Abstract
BACKGROUND
Routine management of patients with acute decompensated heart failure (ADHF) requires careful attentiveness to fluid status and diuretic treatment efficacy. New advances in ultrasound have made ultraportable echocardiography (UE) available to physicians for point-of-care use. The purpose of this study is to explore physiologic measures of intravascular fluid volume derived from UE and explore predictors of diuretic response in ADHF.
METHODS
Various echocardiography imaging measurements, particularly diameter and collapse of inferior vena cava (IVC), were collected from 77 patients admitted with a primary diagnosis of ADHF. Patients were divided into two groups based on whether or not they achieved a net negative fluid output of 3 L within 48 hours. The demographic information, serum laboratory markers, and physical characteristics of the subjects were obtained to correlate with daily ultrasound measurements. Univariate and multivariate analyses were used to compare diuretic “responders” to “nonresponders.”
RESULTS
A negative change in the IVC diameter at 48 hours was robust in prediction of diuretic response. For every 1 mm decrease in the IVC diameter at 48 hours, there was an odds ratio of 1.62 (95% CI: 1.20–2.19) for responding to diuretic therapy independent of other variables including baseline renal filtration function and blood B-type natriuretic peptide.
CONCLUSION
Assessment of central venous pressure as a proxy for passive renal congestion independently predicts initial diuretic response in ADHF. Future research is needed to further understand the individual variation in response to loop diuresis and to identify optimal treatment approaches that utilize anatomic and physiologic measures such as venous ultrasound.
Keywords: ultraportable echocardiography, cardiac ultrasound, heart failure
Introduction
The prevalence of heart failure (HF) continues to increase and is now the most common cause of hospitalization in the United States.1,2 Routine management of patients with HF requires careful attentiveness to fluid status and diuretic treatment efficacy. Although diuretics have been in clinical use for decades, patient response and clinical effectiveness are uncertain.3 Although the physical examination is a critical component for decision-making, it is limited by body habitus or subclinical hemodynamic fluid changes. In addition to rales, edema, and elevated jugular venous pulse, clinicians measured weight, urine output, and serum creatinine on a daily basis in order to determine when euvolemia is achieved.
Heterogeneity of HF and disease management present significant challenge, and often other comorbidities and conditions complicate presentation of acute decompensated heart failure (ADHF; ie, cardiorenal syndrome).4 The two basic hemodynamic explanations for the development of cardiorenal syndrome are (1) inadequate forward perfusion typically after one or two parenteral doses of loop diuretic and (2) passive venous congestion that temporarily worsens with plasma refill from the extravascular tissues in the setting of diuresis for ADHF. Daily point-of-care ultrasound is ideal to better explore these hemodynamic changes in this patient population.
Assessment of cardiac structure and function is an important component in the clinical management of ADHF and to understand the pathophysiology of the individual patient. Transthoracic echocardiography is the primary method of imaging individuals with HF; however, echocardiography is used infrequently and typically not serially secondary to cost and resource utilization.5 Current appropriateness guidelines do not address the use of point-of-care echocardiography in clinical scenarios of ADHF to guide acute response to therapy.6 The recent availability of miniaturized ultrasound technology enables physicians to perform real-time, point-of-care UE assessment of patients.7 When applied to patients with dynamic cardiovascular disorders such as ADHF, this technology allows for daily objective assessment of intravascular fluid status.
Although the benefit of these devices seems obvious, little data exist on the type of information or additive benefit these devices provide in the routine care of HF patients particularly with respect to inferences concerning passive venous congestion of the kidneys. This prospective, observational trial examined the use of handheld, ultraportable echocardiography (UE) by physicians in patients admitted with ADHF. The purpose of this study was to measure daily changes in UE in patients with ADHF and compare those who respond to diuretic therapy to those who did not.
Methods
This prospective comparative trial examines physiologic measures of intravascular fluid volume derived from UE and explores predictors of diuretic response in ADHF. The research was conducted in accordance with the Declaration of Helsinki. The protocol was reviewed and approved by the Genesys Health System Institutional Review Board.
Setting and participants
This study was conducted from December 2011 to January 2012 at Genesys Regional Medical Center (GRMC), a 410-bed community-based teaching hospital. Symptomatic patients admitted to GRMC with the primary diagnosis of ADHF who were able to provide informed consent were considered for selection during initial cardiac consultation. Subjects with adequate visualization with UE (as determined by preliminary imaging by the echocardiologist study investigator [TEV]) were invited to be a part of the study. Patients were excluded if they had poor, nondiagnostic imaging on UE, unable to provide informed consent, younger than 18 years, or if a prisoner or other institutionalized individual. Ultimately, 77 subjects were enrolled and were typically scanned with the ultrasound device on a daily occurrence during routine physical examination. During data analysis, patients were divided into two groups based on whether or not they achieved a negative fluid output of 3 L within 48 hours. Achievement of 3 L of fluid removal represented the median urine output of all study participants and was therefore selected as the criterion for patient inclusion in the diuretic “responder” cohort.
Device and measurements
The ultraportable two-dimensional ultrasound device used in this study measures 3″ wide by 5.3″ tall and weighs <1 pound (Vscan; GE). This pocket-size echocardiography has the capability of providing high-quality black and white imaging with Doppler color-coded blood flow imaging. The study echocardiologist, board-certified in adult cardiovascular medicine and adult echo cardiography and experienced in echocardiographic acquisition, performed all cardiac ultrasound measurements. Daily acquired images were stored in the device for further analysis.
Daily assessment of left ventricle function with standard parasternal long- and short-axis views and apical two- and four-chamber views was performed. The mitral and aortic valves were measured using color Doppler assessment. Finally, the size and degree of collapse of the IVC and presence/size of pericardial effusions were measured. Subcostal views of the junction of the IVC, hepatic vein and right atrium (RA), and IVC collapse during inspiration were also examined. The long- and short-axis views of the IVC were obtained and measurements were obtained at end-expiration just proximal to the junction of the hepatic veins (approximately 0.5–3.0 cm before the ostium of the RA). These measurements were taken at the bedside with a caliper tool available on the device, and snapshots of the actual measurements were recorded in the device. In addition, video image clips of the IVC were acquired to access IVC variability with respiration. IVC function was subjectively graded as having no collapse (IVC collapse score = 2), IVC collapse <20% with quiet respiration (IVC collapse score = 1), and IVC collapse (IVC collapse score = 0) ≥20% (IVC collapse score = 0) with quiet respiration. Daily measurements were recorded in the progress notes of the patient’s clinical chart during routine evaluation.
Data collection
Study investigators collected subject data including demographic information, medical history, and standard inpatient HF clinical data with a structured database. Ultrasound measurements, subject variables, and clinical parameters were entered into a database on a daily basis.
Patient demographic information collected included age, gender, weight, and height to calculate body mass index. Factors regarding patient medical history such as documented history of HF, relevant comorbid conditions listed in the medical record, social history of smoking or alcohol use, family history of heart disease, and presence of an implantable device such as a pacemaker or automatic implantable cardioverter defibrillator were obtained. Laboratory values collected included daily serum sodium, potassium, blood urea nitrogen, and creatinine, as well as serial troponin-T and brain natriuretic peptide level at admission. Variables from chest radiography included presence of pleural effusion, cardiomegaly, and pulmonary edema as indicated by the final radiology report.
Data points collected from the most recent formal transthoracic echocardiogram (within five years) included: ejection fraction, presence and graded severity of diastolic dysfunction, left ventricular internal diastolic and systolic dimension, interventricular septum diastolic thickness, left ventricular posterior and relative wall diastolic thickness, right ventricular systolic pressure, and presence and severity of graded valvular pathology. Objective information collected daily included vital signs at the time of examination such as oxygen requirements, 24-hour fluid intake and output per nursing records, and positive findings of jugular venous distension, a third heart sound, crackles in the lungs, hepatosplenomegaly, hepatojugular reflex, and lower extremity edema.
Statistical analysis
Given the small sample size and observational design, most of the data presented as continuous variables are expressed as mean ± standard deviation or counts with population as appropriate. Inter and intraobserver variability between ejection fraction on UE and standard echocardiography was examined. An intraclass correlation coefficient was calculated to measure the UE ejection fraction reproducibility against standard formal echocardiography on each patient.
Variables with P-values less than 0.1 on univariate analysis are considered for inclusion in the multiple regression model. Using forward conditional method, variables were entered into the model and the regression analysis was performed. All potential explanatory variables were assessed for collinearity, and only independent variables were included in the regression model. Hosmer–Lemeshow goodness-of-fit test was used to measure how well the model reflects the data on which it was created and the area under the receiver operating characteristics curve was calculated. SPSS 16.0 computer software was used to perform statistical calculations.
Results
Characteristics and outcomes
There were a total of 77 patients who fulfilled study criteria and were included in this analysis. The overall mean age was 67.0 (SD ± 13.1) years, 58% of patients were male, and the average body mass index (BMI) was 29.5 kg/m2. The minimum, average, and maximum inpatient length of stay was 3.0, 6.7, and 14 days, respectively. Of the total 77 patients, 25 patients (32.4%) presented with new-onset ADHF and the remainder presented with acute exacerbation of chronic HF. Baseline demographic information of the patients are presented in Table 1.
Table 1.
Demographics and characteristics of study population stratified by patients who responded to diuretic therapy (≥3,000 mL of net fluid output within 48 hours) versus those who did not.
| FACTOR | % DIURETIC RESPONDERS (n = 29) |
% DIURETIC NON-RESPONDERS (n = 48) |
P-VALUE |
|---|---|---|---|
| Demographic data | |||
| Male | 59.0 | 58.5 | 0.981 |
| New onset heart failure | 45.3 | 25.6 | 0.073 |
| Existing implanted device | 34.1 | 27.3 | 0.469 |
| Past medical history | |||
| Hypertension | 38.9 | 62.3 | 0.037 |
| Atrial fibrillation | 17.3 | 42.1 | 0.027 |
| Hyperlipidemia | 34.2 | 50.5 | 0.189 |
| Coronary artery disease | 45.2 | 40.1 | 0.656 |
| Anemia | 31.2 | 50.0 | 0.106 |
| Tobacco | 45.6 | 40.2 | 0.656 |
| Diabetes | 17.3 | 21.7 | 0.704 |
| COPD | 10.0 | 12.1 | 0.779 |
| Chronic kidney disease | 17.2 | 40.2 | 0.095 |
| Physical exam | |||
| Crackles | 66.6 | 58.8 | 0.537 |
| Third heart sound | 41.2 | 60.0 | 0.107 |
| Jugular venous distension | 48.0 | 38.2 | 0.359 |
| Lower extremity edema | 84.3 | 89.3 | 0.339 |
| Chest radiograph | |||
| Cardiomegaly | 62.2 | 60.1 | 0.877 |
| Pulmonary edema | 38.8 | 35.5 | 0.841 |
| Pleural effusion | 44.4 | 44.9 | 0.211 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In total, with all patient days combined, the study population consisted of 544 inpatient days, in which 431 days (79.2%) were spent for UE. In total, 73 of 77 patients survived to hospital discharge and one patient expired during the study. Patients with new-onset HF comprised 32.4% of the study, while 67.5% were found to have an acute exacerbation of chronic HF. The most frequent comorbid conditions were hypertension (53.3%), hyperlipidemia (44.2%), coronary artery disease (41.6%), anemia (42.9%), and tobacco use (41.6%). Atrial fibrillation was present in 32.5%, chronic obstructive pulmonary disease was present in 11.7%, and diabetes was present in 19.5%.
Inferior vena cava measurements
Data obtained during initial physical examination and diagnostic testing are listed in Table 2. Overall formal echocardiography showed a decreased mean left ventricular ejection fraction (LVEF) of 39.6% (SD ± 16.7%). The majority of patients (79.2%) also had diastolic dysfunction and mitral valve regurgitation was another common finding. The mean left ventricular internal diastolic dimension was 6.22 cm (SD ± 1.12 cm), mean left ventricular internal systolic dimension was 4.89 cm (SD ± 1.52 cm), interventricular septum diastolic thickness was 1.20 cm (SD ± 0.12 cm), left ventricular posterior wall diastolic thickness was 1.14 cm (SD ± 0.11 cm), left ventricular relative wall thickness was 0.38 cm (SD ± 0.062 cm), and mean right ventricular systolic pressure was 45.5 mmHg.
Table 2.
Clinical variables of study population stratified by patients who responded to diuretic therapy (≥3,000 mL of net fluid output within 48 hours) versus those who did not.
| FACTOR | RESPONDER (n = 29) |
NON-RESPONDER (n = 48) |
P-VALUE |
|---|---|---|---|
| Demographic data | Mean (SD) | Mean (SD) | |
| Inpatient length of stay (days) | 5.5 (1.4) | 6.2 (2.1) | 0.076 |
| Age (years) | 68.8 (13.8) | 66.1 (12.7) | 0.399 |
| BMI (kg/m2) | 29.3 (7.8) | 30.6 (6) | 0.460 |
| Clinical data | |||
| Systolic blood pressure (mmHg) | 113.1 (14.3) | 122.3 (19.7) | 0.021 |
| Diastolic blood pressure (mmHg) | 64.4 (15) | 71.9 (17.3) | 0.051 |
| Heart rate (bpm) | 93.7 (19.5) | 88.2 (19.7) | 0.233 |
| Respiratory rate (bpm) | 19.0 (3.2) | 18.6 (2.8) | 0.614 |
| Input (mL) | 1548.3 (317.6) | 1667.9 (361.8) | 0.134 |
| Output (mL) | 4561.2 (523.9) | 2199.7 (711.8) | 0.000 |
| Daily average fluid output (mL) | 1506.5 (289.5) | 265.9 (397.6) | 0.000 |
| Net fluid loss (mL) | 3012.9 (579.1) | 531.8 (795.3) | 0.000 |
| Admission IVC diameter (cm) | 2.0 (0.4) | 2.1 (0.4) | 0.781 |
| Day 2 IVC diameter (cm) | 1.9 (0.5) | 2.1 (0.5) | 0.026 |
| IVC Diameter (net change) | −0.2 (0.3) | 0.1 (0.2) | 0.000 |
| Admission weight (kg) | 84.1 (19.3) | 87.5 (15.9) | 0.426 |
| Day 2 weight (kg) | 83.0 (19.6) | 86.7 (15.8) | 0.394 |
| Net weight loss (kg) | 1.1 (1.6) | 0.8 (1.7) | 0.471 |
| Laboratory Data | |||
| Sodium | 138.6 (0.8) | 138.1 (4.3) | 0.627 |
| Potassium | 3.9 (0.1) | 4.0 (0.5) | 0.759 |
| Blood urea nitrogen | 21.4 (1.5) | 27.3 (16.1) | 0.073 |
| Creatinine | 1.6 (0.1) | 1.7 (1.1) | 0.500 |
| Troponin | 0.4 (0.3) | 0.3 (1.4) | 0.829 |
| BNP | 891.9 (105.6) | 531.9 (379.8) | 0.001 |
Abbreviations: BMI, body mass index; BNP, B-type natriuretic peptide.
Changes in cardiac function
Of the 77 subjects, 12 patients were observed to have noticeable improvement in cardiac function during the course of the hospital stay by UE. Of the 12 patients with improved LV function, the majority of these patients had new-onset HF and most were presumed to be nonischemic cardiomyopathy (only one patient had coronary angiography performed). Improvement in left ventricle function preceded recognizable clinical improvement. One patient demonstrated day-to-day deterioration of cardiac function. This patient presented with acute on chronic decompensated HF secondary to acute on chronic myocardial ischemia/infarction. This patient had an initial LVEF of approximately 45% visualized by UE, and over the next three days, the LVEF deteriorated to approximately 15%. Unfortunately, the patient was deemed nonrevascularizable, and despite maximal therapy, the patient developed cardiogenic shock and expired from a malignant arrhythmia on hospital day 4.
Changes in IVC diameter
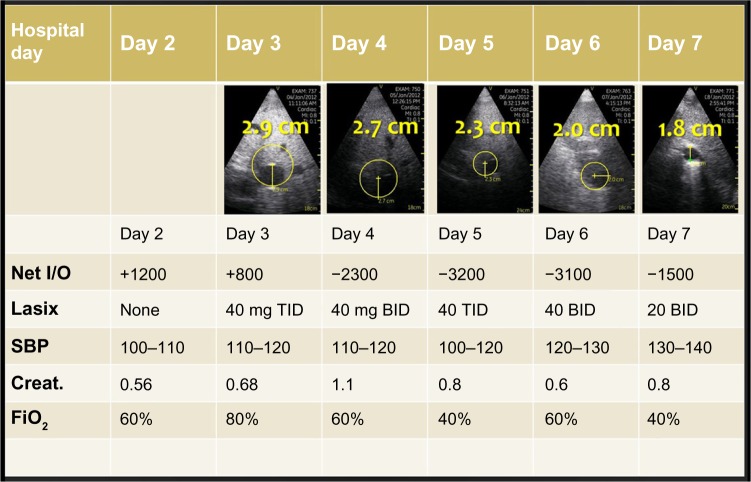
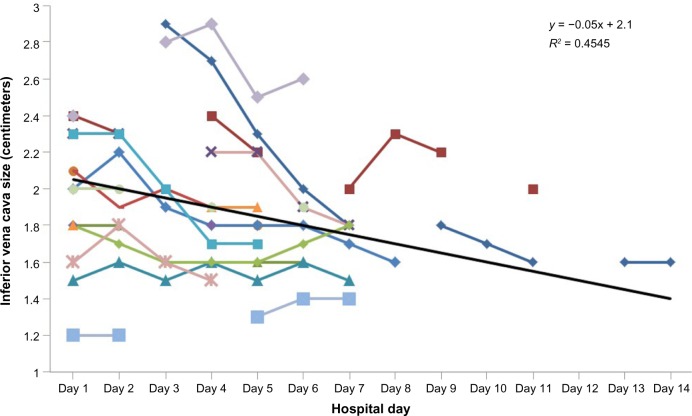
The diameter of the IVC was measured on each participant on day 1 of hospitalization and on subsequent hospital days. The change in IVC diameter at 48 hours was calculated and reported in Table 2. The mean IVC diameter for all subjects upon initial hospitalization was 2.04 cm (±0.37 cm), the mean IVC diameter for all subjects after 48 hours was 2.03 cm (±0.49 cm), and the mean 48-hour change in IVC diameter was −0.013 cm (±0.270 cm). Figure 1 demonstrates a patient who was treated over four days and the change in IVC diameter and other relevant clinical variables.
Figure 1.
Serial ultrasound measurement of the IVC and other clinical data obtained in a patient over first four days of hospitalization.
Abbreviations: *IVC, inferior vena cava; TID, three times daily; BID, twice daily.
On average, the IVC dimension decreased by 0.23 cm (±0.37 cm) over the study course. In all but three patients, the IVC dimension decreased over the total examination period. In the three patients in whom the IVC dimension increased, the absolute measurement on the final day study remained within the normal range (<2.0 cm) for IVC dimension. In the remaining one patient, the IVC measured 2.4 cm upon study enrollment and measured 2.6 cm on the last day of hospitalization (hospital day 6).
Diuretic effect and comparison between responders and nonresponders
Mean 48-hour urine output for the study group was 3,089 mL (SD ± 1,319 mL), 48-hour fluid intake was 1,622 (±348.5), and 48-hour net fluid balance was −1,466 mL (±1,406 mL). The study cohort was divided by negative fluid balance at 48 hours. A total of 29 (37.7%) in the study population achieved the goal of a negative fluid balance of greater than 3,000 mL within the first 48 hours and were labeled the responder cohort. The remaining 48 (N = 62.3%) participants did not achieve a negative fluid balance of at least 3,000 mL and were described as the nonresponder cohort.
As described in Table 1, univariate comparisons were made between two study cohorts and atrial fibrillation and hypertension were less common in the responder cohort. There was a greater change in the IVC diameter after 48 hours in the responder versus nonresponder cohort (−0.2 vs. 0.1, P < 0.001). There was higher presenting B-type natriuretic peptide (BNP) levels in responder cohort. On the other hand, the presenting systolic blood pressure was less in the responder cohort than the nonresponder group. Although it did not achieve statistical significance, the presence of new-onset HF was present with a higher frequency in the responder cohort as compared to the nonresponder cohort (25.6% vs. 45.3%, P = 0.073).
Multiple regression model
The variables that achieved significance with P-values less than 0.10 were used to construct multiple regression model. These included presence of hypertension, atrial fibrillation, new-onset HF, chronic kidney disease (CKD), in addition to the BNP level, and change in IVC dimension. Fluid input/output measurements and pre/post IVC diameter were excluded from the multivariable model secondary to collinearity with other variables. As described in Table 3, negative change in IVC diameter, the absence of CKD, and increased BNP value independently predicted higher likelihood of responding to diuretic therapy. Based on this regression model for every 1 mm decrease in the IVC diameter at 48 hours, there was an odds ratio of 1.62 (95% CI: 1.20–2.19) for responding to diuretic therapy (>3,000 mL of fluid output at 48 hours), independent of other variables. Goodness-of-fit calculation revealed that the model appropriately fit the data, and the area under the receiver operating characteristics curve was 0.85 as described in Table 3 and Figure 2.
Table 3.
Score performance for the prediction of responder to diuretic therapy for patients with acute exacerbation of HF (N = 77).
| COEFFICIENT (B) |
WALD X2 |
SIG. | ODDS RATIO | 95.0% C.I. FOR EXP(B) | ||
|---|---|---|---|---|---|---|
| LOWER | UPPER | |||||
| BNP level | −0.002 | 9.33 | 0.002 | 1.002 | 1.001 | 1.004 |
| Negative change in IVC (by mm) | 0.483 | 0.15 | 0.002 | 1.62 | 1.20 | 2.19 |
| CKD Stage II or III | 2.79 | 6.00 | 0.014 | 0.061 | 0.064 | 0.571 |
| CKD Stage IV or V | 0.771 | 0.37 | 0.544 | 0.463 | 0.038 | 5.55 |
| Area under the ROC curve | Hosmer-Lemeshow Goodness-of-Fit Test | |||||
| Full multivariable model | 0.85 (0.75–0.94) | X2 = 2.97, df = 8, P = 0.94 | ||||
Figure 2.
Area under the receiver operating curve for the multivariable model for prediction of diuretic response (N = 77), area = 0.85 (0.75–0.94).
Discussion
Attributable to its novelty, no previous studies have described the use of the latest generation of UE in routine clinical management of ADHF. The data from this small observational study demonstrate the ability of UE to predict patient response to diuretics when treated for ADHF. At the present time, UE is approved by regulatory bodies for clinical use in medicine and is commercially available7; however, little data exist on the type of information or additive benefit these devices provide in the routine care of HF patients.
When used as an extension of the daily physical examination, fluid overload can be monitored noninvasively with ultrasound measurements of global left ventricular function and change in IVC diameter.8 Until recently, significant barriers to ultrasound device technology prevented their implementation for routine use. Reduction in device size, weight, and cost, with improvements in battery life, and ease of use are making inroads for this bedside tool. Factors such as time constraints, noisy intensive care units, and patient body habitus have threatened the time-honored physical examination. This movement away from relying solely on older practices may be justifiable, as evidence-based scrutiny is lacking for many of these subjective examination techniques when applied in contemporary settings.9,10 This new era is moving ultrasound technology from the realm of formalized diagnostic imaging into the realm of a tool used to supplement the physical examination much the same as the stethoscope (Table 4).11 UE ultimately fulfills its promise by allowing us to finally visualize pathology directly than traditional methods and adds to other established examination methods (ie, percussion, palpation, and auscultation).
Table 4.
Comparison of stethoscope versus UE.
| TRADITIONAL STETHOSCOPE | ULTRAPORTABLE ECHOCARDIOGRAPHY | |
|---|---|---|
| Method | Auscultation of heart sounds | Visual 2-D ultrasound images with color doppler of blood flow |
| Approximate time required to perform exam | 1–4 minutes | 1–5 minutes |
| Subjectivity of data gathered | ++++ | ++ |
| Training required to perform | +++ | +++++ |
| Cost of equipment | $85–$300 | ∼$8500 |
| Patient exposure/privacy | ++ | +++ |
| Fragility of equipment | + | +++ |
| Portability/Burden to carry | Fits in lab coat, able to wear around neck | Fits in lab coat |
| Patient exposure/privacy | ++ | +++ |
| Additional advantages | Images/video can be stored, replayed and shown to patients, family and other caregivers | |
| Additional limitations | Subject to body habitus, positioning and other factors | Subject to body habitus, positioning and other factors Battery lasts ∼1 hour with continuous scanning Requires ultrasound gel |
Most admissions for ADHF occur because of clinical signs and symptoms of fluid overload. HF is a very heterogenous clinical disorder and requires physicians to utilize multiple data points to make the diagnosis including history, physical examination, and testing. Previous literature suggests that ultrasound assessment of structures like the IVC can provide valuable supplemental data to aid in diagnosis and management.12–14 As described, the significant change observed in these patients was a decrease in mean IVC size over the study period. Novel to our report, we demonstrate the ability of the UE to provide these data. IVC diameter is an estimation of right atrial pressure and is analogous to the changes that occur with jugular venous distention in patients treated for fluid overload. The value of sequential assessment of the jugular venous distention is that it helps monitor patient clinical status and individually tailor diuretic therapy.15
It can be very difficult to assess response to diuretic therapy within the first few days of initiation. It can also be very difficult to predict which patients will respond favorably to diuretic therapy as evident by the lack of difference in clinical variables between the responders and nonresponders observed in this study. As expected, impaired renal function and lower BNP levels in patients predicted less responsiveness to diuretic therapy based on the multivariate regression. The change in the IVC diameter, however, was very robust in prediction of diuretic response. Importantly, if the IVC diameter remained relatively unchanged or increased with diuresis, it predicted a poor response in urine output over the next several days, suggesting increased plasma refill and temporary increases in passive renal congestion. This is consistent with a previous report that BNP, IVC size, and collapsibility were able to predict 30-day readmission in ADHF patients.16
Limitations
Our study has all the limitations of small prospective cohort studies and unblinded assessments using novel technology. We present data from a small number of patients where clinical utility was observed; therefore, a considerably larger study without selection bias is still needed. This study did not capture data on the exact dose of diuretic administered, daily medication changes, and many other clinical variables that would be expected to be present in patients admitted with ADHF. Patients with poor acoustic visualization were excluded, so future studies should compare conventional 3D echocardiography to its pocket counterpart to further describe limitations. Showing raw data obtained from routine clinical care does not yield consistent information among all patients enrolled, particularly with variations in daily laboratories and the number of days patients scans. Additionally, our study used an experienced cardiologist trained in echocardiography to perform UE. It is thought that primary care providers and residents would benefit from UE,17 so further investigation is needed to support what level of basic training is necessary for reproducible results in the HF population. While ultrasound is noninvasive and cost-effective, use of handheld echocardiography requires initial investment by the provider. Finally, we did not have advanced biomarkers of acute kidney injury (AKI) or imaging measures of intrarenal venous congestion or intracapsular pressure, and thus, our inference on passive renal congestion and AKI is indirect.
Conclusion
With daily ultrasound measurement, this study demonstrates that a negative change in IVC diameter, the absence of diagnosed CKD, and BNP value independently predicted higher likelihood of responding to diuretic therapy within the first 48 hours.
Acknowledgments
We acknowledge the support of Genesys Regional Medical Center’s Departments of Medical Education and Clinical Research, specifically, Dr. Heather Kirkpatrick, PhD, and Dr. Kimberly Barber, PhD, in the preparation of this manuscript.
Abbreviations
- AKI
acute kidney injury
- ADHF
acute decompensated heart failure
- BNP
B-type natriuretic peptide
- CKD
chronic kidney disease
- RA
right atrium
- IVC
inferior vena cava
- UE
ultraportable echocardiography
Footnotes
ACADEMIC EDITOR: Thomas E Vanhecke, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled words, 2016 excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: DK, TV, AK. Analyzed the data: TV. Wrote the first draft of the manuscript: DK. Contributed to the writing of the manuscript: DK, TV. Agree with manuscript results and conclusions: BP, SE, AK, PM. Jointly developed the structure and arguments for the paper: TV, DK. Made critical revisions and approved final version: All authors. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCullough PA, Philbin EF, Spertus JA, Sandberg KR, Weaver WD. Confirmation of a heart failure epidemic: findings from the resource utilization among congestive heart failure (REACH) study. J Am Coll Cardiol. 2002;39:60. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 3.Joffe SW, Dewolf M, Shih J, et al. Trends in the medical management of patients with heart failure. J Clin Med Res. 2013;5(3):194–204. doi: 10.4021/jocmr1376w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sambandam KK. Effective use of loop diuretics in heart failure exacerbation: a nephrologists view. Am J Med Sci. 2014 Feb;347(2):139–45. doi: 10.1097/MAJ.0b013e31828a2962. [DOI] [PubMed] [Google Scholar]
- 5.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–9. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heart Failure Society of America. Lindenfeld J, Albert NM, et al. Heart Failure Society of America comprehensive heart failure practice guideline. J Card Fail. 2010;16:475–539. [Google Scholar]
- 7.Innovative Technology: GE Healthcare. [Accessed November 1, 2012]. Available at: http://vscanultrasound.gehealthcare.com/
- 8.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–73. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 9.Marantz PR, Kaplan MC, Alderman MH. Clinical diagnosis of congestive heart failure in patients with acute dyspnea. Chest. 1990;97:776–81. doi: 10.1378/chest.97.4.776. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884–8. [PubMed] [Google Scholar]
- 11.Liebo MJ, Israel RL, Lillie EO, Smith MR, Rubenson DS, Topol EJ. Is pocket mobile echocardiography the next-generation stethoscope? A cross-sectional comparison of rapidly acquired images with standard transthoracic echocardiography. Ann Intern Med. 2011;5(155):33–8. doi: 10.7326/0003-4819-155-1-201107050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen V, Ho J, Ho C, Givertz M, Stevvenson L. Handheld echocardiography offers rapid assessment of clinical volume status. Am Heart J. 2008;156:537–42. doi: 10.1016/j.ahj.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Prinz C, Voigt J. Diagnostic accuracy of a hand-held ultrasound scanner in routine patients referred for echocardiography. J Am Soc Echocardiogr. 2011;24:111–6. doi: 10.1016/j.echo.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Skjetne K, Graven T, Haugen BO, Salvesen Ø, Kleinau JO, Dalen H. Diagnostic influence of cardiovascular screening by pocket-size ultrasound in a cardiac unit. Eur J Echo. 2011;12:737–43. doi: 10.1093/ejechocard/jer111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Goonewardena S, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and n-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595–601. doi: 10.1016/j.jcmg.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Razi R, Estrada J, Doll J, Spencer K. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24:1319–24. doi: 10.1016/j.echo.2011.07.013. [DOI] [PubMed] [Google Scholar]