Abstract
Background
The median survival for patients with stage IV metastatic melanoma is usually limited to approximately 1 year. In the case of liver metastasis, resection and ablation can achieve long-term survival. This study aimed to describe the outcomes after liver resection or ablation for metastatic melanoma to the liver and identify preoperative prognostic factors.
Methods
Forty eight patients who underwent liver resection (n = 32) or percutaneous ablation (n = 16) were identified from the 1,523 patients with melanoma liver metastases evaluated between January1993 and January 2013.
Results
Median OS was 25.9 months. Median OS was not different after ablation (18 months) and resection (26 months; P > 0.2). Patients in the ablation group more often presented with extrahepatic disease (EHD) (P = 0.008) and received more frequently systemic therapy before ablation (P = 0.005). Patients without EHD tended to have longer OS (26.5 vs. 12 months; P = 0.076) and PFS (13 vs. 5 months; P = 0.11) in the whole cohort. EHD was significantly associated with a worse OS in the resection group (P = 0.034).
Conclusion
Liver resection is associated to prolonged survival over 24 months and should be considered only in selected patients with metastatic disease confined to the liver. In patients not candidate for surgery, tumor ablation can be considered.
Keywords: melanoma liver metastasis, liver resection, ablation therapy, extrahepatic disease
Introduction
The prognosis for metastatic melanoma is poor and metastases can occur long after the primary diagnosis. Once metastatic disease from ocular melanoma is diagnosed, median survival is less than 5 months, with a 1-year survival of 10–15% [1,2]. Long-term outcomes have been recently improved in stage IV cutaneous melanoma but remain poor with a 5-year overall survival up to 28.4% and median survival ranging from 10 to 13.6 months [3–5]. Primary cutaneous and ocular melanomas are known to have different biological behaviors and recurrence patterns [6,7]. Cutaneous melanoma spreads primarily to lymph nodes and soft tissues, with liver disease found in only 15% of patients with metastases [8]. Conversely, hepatic disease is observed in up to 80% of patients with metastatic ocular melanoma, often as the first and only site of metastasis [9]. Until recently, given the lack of effective therapy available in stage IV melanoma management, several retrospective studies have previously advocated that complete resection should be considered in highly selected patients in an attempt to improve overall survival. This approach is associated with a 24–28% 5-year overall survival [10–12]. A recent meta-analysis reported outcomes were similar after liver resection for liver metastases from both primary ocular and cutaneous melanoma. While many biases hampered an accurate comparison between these two groups, overall survival was higher in patients undergoing resection, when compared to a non-operative group [13]. Unfortunately, resectable stage-IV disease remains scarce [14].
For the uncommon highly selected patient with limited and resectable hepatic metastases, resection is considered the treatment of choice. Any patient deemed unresectable or unfit for surgery can undergo other locoregional therapies such as ablation, chemoembolization, or radio embolization [15–17]. Liver ablation therapy is currently validated in the management of small HCC (<3 cm) and also used for colorectal liver metastases (CRLM) [18,19]. To date, several retrospective studies have reported that thermal ablation for melanoma metastasis through a percutaneous or laparoscopic approach is effective, achieving local control in patients not amenable to surgery or refractory to systemic therapy [15,20]. More recently, Faries et al. [21] reported that combined liver resection and ablation can be performed, with an estimated 5-year survival rate of 28%. However, while novel therapies including BRAF/MEK inhibitors and monoclonal antibodies that block CTLA-4 and PD1 are radically changing the management of metastatic cutaneous melanoma, the role of liver metastasectomy for melanoma is still unclear [22]. This retrospective study aimed to report our experience, analyze how liver resection and ablation influence long-term outcomes and identify preoperative prognostic factors to improve patient selection.
Methods
All patients who had a liver resection or ablation for metastatic melanoma between January 1993 and January 2013 were identified in liver resection and percutaneous liver ablation databases and reviewed for inclusion. Both ocular and cutaneous primary melanomas were included.
Data Collection
The following data were recorded: epidemiologic data, information regarding the primary tumor (Demographics, AJCC stage, primary tumor site, and treatment modalities) and data about liver metastases (interval from primary to liver metastases management, previous metastases, number, size, preoperative serum lactate dehydrogenase (LDH), mutational status, and preoperative therapy). Response to preoperative systemic therapy was assessed according to RECIST 1.0 criteria when pre- and post-treatment imaging was available [23]. Surgical morbidity was prospectively recorded in the Memorial Sloan Kettering Secondary Surgical Events program database using a grading system consistent with the “Common Terminology Criteria for Adverse Events Version 4.0” [24]. Morbidity related to percutaneous ablation was retrospectively collected from medical records and graded using the same system as above. Major morbidity was defined as grade 3 or 4. Postoperative mortality was defined as death within 90 days of surgery or ablation that was clearly related to the procedure [25]. Pathologic data, postoperative treatment, recurrence data, and follow-up were also reviewed. Progression was defined as any new intrahepatic or extrahepatic metastatic site diagnosed after liver therapy or postprocedural progression of metastatic sites present preoperatively. This was proven either radiologically or pathologically.
Treatment Modalities
Prior to liver therapy, all patients were reviewed by a multidisciplinary disease management team for performance status, surgical resectability, tumor response to therapy, and biological behavior of the disease. Liver resection was considered in patients with technically resectable liver disease without extrahepatic disease (EHD). With limited EHD, associated resection was discussed on a case-by-case basis. Conversely, liver resection was precluded when hepatic disease was rapidly progressing or deemed surgically unresectable because of extensive bilobar liver disease or associated with multiple sites of EHD on preoperative work up. Major liver resection was defined as a resection of three or more segments.
Percutaneous liver ablation was performed in patients with liver disease amenable to local ablation associated with EHD that was low volume or responding to therapies. Liver ablation was also performed instead of resection when patient's condition precluded surgery. CT-guided local ablation was percutaneously delivered under general anesthesia using radiofrequency, microwave, or cryoablation and was combined with trans-arterial hepatic embolization (TAE) at the discretion of the radiologist. Patients with embolization alone were not included in this series.
Statistical Analysis
Utilizing SPSS Statistics software (version 22.0, IBM, Armonk, New York), continuous variables were expressed as median or mean ± standard deviation and were compared using an independent samples t-test. Categorical variables were compared using a χ2 test. Univariate and multivariate analyses were performed. Overall survival (OS) and progression-free survival (PFS) were computed from the time of operative resection or ablation to the date of death or last follow-up and to the first recurrence/progression date, respectively. Estimates of OS and PFS probabilities were calculated using the Kaplan–Meier method; the log rank test was used to compare survival data. The threshold for statistical significance was set at P < 0.05 Our institutional review board approved this study.
Results
Preoperative Data
Comparison regarding primary origin
During the study period, 1,523 patients with melanoma liver metastases with or without extrahepatic metastatic sites were evaluated at our institution. Overall, only 48 patients (3.2%) undergoing complete resection or percutaneous ablation were identified (Table I). Fifteen liver resections and 11 percutaneous ablations were performed in 26 patients for liver metastases from primary cutaneous melanoma (median Breslow index = 3 mm; range, 0.4–6.2). Seventeen liver resections and five percutaneous ablations were performed in 22 patients presenting with liver metastases from ocular melanoma (median basal tumor diameter = 16 mm; range, 9–18). Although symptomatic in six patients (12.5%), metastatic disease to the liver was diagnosed most frequently during routine melanoma surveillance imaging (79.5%). Patients with metastatic disease from ocular origin were younger (47 years; range 20–67) than patients with metastatic cutaneous melanoma (64 years; range, 34–83; P < 0.001) and the disease-free interval before liver therapy was significantly longer in patients with metastatic disease from ocular origin (54 months vs. 14 months; P = 0.006). No difference in terms of EHD, liver tumor size and number, and liver therapy modality was found between these two subgroups. Twenty patients (41.3%) had mutational screening, of which three patients (11.5%) with metastatic cutaneous melanoma harbored BRAF V600K mutation (n = 2) and NRAS pQ61.3 mutation (n = 1) whereas six patients (27.3%) presented with mutated GNA11 Q209 metastatic ocular melanoma.
Table I. Descriptive Data According to Primary Melanoma Origin and Treatment Modality.
| Total (n = 48) | Resection (n = 32) | Ablation (n = 16) | P-value | |
|---|---|---|---|---|
| Age, years | 55 (20–83) | 55 (20–77) | 54 (40–83) | ns |
| Sex (male/female) | 27/21 | 17/15 | 10/6 | ns |
| Primary | 0.15 | |||
| Cutaneous | 26 | 15 (57%) | 11 (43%) | |
| Ocular | 22 | 17 (81%) | 5 (19%) | |
| Primary to LM interval, months | 78 (5–319) | 86 (5–319) | 45 (5–93) | 0.017 |
| DFI before Liver Mets, months | 16 (5–283) | 31 (5–283) | 11 (5–248) | 0.011 |
| Stage III-IV before LM | 21 (43.8%) | 10 (31.3%) | 11 (68.8%) | 0.03 |
| Preoperative therapy | 19 (39.6%) | 8 (25%) | 11 (68.8%) | 0.005 |
| EHD | 15 (31.3%) | 6 (18.8%) | 9 (56.3%) | 0.008 |
| Number of liver lesions | ns | |||
| 1 | 29 (60.4%) | 20 (62.5%) | 9 (56.3%) | |
| 2 | 13 (27.1%) | 7 (21.9%) | 6 (37.5%) | |
| >2 | 6 (12.5%) | 5 (15.7%) | 1 (6.3%) | |
| Largest LM, mm | 25.5 (4–105) | 40 (4–105) | 22 (10–50) | 0.004 |
| Postoperative therapy | 14 (29.2%) | 13 (40.6%) | 6 (37.5%) | ns |
DFI, disease-free interval; EHD, extrahepatic disease; LM, liver metastasis; ns, non significant with P > 0.2.
Clinical characteristics of our cohort regarding treatment modality of liver disease.
Continuous variables are reported as median (range) when appropriate.
Categorical variables are reported with (%).
Comparison regarding treatment modalities
A total number of 32 patients underwent liver resection and 16 were treated by percutaneous ablation (radiofrequency, n = 8; microwave, n = 6; cryoablation, n = 2) along with TAE in three cases. One patient in the resection group received concomitant laparoscopic resection and radiofrequency ablation (RFA). Characteristics of both cohorts are outlined in Table I. Notably, patients who underwent ablation presented with more adverse disease characteristics with a significantly shorter disease-free interval between primary melanoma diagnosis and treatment initiation for liver metastases (11 months vs. 31 months; P = 0.011) and more often harbored EHD (56.3% vs. 18.8%; P = 0.008). There was no difference regarding the number of liver tumors between both treatment groups. Tumor size, however, was significantly greater in the resection group (40 mm vs. 25.5 mm, P = 0.004). Preprocedural systemic therapy was more often delivered (P = 0.005) in the ablation group (68%, 11 patients receiving respectively chemotherapy alone, n = 4; BRAF inhibitor alone, n = 4; anti-CTLA4 agent alone, n = 1; anti-CTLA4 agent combined with chemotherapy, n = 2) than in the resection group (25%, eight patients receiving respectively systemic chemotherapy alone, n = 4; anti-CTLA4 alone, n = 3; combined anti-CTLA4 and PD1 blocker, n = 1). In the nine patients harboring EHD before percutaneous liver ablation, partial response was observed in seven cases while two patients remained stable under treatment. No patient experienced progression of disease on their preoperative therapy in either group.
Procedural Data
Of the 32 patients resected, nine (28.1%) underwent major liver resection. Associated EHD resection was performed after intraoperative EHD diagnosis in four patients (partial gastrectomy, n = 1; peritoneal resection, n = 1; small bowel resection, n = 1; right adrenalectomy, n = 1) and in two patients for preoperatively suspected limited EHD (cervical lymphadenectomy, n = 1; distal pancreatectomy, n = 1). Portal lymphadenectomy was carried out in eight patients. Major morbidity occurred in three cases (9.4%). No mortality was observed within 90 days of surgery. Complete microscopic resection (R0) was achieved in 30 patients (93.8%).
Of the 16 patients in the ablation group, eight were treated with radiofrequency ablation, six received microwave-ablation, and two underwent cryoablation. Concurrent TAE was performed in three cases. Seven patients (43.8%) had at least two tumors treated in the liver. No patient experienced major morbidity. Nine patients harbored EHD at the time of procedure (lung, n = 4; bone, n = 1; retroperitoneal lymph nodes, n = 2; multiple sites, n = 2) and all were under systemic therapy, as aforementioned. Postprocedural therapy was delivered equally in 40.6% of patients after resection and 37.5% after ablation (P = 0.7).
Survival and Progression
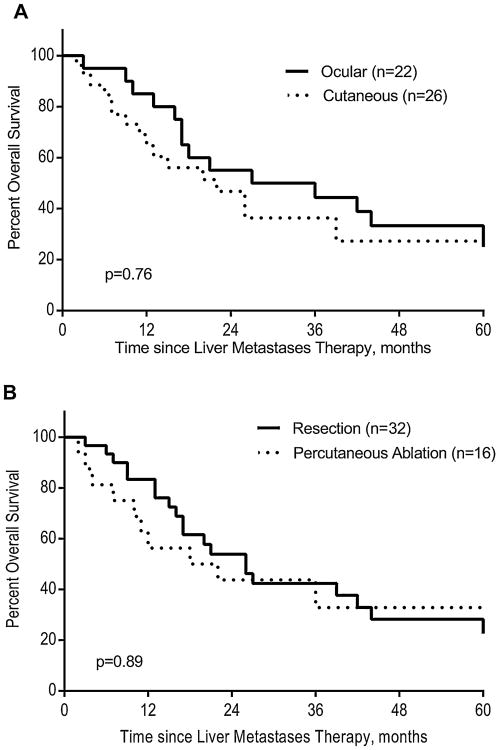
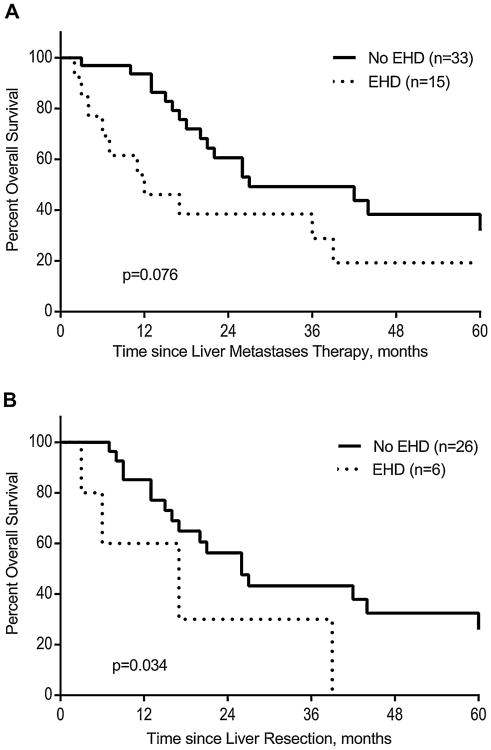
Median overall survival in the whole cohort was 25.9 months. Estimated 5-year OS was 30%. Among the 16 patients alive at last follow-up (33.3%), (10 after resection and 6 after percutaneous ablation), the median follow-up was 32.8 months (range, 12–174). Figure 1A demonstrates the similar outcomes between ocular and cutaneous primary melanomas (P = 0.73). There was a trend towards improved overall survival among the resection patients with a median overall survival of 26 months (range, 3–174) and 18 months (range, 2–70) after resection and ablation, respectively (Fig. 1B). Age was the only factor significantly associated with OS in univariate analysis (P = 0.032; HR = 0.97, CI95% 0.95–0.99) and EHD tended to shorten OS (P = 0.076; HR = 3.68; Fig. 2A). Results of univariate analysis using different clinical variables are listed in Table III. In subset analysis, only EHD was significantly associated with lower overall survival in the resected group (P = 0.034; HR = 4.4; Fig. 2B). EHD had no association with survival in the percutaneous ablation group (P = 0.72). Conversely, the number of liver metastases tended to be associated with a worse OS in patients treated with percutaneous ablation (P = 0.08).
Fig. 1.
Neither melanoma type (A) nor treatment modality (B) impact significantly overall survival.
Fig. 2.
Kaplan Meier overall survival after liver resection and ablation (A) and after liver resection alone (B) in patients stratified by the presence of extrahepatic disease.
Table III. Progression Patterns After Melanoma Liver Metastasis Therapy According to the Primary Origin.
| Cutaneous melanoma (n = 26) | Ocular melanoma (n = 22) | P-value | |||
|---|---|---|---|---|---|
|
|
|
||||
| Resection = 15/Ablation = 11 | TTP | Resection = 17/Ablation = 5 | TTP | ||
| IH recurrence only | 2 (7.7%) | 10.6 | 9 (36.4%) | 10.4 | 0.005 |
| After liver resection | 1 | 7 | |||
| After liver ablation | 1 | 2 | |||
| EH recurrence only | 10 (38.5%) | 6.6 | 5 (22.7%) | 18.7 | ns |
| After liver resection | 6 | 4 | |||
| After liver ablation | 4 | 1 | |||
EH, extrahepatic; IH, intrahepatic; ns, non significant with P > 0.2; TTP, time to recurrence in months after liver metastasis therapy.
Overall, 37 patients (77.1%) experienced disease progression at a median time of 9.1 months (range; 2–35) after liver metastasis therapy. The estimated 3-year PFS rate was 8%. After resection or ablation, nine (18.7%) and two patients (4.2%) have not recurred after a median follow-up of 31 months (range, 16–78). No clinical variable was significantly associated with PFS in the whole cohort (Table II) and in the resection subset. In the ablation subset, the number of liver metastases tended to correlate with worse PFS (P = 0.07). Time to progression appeared to be slightly shorter after percutaneous ablation than resection (7.1 months vs. 10.6 months; P = 0.054). Depending on melanoma origin, ocular melanoma patients tend to progress more in the liver than cutaneous melanoma (82% vs. 65%; P = 0.07) whereas cutaneous melanoma tended to progress earlier than ocular melanoma after metastases liver therapy (5.1 months vs. 11 months; P = 0.036). Progression patterns after liver metastasis therapy are summarized in Table III. Notably, five patients (31.3%) relapsed at the site of percutaneous liver ablation (after radiofrequency ablation, n = 3; after micro-wave ablation, n = 1; after cryoablation, n = 1) and one patient (3.1%) recurred after resection.
Table II. Predictors of OS and PFS in Univariate Analysis (n = 48).
| Median OS, months | Overall survival | Median DFS, months | Progression-free survival | |
|---|---|---|---|---|
| Primary melanoma type | ||||
| Cutaneous | 22 | ns | ns | |
| Ocular | 27.2 | |||
| Sex | ||||
| Male | 26 | ns | 11 | ns |
| Female | 18 | 9 | ||
| Age | ||||
| <60 years | 17.4 | 0.03 | 11 | 0.09 |
| >60 years | 27.2 | 0.07 | 12.1 | 0.07 |
| Primary to liver metastases interval, months | 0.19 | 0.19 | ||
| Preoperative LDH level > 400U/l | ||||
| Yes | 18 | ns | 4 | ns |
| No | 22 | 10 | ||
| Preoperative therapy | ||||
| Yes | 36 | ns | 11 | ns |
| No | 20 | 10 | ||
| EHD | ||||
| Yes | 12 | 0.076 | 5 | 0.11 |
| No | 26.5 | 13 | ||
| Number of liver metastases | ||||
| Solitary | 27.2 | 0.16 | 12 | ns |
| Multiple | 17.4 | 0.16 | 4 | 0.13 |
| Largest liver metastases, cm | ns | ns | ||
| Postoperative therapy | ||||
| Yes | 20 | ns | 11 | ns |
| No | 36 | 11 | ||
EHD, extrahepatic disease; LDH, lactate dehydrogenase; ns, non significant with P > 0.2.
Discussion
Liver metastases can arise from both primary cutaneous and ocular melanomas and their behavior is often different. Ocular melanoma frequently affects younger patients with often a significantly longer interval to the first recurrence and a clear metastatic organotropism for the liver. Liver metastases from cutaneous melanoma, however, are less frequent, and occur shortly after the diagnosis of the primary lesion. As several other series have reported, survival after regional therapy for liver metastasis are similar in both melanoma types [26].
The scarcity of patients undergoing surgery or local therapy for stage IV disease hampers more comprehensive investigation on the impact of surgery on long-term outcomes. The median overall survival of 25.9 months and an actuarial 5-year overall survival reaching 30% in our cohort were both similar to those observed in previous reports [10,11]. To date, one phase II trial and retrospective studies have been conducted in small cohorts of resected patients and favor complete surgical resection [11,27,28]. Howard et al. [12] reported a comparative analysis in 291 stage IV cutaneous melanoma patients that were enrolled in the Multicenter Selective Lymphadenectomy Trial (MSLT-1]. In total, 161 underwent resection ± systemic medical therapy (SMT) versus 130 treated by systemic therapy alone. Median survival was 15.8 versus 6.9 months and 4-year survival was 20.8% versus 7.0% for patients receiving surgery ± SMT versus SMT alone (P < 0.0001; HR = 0.406). Both groups, however, were not equivalent in terms of number of metastatic sites and tumors, suggesting different tumor biology.
In the current study, only 32 (2.1%) and 16 patients (1.1%) were selected for liver resection and percutaneous tumor ablation, respectively. No mortality was observed in any patients in this series. Major morbidity was low after resection (9.4%) and nonexistent after ablation. Survival tended to be longer in the resection group (26 months) when compared to the ablation group (18 months, P = 0.89).
Multimodal therapy for melanoma liver metastasis including liver resection or ablation improves long-term outcomes in some selected patients. Six patients (12.5%), respectively four after resection and two after percutaneous ablation, survived longer than 5 years. Additionally, among the 16 patients alive (33.3%) after a median follow-up of 32.8 months (range, 12–174), nine patients (18.7%) and two patients (4.2%) did not recur after resection or ablation, respectively. However, prognostic criteria reflecting favorable tumor biology to select those patients benefiting from such therapies are lacking.
In the current series, EHD was a negative prognostic factor in the resection group (HR = 4.2; P = 0.04) and tended towards a worse OS (P = 0.076) and PFS (P = 0.11) in the whole cohort. When disease was not confined to the liver, median overall survival after resection or ablation was only 6 and 12 months, respectively. Thus, in patients with EHD, liver resection was associated with a short survival time. A previous report by Adam et al. similarly reported that EHD was associated with survival in a large cohort of patients that underwent liver resection for non-colorectal, non-neuroendocrine liver metastases. This series included 148 patients with melanoma liver metastases [29]. Hence, a careful imaging work-up should be performed to assess the extent of the disease before liver resection. Bronstein et al. [30] recommended PET-CT, especially in the staging of patients with cutaneous melanoma, due to its tendency to metastasize to lymph nodes and soft tissues.
Preoperative systemic therapy was predominantly used within the ablation group. Eleven patients (68.8%) in the ablation group received preoperative therapy, of whom seven were treated with novel therapies such as BRAF-inhibitors (n = 4) and CTLA4 blockade agents (n = 3). In contrast, only eight patients (25%) received systemic therapy in the resection group. All patients with EHD (n = 9) in the ablation group received preoperative therapy and seven (78%) experienced tumor response in both hepatic and extrahepatic tumor site. It is possible that improved systemic control of the disease related to preoperative systemic therapy might explain the similarity in outcomes between both resection and ablation groups, despite the higher risk nature of the ablation group. Alternatively, one can argue that possible synergistic effects between systemic therapy and thermal ablation may improve disease control as recently reported about the combination of radiotherapy with immunotherapy [31]. In addition, this could explain the longer median OS observed in patients with EHD undergoing liver ablation instead of resection. Finally, ablation therapy might be favored in patients with multiple metastatic sites experiencing EHD improvement under medical therapy while needing further treatment in the liver. This approach was adopted in two patients presenting with stable disease in the liver with BRAF-inhibitor therapy and responding tumor in the lung. One patient is still alive 31 months after microwave ablation of two liver lesions. The second did progress 4 months post ablation in the lungs and the liver and eventually died after 12 months. Nevertheless, multimodal therapies for metastatic melanoma are not established. Several clinical trials analyzing novel therapies impact in stage IV melanoma patients including high rates of patients with visceral metastases have recently reported objective response rate up to 40% and median OS reaching 16.8 months namely with PD-1 blockade [32–35]. Preoperative induction immunochemotherapy followed by resection has been associated with prolonged survival and complete remission in advanced metastatic melanomas [36–39]. Such an approach using induction therapies before metastasectomy will need further investigation.
Our current results are limited for several reasons. First, the small number of patients undergoing resection and ablation to treat hepatic melanoma metastases hampered the identification of prognostic factors, especially in multivariate analysis. This emphasizes the difficulty in evaluating the impact of complete resection or ablation to treat metastatic melanoma to the liver. Secondly, the study is retrospective in nature, and thus associations are correlative rather than conclusive. Additionally, the study time period over 20 years may implicate time-lead bias especially regarding follow-up protocols and systemic therapy modalities which have changed overtime. Finally, the resection and ablation groups were not comparable in term of disease characteristics and our findings comparing liver resection and percutaneous ablation should be carefully considered.
In conclusion, liver resection should be considered first in patients with resectable hepatic disease only. Presence of any EHD should preclude surgery. Patients unfit for surgery with limited disease in the liver or in patients with EHD responding to systemic therapy needing liver disease control can be considered for percutaneous tumor ablation but survival benefit remains unclear. Combining surgery or tumor ablation for metastatic melanoma to the liver with novel therapies in a tailored multimodal approach remains to be evaluated in the future.
Acknowledgments
Dr. Doussot reported completing this work when supported by research fellowship grants from the French Association of Hepatobiliary Surgery and Transplantation (ACHBT) and the Universite de Bourgogne.
Dr. Nardin reported completing this work when supported by research fellowship grants from the French Society of Dermatology (SFD) and the College des Enseignants en Dermatologie de France (CEDEF).
Grant sponsor: French Association of Hepatobiliary Surgery and Transplantation (ACHBT).; Grant sponsor: Universite de Bourgogne.; Grant sponsor: French Society of Dermatology (SFD).; Grant sponsor: College des Enseignants en Dermatologie de France (CEDEF).
Footnotes
All other authors have no conflict of interest to disclose.
References
- 1.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123:1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 2.Singh AD, Borden EC. Metastatic uveal melanoma. Ophthalmol Clin N Am. 2005;18:143–150. ix. doi: 10.1016/j.ohc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Lebbé C, Weber JS, Maio M, et al. Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2014;25:2277–2284. doi: 10.1093/annonc/mdu441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuidervaart W, van Nieuwpoort F, Stark M, et al. Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. Br J Cancer. 2005;92:2032–2038. doi: 10.1038/sj.bjc.6602598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel JK, Didolkar MS, Pickren JW, et al. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 8.Leiter U, Meier F, Schittek B, et al. The natural course of cutaneous melanoma. J Surg Oncol. 2004;86:172–178. doi: 10.1002/jso.20079. [DOI] [PubMed] [Google Scholar]
- 9.Eskelin S, Pyrhönen S, Summanen P, et al. Screening for metastatic malignant melanoma of the uvea revisited. Cancer. 1999;85:1151–1159. [PubMed] [Google Scholar]
- 10.Wood TF, DiFronzo LA, Rose DM, et al. Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival? Ann Surg Oncol. 2001;8:658–662. doi: 10.1007/s10434-001-0658-4. [DOI] [PubMed] [Google Scholar]
- 11.Sanki A, Scolyer RA, Thompson JF. Surgery for melanoma metastases of the gastrointestinal tract: Indications and results. Eur J Surg Oncol EJSO. 2009;35:313–319. doi: 10.1016/j.ejso.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Howard JH, Thompson JF, Mozzillo N, et al. Metastasectomy for distant metastatic melanoma: Analysis of data from the first multicenter selective lymphadenectomy trial (MSLT-I) Ann Surg Oncol. 2012;19:2547–2555. doi: 10.1245/s10434-012-2398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aubin JM, Rekman J, Vandenbroucke-Menu F, et al. Systematic review and meta-analysis of liver resection for metastatic melanoma. Br J Surg. 2013;100:1138–1147. doi: 10.1002/bjs.9189. [DOI] [PubMed] [Google Scholar]
- 14.Wevers KP, Hoekstra HJ. Stage IV melanoma: Completely resectable patients are scarce. Ann Surg Oncol. 2013;20:2352–2356. doi: 10.1245/s10434-013-2881-1. [DOI] [PubMed] [Google Scholar]
- 15.Amersi FF, McElrath-Garza A, Ahmad A, et al. Long-term survival after radiofrequency ablation of complex unresectable liver tumors. Arch Surg Chic Ill 1960. 2006;141:581–587. doi: 10.1001/archsurg.141.6.581. discussion 587–588. [DOI] [PubMed] [Google Scholar]
- 16.Kamat PP, Gupta S, Ensor JE, et al. Hepatic arterial embolization and chemoembolization in the management of patients with large-volume liver metastases. Cardiovasc Intervent Radiol. 2008;31:299–307. doi: 10.1007/s00270-007-9186-3. [DOI] [PubMed] [Google Scholar]
- 17.Gonsalves CF, Eschelman DJ, Sullivan KL, et al. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: A single-institution experience. AJR Am J Roentgenol. 2011;196:468–473. doi: 10.2214/AJR.10.4881. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. doi: 10.1186/1471-230X-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stang A, Oldhafer KJ, Weilert H, et al. Selection criteria for radiofrequency ablation for colorectal liver metastases in the era of effective systemic therapy: A clinical score based proposal. BMC Cancer. 2014;14:500. doi: 10.1186/1471-2407-14-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berber E, Siperstein AE. Perioperative outcome after laparoscopic radiofrequency ablation of liver tumors: An analysis of 521 cases. Surg Endosc. 2007;21:613–618. doi: 10.1007/s00464-006-9139-y. [DOI] [PubMed] [Google Scholar]
- 21.Faries MB, Leung A, Morton DL, et al. A 20-year experience of hepatic resection for melanoma: Is there an expanding role? J Am Coll Surg. 2014;219:62–68. doi: 10.1016/j.jamcollsurg.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karimkhani C, Gonzalez R, Dellavalle RP. A review of novel therapies for melanoma. Am J Clin Dermatol. 2014;15:323–337. doi: 10.1007/s40257-014-0083-7. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayo SC, Shore AD, Nathan H, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB. 2011;13:473–482. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu SW, Saw R, Scolyer RA, et al. Liver resection for metastatic melanoma: Equivalent survival for cutaneous and ocular primaries: Liver resection for metastatic melanoma. J Surg Oncol. 2013;108:129–135. doi: 10.1002/jso.23361. [DOI] [PubMed] [Google Scholar]
- 27.Sosman JA, et al. A phase 2 trial of complete resection for stage IV melanoma. Cancer. 2011:1–7. doi: 10.1002/cncr.26111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawlik TM, Zorzi D, Abdalla EK, et al. Hepatic resection for metastatic melanoma: Distinct patterns of recurrence and prognosis for ocular versus cutaneous disease. Ann Surg Oncol. 2006;13:712–720. doi: 10.1245/ASO.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: Analysis of 1452 patients and development of a prognostic model. Trans Meet Am Surg Assoc. 2006;124:189–200. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bronstein Y, Ng CS, Rohren E, et al. PET/CT in the management of patients with stage IIIC and IV metastatic melanoma considered candidates for surgery: Evaluation of the additive value after conventional imaging. Am J Roentgenol. 2012;198:902–908. doi: 10.2214/AJR.11.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: A review of clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;88:986–997. doi: 10.1016/j.ijrobp.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 33.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 35.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwu WJ, Panageas KS, Menell JH, et al. Phase II study of temozolomide plus pegylated interferon-alpha-2b for metastatic melanoma. Cancer. 2006;106:2445–2451. doi: 10.1002/cncr.21909. [DOI] [PubMed] [Google Scholar]
- 37.Gutman H, Ben-Ami E, Shapira-Frommer R, et al. Multidisciplinary management of very advanced stage III and IV melanoma: Proof-of-principle. Oncol Lett. 2012;4:307–310. doi: 10.3892/ol.2012.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koers K, Francken AB, Haanen JBAG, et al. Vemurafenib As neoadjuvant treatment for unresectable regional metastatic melanoma. J Clin Oncol. 2013;31:e251–e253. doi: 10.1200/JCO.2012.45.3845. [DOI] [PubMed] [Google Scholar]
- 39.La Greca M, Grasso G, Antonelli G, et al. Neoadjuvant therapy for locally advanced melanoma: New strategies with targeted therapies. Onco Targets Ther. 2014;7:1115–1121. doi: 10.2147/OTT.S62699. [DOI] [PMC free article] [PubMed] [Google Scholar]