or if they were pregnant, breast-feeding, or unable or unwilling to consider the use of effective contraception during treatment.
TREATMENT
The radiation dose was 54 Gy, administered in 30 fractions of 1.8 Gy each (prescribed to the isocenter) over a period of 6 weeks. The radiation volume was defined according to the abnormality on the T2-weighted magnetic resonance imaging (MRI) signal, including any surgical defect. Patients who had been randomly assigned to receive chemotherapy received the treatment after they completed radiation therapy. Chemotherapy consisted of six cycles of procarbazine (at a dose of 60 mg per square meter of body-surface area orally per day on days 8 through 21 of each cycle), CCNU (at a dose of 110 mg per square meter orally on day 1 of each cycle), and vincristine (at a dose of 1.4 mg per square meter [maximum dose, 2.0 mg] administered intravenously on days 8 and 29 of each cycle). The cycle length was 8 weeks.
EVALUATION AND FOLLOW-UP OF THE PATIENTS
A pathological review of all tumor samples was performed at a central laboratory before the initiation of treatment; tumor blocks, cores, or slides were requested for correlative laboratory studies. Patients underwent a Mini–Mental State Examination (MMSE) as well as preoperative and postoperative MRIs that were performed with and without the use of contrast material. MRIs and the MMSE were repeated before cycles three and five, after the completion of therapy, and then every 4 months for 1 year, every 6 months for 2 years, and every year thereafter until tumor progression.
LABORATORY METHODS
To determine IDH1 mutational status, we performed immunohistochemical testing with the mutation-specific monoclonal antibody IDH1 R132H (Dianova; dilution, 1:50) for 30 minutes at room temperature. All immunostaining was performed with the use of a Bond RX autostainer and included the Bond Epitope Retrieval Solution 1 (for 20 minutes) and the Bond Polymer Refine Detection Kit (all from Leica Biosystems). Immunoreaction was scored as positive when tumor cells showed cytoplasmic staining for IDH1 R132H mutation. Staining of macrophages was not scored as positive.
STATISTICAL ANALYSIS
In the initial analysis,7 overall survival and progression-free survival were estimated with the use of the Kaplan–Meier method,8 and differences between treatment groups were tested with the use of the modified Wilcoxon test,9 as specified in the protocol. In this exploratory analysis with long-term follow-up, overall survival and progression-free survival were assessed with the use of the log-rank test, which has the ability to consider late separation in the survival curves.10
Overall survival was measured from the date of randomization to the date of death or the last follow-up date on which the patient was reported to be alive. Progression-free survival was measured from the date of randomization to the date of disease progression or death or the last follow-up date on which the patient was reported to be alive. Patients who did not receive treatment according to the protocol or who discontinued treatment were followed until death. Patients who were lost to follow-up had their data censored on the last date of contact.
Cox proportional-hazards models11 were used to assess the effect of treatment, histologic findings, age, sex, IDH1 R132H mutation status, Karnofsky performance-status score, contrast enhancement, and extent of surgery on progression-free survival and overall survival. Since the proportionality assumption of the Cox proportional-hazards model is not reasonable for both progression-free survival and overall survival, a time-varying treatment effect was added to the models. The cutoff point for this effect was chosen to be 1 year on the basis of the largest log (partial) likelihood. Sensitivity analyses were performed to determine the effect that patients who were lost to follow-up had on the treatment effect for overall survival.
RESULTS
CHARACTERISTICS OF THE PATIENTS
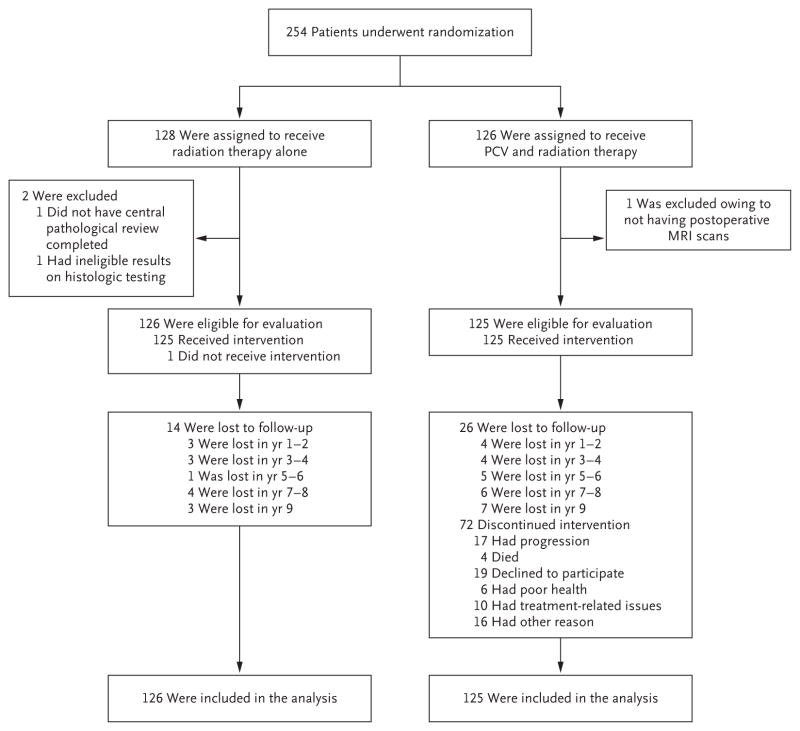
Between October 31, 1998, and June 27, 2002, a total of 254 patients underwent randomization; 128 patients were assigned to radiation therapy alone, and 126 to radiation therapy plus combination chemotherapy with procarbazine, CCNU, and vincristine. Two patients in the group that received radiation therapy alone were found to be ineligible (in 1 patient, pathological review was not performed before randomization, and in another patient, results from the pathological review did not meet the criteria for eligibility), and 1 patient in the group that received radiation therapy plus chemotherapy was found to be ineligible (no postoperative MRI was available). The 251 remaining patients had data that could be evaluated for treatment outcomes and toxic effects.
The median follow-up time of the surviving patients was 11.9 years; at the time of analysis, 67% of the enrolled patients had had tumor progression, and 55% had died (Fig. 1). As summarized in Table 1, patients in the two groups were balanced with regard to previously verified prognostic variables, including age, Karnofsky performance-status score, neurologic function, MMSE score, extent of surgery (as assessed by the operating neurosurgeon), histologic findings, and IDH1 R132H mutation status.
Figure 1. Randomization, Treatment, and Analysis Populations.
PCV denotes procarbazine, lomustine (also called CCNU), and vincristine.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Radiation Therapy Alone (N = 126) | Radiation Therapy plus PCV (N = 125) |
|---|---|---|
| Median age — yr | 40 | 41 |
| Sex — no. (%) | ||
| Male | 77 (61) | 65 (52) |
| Female | 49 (39) | 60 (48) |
| Race or ethnic group — no. (%)† | ||
| White | 116 (92) | 111 (89) |
| Hispanic | 5 (4) | 6 (5) |
| Black | 5 (4) | 5 (4) |
| Asian, American Indian, or Alaska Native | 0 | 3 (2) |
| Karnofsky performance-status score — no. (%)‡ | ||
| 60–80 | 33 (26) | 31 (25) |
| 90 or 100 | 93 (74) | 94 (75) |
| Neurologic function — no. (%) | ||
| No symptoms | 49 (39) | 62 (50) |
| Minor symptoms | 67 (53) | 49 (39) |
| Moderate symptoms and fully active | 5 (4) | 10 (8) |
| Moderate symptoms and not fully active | 5 (4) | 4 (3) |
| Extent of surgery — no. (%) | ||
| Biopsy | 59 (47) | 60 (48) |
| Partial resection | 56 (44) | 51 (41) |
| Total resection | 11 (9) | 14 (11) |
| Histologic findings — no. (%) | ||
| Astrocytoma | 29 (23) | 36 (29) |
| Oligodendroglioma | 57 (45) | 50 (40) |
| Oligoastrocytoma | ||
| Astrocytoma features dominant | 19 (15) | 19 (15) |
| Astrocytoma features equivalent to oligodendroglioma features | 5 (4) | 1 (1) |
| Oligodendroglioma features dominant | 16 (13) | 19 (15) |
| IDH1 R132H mutation — no./total no. (%) | ||
| Present | 35/57 (61) | 36/56 (64) |
| Absent | 22/57 (39) | 20/56 (36) |
| MMSE score — no. (%)§ | ||
| <27 | 11 (9) | 17 (14) |
| 27–30 | 111 (88) | 99 (79) |
| Not completed | 4 (3) | 9 (7) |
There were no significant between-group differences at baseline. IDH1 denotes isocitrate dehydrogenase 1 gene, and PCV procarbazine, lomustine (also called CCNU), and vincristine.
Race or ethnic group was self-reported.
Karnofsky performance-status scores range from 0 to 100, with lower numbers indicating greater disability.
Scores on the Mini–Mental State Examination (MMSE) range from 0 to 30, with lower scores indicating poor cognitive performance. Scores of 27 to 30 are considered to be in the normal range; a score of less than 27 is considered to be abnormal.
TREATMENT DELIVERED
In the group that received radiation therapy alone, 98% of the patients received it per protocol or with an acceptable variation. In the group that received radiation therapy plus chemotherapy, 93% of the patients received radiation therapy per protocol or with an acceptable variation and 56% received chemotherapy per protocol. The median number of chemotherapy treatment cycles was three for procarbazine, four for CCNU, and four for vincristine.
IDH1 MUTATION AND 1P/19Q CODELETION
Tumor blocks, cores, or slides were submitted for 117 patients and were suitable for IDH1 R132H immunohistochemical interpretation for 113 (57 of 126 patients [45%] in the group that received radiation therapy alone and 56 of 125 [45%] in the group that received radiation therapy plus chemotherapy). IDH1 R132H mutations were detected in 35 of 57 patients (61%) in the group that received radiation therapy alone and in 36 of 56 (64%) in the group that received radiation therapy plus chemotherapy (Table 1). IDH1 R132H mutations were detected in 78% of patients with oligodendroglioma, 54% of those with oligoastrocytoma, and 48% of those with astrocytoma (Table S2 in the Supplementary Appendix). Tissue that was sufficient for the determination of other IDH mutations or the codeletion of chromosome arms 1p and 19q (1p/19q codeletion) was available for analysis in 63 patients (29 patients in the group that received radiation therapy alone and 34 in the group that received radiation therapy plus chemotherapy). Because the number of samples and events in each subgroup was so small, reliable assessment could not be made of the effect of these molecular markers on outcomes.
PROGRESSION-FREE SURVIVAL
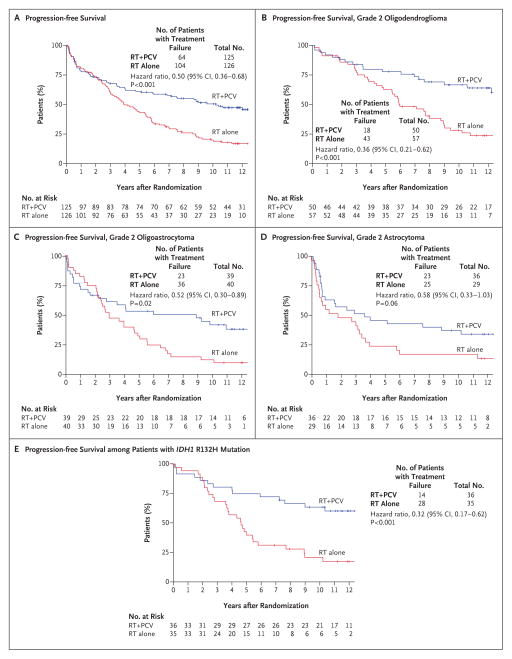
Consistent with what was noted in the previous report,7 patients receiving radiation therapy plus chemotherapy had longer progression-free survival than did those who received radiation therapy alone (Fig. 2A). The median progression-free survival was 10.4 years (95% confidence interval [CI], 6.1 to not reached) among patients in the group that received radiation therapy plus chemotherapy versus 4.0 years (95% CI, 3.1 to 5.5) among those who received radiation therapy alone (hazard ratio for disease progression or death, 0.50; P<0.001). The rate of progression-free survival at 5 years was 61% (95% CI, 53 to 70) among patients who received radiation therapy plus chemotherapy versus 44% (95% CI, 35 to 53) among those who received radiation therapy alone; the corresponding rates at 10 years were 51% (95% CI, 42 to 59) versus 21% (95% CI, 14 to 28). There did not appear to be a between-group difference in progression-free survival during the first 2 years after randomization; however, the difference became apparent after approximately 25% of patients had disease progression, and the difference continued to increase over time.
Figure 2. Progression-free Survival, According to Treatment Group.
All hazard ratios for the comparison of radiation therapy alone (RT) or radiation therapy plus PCV in the analyses of progression-free survival are for disease progression or death, and all P values are two-sided. Tick marks indicate censored data. There were too few events among patients without the R132H mutation in the isocitrate dehydrogenase 1 gene (IDH1)to assess the association with treatment.
In an exploratory analysis of progression-free survival according to individual histologic type, patients who received radiation therapy plus chemotherapy had apparently longer progression-free survival than did patients who received radiation therapy alone, regardless of histologic type (Fig. 2B, 2C, and 2D). In a second exploratory subgroup analysis of progression-free survival according to IDH1 R132H mutation status, patients with tumoral IDH1 R132H mutations had significantly longer progression-free survival than did those without the mutation (P = 0.003) (Fig. S1A in the Supplementary Appendix), regardless of treatment. Among patients with tumoral IDH1 R132H mutations, those in the group that received radiation therapy plus chemotherapy had longer progression-free survival than did those who received radiation therapy alone (P<0.001) (Fig. 2E). The number of events among patients without the IDH1 R132H mutation was too small to determine an association with treatment effect in this subgroup.
In a multivariable analysis of progression-free survival at 1 year of follow-up, the following were identified as favorable prognostic variables: radiation therapy plus chemotherapy (hazard ratio for the comparison with radiation therapy alone, 0.24; 95% CI, 0.12 to 0.45; P<0.001), histopathological findings of oligodendroglioma (hazard ratio for the comparison with oligoastrocytoma, 0.58; 95% CI, 0.34 to 0.98; P = 0.04; hazard ratio for the comparison with astrocytoma, 0.50; 95% CI, 0.26 to 0.95; P = 0.04), and IDH1 R132H mutation (hazard ratio for the comparison with no IDH1 R132H mutation, 0.58; 95% CI, 0.36 to 0.93; P = 0.02) (Table S3A in the Supplementary Appendix). IDH1 R132H mutation was identified as an independent prognostic variable.
OVERALL SURVIVAL
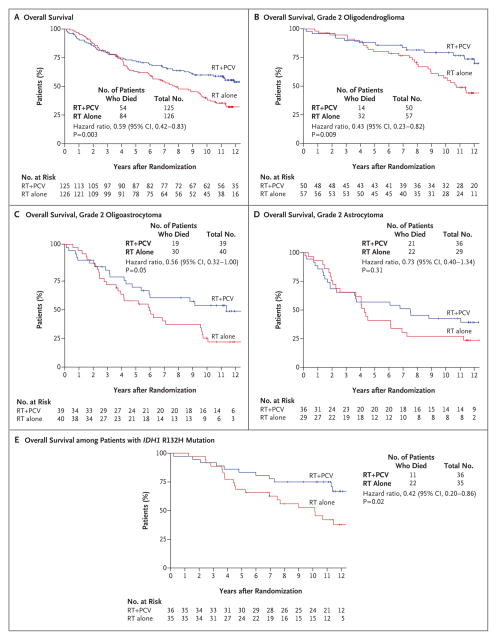
In contrast to outcomes reported previously,7 in the analysis with additional follow-up, patients who received radiation therapy plus chemotherapy had longer overall survival than did those who received radiation therapy alone (Fig. 3A). The median overall survival was 13.3 years (95% CI, 10.6 to not reached) with radiation therapy plus chemotherapy versus 7.8 years (95% CI, 6.1 to 9.8) with radiation therapy alone (hazard ratio for death, 0.59; P = 0.003). The rate of overall survival at 5 years was 72% (95% CI, 64 to 80) in the group that received radiation therapy plus chemotherapy versus 63% (95% CI, 55 to 72) in the group that received radiation therapy alone. The corresponding rates at 10 years were 60% (95% CI, 51 to 69) and 40% (95% CI, 31 to 49). Similar to the distributions for progression-free survival, the distributions for overall survival did not begin to show clear differences between the treatment groups until approximately 4 years after randomization, by which time approximately 25% of the patients had died. Thereafter, the survival distributions continued to diverge in favor of the group that received radiation therapy plus chemotherapy (Fig. 3A).
Figure 3. Overall Survival, According to Treatment Group.
All hazard ratios in the analyses of overall survival are for death, and all P values are two-sided. Tick marks indicate censored data. There were too few events among patients without the IDH1 R132H mutation to assess the association with treatment.
The significant treatment effect was maintained in a sensitivity analysis that categorized all the patients in the two groups who were lost to follow-up as having died (hazard ratio for death in the group that received radiation therapy plus chemotherapy, 0.72; P = 0.03). However, the significant treatment effect was not maintained in a sensitivity analysis in which only patients who received radiation therapy plus chemotherapy and were lost to follow-up were categorized as having died and the patients in the group that received radiation therapy alone had their data censored (hazard ratio, 0.85; P = 0.32). The significant treatment effect was maintained in an analysis that balanced the number of patients who were lost to follow-up in the groups (so that each group would have the same number of patients lost to follow-up) by changing the status of 12 patients in the group that received radiation therapy plus chemotherapy and were lost to follow-up to the status of having died (hazard ratio, 0.68; P = 0.02) (Table S4 in the Supplementary Appendix).
In an exploratory analysis of overall survival according to individual histologic type, the superiority of radiation therapy plus chemotherapy over radiation therapy alone was seen with all histologic diagnoses, although the difference did not reach significance among patients with astrocytoma (Fig. 3B, 3C, and 3D). In a second exploratory subgroup analysis of overall survival according to IDH1 R132H mutation status, patients with tumoral IDH1 R132H mutations had significantly longer overall survival than did those without the mutation, regardless of treatment (P=0.02). The median survival was 13.1 years (95% CI, 10.1 to not reached) among patients with the mutation versus 5.1 years (95% CI, 1.9 to 11.5) among those without the mutation (Fig. S1B in the Supplementary Appendix). Among patients with tumoral IDH1 R132H mutations, those who received radiation therapy plus chemotherapy had longer overall survival than did those who received radiation therapy alone (P = 0.02) (Fig. 3E). The number of events among patients without the IDH1 R132H mutation was too small to determine the association of treatment effect in this subgroup.
Multivariable analyses of overall survival revealed the following favorable prognostic variables: radiation therapy plus chemotherapy after 1 year of follow-up (hazard ratio for death for the comparison with radiation therapy alone, 0.35; 95% CI, 0.19 to 0.66; P = 0.001), histologic findings of oligodendroglioma (hazard ratio for the comparison with oligoastrocytoma, 0.35; 95% CI, 0.19 to 0.66; P = 0.001; hazard ratio for the comparison with astrocytoma, 0.38; 95% CI, 0.18 to 0.81; P = 0.01), and age of less than 40 years (hazard ratio for the comparison with age of ≥40 years, 0.50; 95% CI, 0.29 to 0.87; P=0.01) (Table S3B in the Supplementary Appendix). IDH1 R132H mutation was forced into the model, but the results were not significant (hazard ratio for the comparison with no mutation, 0.66; 95% CI, 0.39 to 1.12; P=0.12).
TREATMENT AFTER DISEASE PROGRESSION
In conjunction with other data items collected after disease progression, treatment after disease progression was reported prospectively. More patients who received radiation therapy alone than patients who received radiation therapy plus chemotherapy underwent surgery or reirradiation or received chemotherapy after tumor progression (Table S5 in the Supplementary Appendix).
TOXIC EFFECTS AND MMSE RESULTS
Table 2 summarizes toxic effects according to treatment group. The most common symptomatic toxic effects were fatigue, anorexia, nausea, and vomiting, which were primarily of grade 1 or 2. Three patients required red-cell transfusions, and one required platelet transfusions. There was one case of neutropenic fever. All these events occurred in patients who were treated with radiation therapy plus chemotherapy. There were no grade 5 toxic effects or reports of myelodysplasia or leukemia.
Table 2.
Most Common Toxic Effects, According to Treatment Group.
| Event | Radiation Therapy Alone (N = 126)
|
Radiation Therapy plus PCV (N = 125)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| no. of patients with event | ||||||||||
| Constitutional symptoms | 43 | 20 | 4 | 1 | 0 | 46 | 30 | 10 | 1 | 0 |
|
| ||||||||||
| Fatigue | 42 | 20 | 3 | 1 | 0 | 47 | 25 | 7 | 1 | 0 |
|
| ||||||||||
| Weight loss | 8 | 0 | 1 | 0 | 0 | 14 | 10 | 4 | 0 | 0 |
|
| ||||||||||
| Blood or bone marrow disorder | 2 | 2 | 1 | 0 | 0 | 11 | 20 | 52 | 12 | 0 |
|
| ||||||||||
| Hemoglobin decreased | 2 | 0 | 0 | 0 | 0 | 32 | 11 | 5 | 1 | 0 |
|
| ||||||||||
| Packed red-cell transfusion required | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 |
|
| ||||||||||
| Platelet count decreased | 1 | 1 | 0 | 0 | 0 | 20 | 12 | 23 | 0 | 0 |
|
| ||||||||||
| Platelet transfusion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
|
| ||||||||||
| Neutropenia | 0 | 0 | 1 | 0 | 0 | 7 | 11 | 44 | 11 | 0 |
|
| ||||||||||
| Febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
|
| ||||||||||
| Infection | 0 | 1 | 0 | 0 | 0 | 11 | 15 | 2 | 0 | 0 |
|
| ||||||||||
| Lymphopenia | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 |
|
| ||||||||||
| Gastrointestinal disorder | 32 | 6 | 2 | 0 | 0 | 45 | 50 | 12 | 0 | 0 |
|
| ||||||||||
| Anorexia | 8 | 1 | 0 | 0 | 0 | 23 | 8 | 1 | 0 | 0 |
|
| ||||||||||
| Constipation | 3 | 0 | 0 | 0 | 0 | 18 | 11 | 0 | 0 | 0 |
|
| ||||||||||
| Nausea | 20 | 4 | 2 | 0 | 0 | 46 | 29 | 3 | 0 | 0 |
|
| ||||||||||
| Vomiting | 3 | 2 | 2 | 0 | 0 | 22 | 15 | 4 | 0 | 0 |
|
| ||||||||||
| Hepatic disorder | 2 | 0 | 0 | 0 | 0 | 27 | 9 | 3 | 2 | 0 |
|
| ||||||||||
| Alanine aminotransferase increased | 0 | 0 | 0 | 0 | 0 | 11 | 1 | 1 | 1 | 0 |
|
| ||||||||||
| Aspartate aminotransferase increased | 0 | 0 | 0 | 0 | 0 | 11 | 1 | 0 | 1 | 0 |
Late events that were attributed to radiation therapy, as assessed by the Radiation Therapy Oncology Group (RTOG)–European Organization for Research and Treatment of Cancer (EORTC) Late Radiation Morbidity Scoring Scheme (Table S6 in the Supplementary Appendix),12 occurred in 245 patients and included toxic effects in the brain in 54 patients (22%): grade 1 events in 38 patients (15%), grade 2 events in 14 (6%), grade 3 events in 1 (<1%), and grade 4 events in 1 (<1%).
Changes in cognitive status, as measured by the MMSE, have been reported in detail previously.13 As compared with their baseline score, patients treated either with radiation therapy alone or with radiation therapy plus chemotherapy had a significantly higher average MMSE score during the first 5 years after randomization. No MMSE data were collected after 5 years.
DISCUSSION
This phase 3 trial showed a survival benefit among patients with grade 2 glioma who were treated with radiation therapy plus chemotherapy, as compared with those who received radiation therapy alone. The treatment effect appeared to be largest in patients with oligodendroglioma or oligoastrocytoma and in patients with IDH1 R132H mutations. The differences in survival cannot be explained on the basis of treatment after tumor progression because patients who received radiation therapy alone had a shorter time to progression and more therapeutic interventions after progression than did those who received radiation therapy plus chemotherapy.
The separation of the progression-free survival curves of the two treatment groups did not begin until 2 to 3 years after randomization, although approximately 25% of the patients in each group had disease progression by then. The differences in outcomes became apparent, as shown by the separation of the progression-free survival curves and the overall survival curves, within each histologic diagnosis category after approximately 15% of patients with oligodendroglioma and approximately 30% of those with either oligoastrocytoma or astrocytoma had disease progression. There was also a delay in the separation of the progression-free survival curves among patients with IDH1 R132H mutations; the same trend of delayed separation was apparent in the overall survival curves. If radiation therapy plus chemotherapy is effective in all patients, we would expect the curves to begin separation much sooner.
In two recently updated phase 3 trials (R940214 and EORTC 2695115), both of which compared radiation therapy alone with radiation therapy plus chemotherapy in patients with newly diagnosed anaplastic oligodendroglioma, patients whose tumors had 1p/19q codeletion, IDH mutations, or both had longer progression-free survival and longer overall survival with radiation therapy plus chemotherapy than with radiation therapy alone. In patients with tumors with neither alteration, there was no significant difference in outcomes between the two treatment groups. However, even in patients with 1p/19q codeletion or IDH mutations, the separation of the progression-free survival curves did not begin until approximately 25% of the events had occurred. Although it is apparent that the patients in the current trial who had IDH1 R132H tumoral mutations also benefited from radiation therapy plus chemotherapy, we cannot determine whether radiation therapy plus chemotherapy was beneficial for patients with wild-type tumoral IDH because of the small number of events in these patients.
A previous retrospective analysis involving patients with low-grade oligodendrogliomas who were treated in clinical trials showed longer survival among patients whose tumors had 1p/19q codeletion than among those whose tumors did not have 1p/19q codeletion.16 Since IDH mutations are associated with the CPG island methylator phenotype, and since most tumors with 1p/19q codeletions also have IDH mutations, it is plausible that DNA-repair enzymes that repair alkylator damage, such as O6-methylguanine–DNA methyltransferase, are silenced by promotor methylation, which results in greater sensitivity to alkylating agents.
As expected, the frequency and severity of toxic effects were greater in the group that received radiation therapy plus chemotherapy than in the group that received radiation therapy alone. Most toxic effects were of grade 1 or 2; toxic effects of grade 3 or 4 were uncommon, except for neutropenia, and were consistent with the toxic effects of other multiagent chemotherapy regimens that have been administered to patients with other cancers. In this trial, which used the RTOG–EORTC Late Radiation Morbidity Scoring Scheme,12 late toxic effects in the central nervous system were reported in 22% of the patients, including grade 1 (mild) events in 15% and grade 2 (moderate) events in 6%. Late toxic effects in the central nervous system that were of grade 3 or 4 were reported in less than 1% of the patients. MMSE scores at 5 years after treatment were as likely to show improvement after treatment as decline.13
Although there appears to be a small cohort of patients with grade 2 glioma who do not benefit from radiation therapy plus chemotherapy, the identification of those patients remains elusive. The magnitude of treatment benefit from combined chemotherapy plus radiation therapy is substantial, but the toxic effects are greater than those observed with radiation therapy alone. Patients and their physicians will have to weigh whether the longer survival justifies the more toxic therapeutic approach.
Supplementary Material
Acknowledgments
Supported by a Radiation Therapy Oncology Group grant (U10 CA21661) and a Community Clinical Oncology Program grant (U10 CA37422) from the National Cancer Institute, a grant (U10 CA25224) from the North Central Cancer Treatment Group, grants (R01CA108633, to Dr. Chakravarti; 1RC2CA148190, to Drs. Chakravarti and Curran; and 1R01CA169368, to Dr. Chakravarti) from the Ohio State University Comprehensive Cancer Center, and a grant (NRG-BN-TS002, to Drs. Bell and Chakravarti) from the Cancer Therapy Evaluation Program of the National Cancer Institute.
We thank Andrea Salavaggione, M.D., for assistance with the IDH immunohistochemical assays.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Cairncross G, Macdonald D, Ludwin S, et al. Chemotherapy for anaplastic oligodendroglioma. J Clin Oncol. 1994;12:2013–21. doi: 10.1200/JCO.1994.12.10.2013. [DOI] [PubMed] [Google Scholar]
- 2.Buckner JC, Brown LD, Kugler JW, et al. Phase II evaluation of recombinant interferon alpha and BCNU in recurrent glioma. J Neurosurg. 1995;82:430–5. doi: 10.3171/jns.1995.82.3.0430. [DOI] [PubMed] [Google Scholar]
- 3.Galanis E, Buckner JC, Burch PA, et al. Phase II trial of nitrogen mustard, vincristine, and procarbazine in patients with recurrent glioma: North Central Cancer Treatment Group results. J Clin Oncol. 1998;16:2953–8. doi: 10.1200/JCO.1998.16.9.2953. [DOI] [PubMed] [Google Scholar]
- 4.Mason WP, Krol GS, DeAngelis LM. Low-grade oligodendroglioma responds to chemotherapy. Neurology. 1996;46:203–7. doi: 10.1212/wnl.46.1.203. [DOI] [PubMed] [Google Scholar]
- 5.Buckner JC, Gesme D, Jr, O’Fallon JR, et al. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol. 2003;21:251–5. doi: 10.1200/JCO.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Stege EM, Kros JM, de Bruin HG, et al. Successful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer. 2005;103:802–9. doi: 10.1002/cncr.20828. [DOI] [PubMed] [Google Scholar]
- 7.Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30:3065–70. doi: 10.1200/JCO.2011.35.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 9.Klein J, Moeschberger M. Survival analysis: techniques for censored and truncated data. New York: Springer; 2003. [Google Scholar]
- 10.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 11.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 12.Fu KK, Pajak TF, Marcial VA, et al. Late effects of hyperfractionated radiotherapy for advanced head and neck cancer: long-term follow-up results of RTOG 83-13. Int J Radiat Oncol Biol Phys. 1995;32:577–88. doi: 10.1016/0360-3016(95)00080-I. [DOI] [PubMed] [Google Scholar]
- 13.Prabhu RS, Won M, Shaw EG, et al. Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: secondary analysis of RTOG 98-02. J Clin Oncol. 2014;32:535–41. doi: 10.1200/JCO.2013.53.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32:783–90. doi: 10.1200/JCO.2013.49.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31:344–50. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–61. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.