Abstract
INTRODUCTION
Oncoplastic breast conservation surgery (OBCS) combines the principles of surgical oncology and plastic surgery. OBCS has now become a growing option for the treatment of breast cancer and forms a part of breast-conserving therapy (BCT). We sought to investigate and report our experience in two breast units in Glasgow (Victoria Infirmary and Western Infirmary) on volume replacement OBCS.
MATERIALS AND METHODS
Details of patients treated with volume replacement OBCS were identified from a prospectively recorded database from November 2010 to October 2015. The clinical records included in the oncoplastic dataset were analyzed for demographics, tumor, treatment characteristics, and recurrences. The data were analyzed for follow-up to determine the pattern and timing of recurrence up to April 2016. The primary outcome of this study was tumor-free margin resection rates, and the secondary outcomes were locoregional and distant recurrence rates as these correlate with the overall oncological safety of volume replacement oncoplastic breast surgery (OPBS).
RESULTS
A total of 30 volume replacement oncoplastic breast conservation procedures have been carried out in this time period. The mean age of the former group was 51 years. Twice as many patients presented symptomatically than had tumors detected on screening. The mean preoperative tumor size on radiology was 25.4 mm. Patients underwent 13 thoracoepigastric flaps, 5 lateral intercostal artery perforator (LICAP) flaps, 2 thoracodorsal artery perforator (TDAP) flaps, 1 lateral thoracic artery perforator (LTAP) flap, 1 crescent flap volume replacement surgery, and 8 matrix rotations. Two patients had neoadjuvant chemotherapy. Fourteen patients had adjuvant chemotherapy, and all patients were treated with adjuvant radiotherapy. Twenty-two patients were treated with hormonal therapy and four patients were treated with Herceptin. The rate of incomplete excision was 10%. Median follow-up time was 48.5 months. Only one regional recurrence was detected. Eight patients encountered some form of complication.
CONCLUSION
This study continues to show the relative oncological safety of volume replacement oncoplastic conservations as an option for reconstruction in breast cancer patients. Further research is urgently needed to build robust evidence supporting the long-term oncological safety.
Keywords: therapeutic mammaplasty, oncoplastic, volume replacement, recurrence, complication
Introduction
Oncoplastic breast conservation surgery (OBCS) describes techniques that combine the principles of surgical oncology with those of plastic surgery in an attempt to achieve a desirable esthetic result while maintaining low cancer recurrence rate.1 OBCS was conceived as a solution to the cosmetic defect from breast conservation surgery (BCS).2 It comprises tumor excision with a wide margin of resection followed by immediate reconstruction of the defect.
OBCS generally comprises two techniques, which are volume displacement and volume replacement. The use of volume displacement OBCS has been well established. Similarly, several volume replacement techniques have also been well established, such as the latissimus dorsi (LD) myocutaneous flap3–5 and the LD myosubcutaneous flap or LD mini (LDm) flap.6–8 Variations of pedicled flaps based on the intercostal artery perforators and thoracodorsal artery perforators (TDAPs) have been described and shown to be reliable in immediate BCS reconstruction.9–12 Additionally, it has been used in combination with other flaps such as the thoracoabdominal advancement flap to achieve desirable results.13 Similarly, the thoracoepigastric flap has also been shown to be another reliable, effective, and relatively simple form of volume replacement.14,15 OBCS is an effective technique used in patients in whom 10% of the breast volume is excised in medial tumors and 20% in lateral tumors, where outcomes with volume displacement techniques would not achieve an acceptable cosmetic outcome.16,17
The current evidence on the oncological outcomes of other forms of volume replacement oncoplastic conservation largely focuses on LD myocutaneous or LDm flaps in multiple study designs. As previously established, the likelihood of conducting a prospective randomized controlled trial for oncoplastic breast conservation is highly unlikely due to the ethical considerations,18,19 and this extends to volume replacement too. We aim to ascertain the recurrence and complication rates after volume replacement oncoplastic breast conservation in our local population. As with all cancer resections, the primary outcome is oncological safety. We sought to investigate and report our experience in two breast units in Glasgow on volume replacement OBCS.
Materials and Methods
This study was designed and reported in line with STROBE criteria.20 Methods for data collection in our centers have previously been described.21–24 The research was conducted in accordance with the principles of the Declaration of Helsinki. Details of patients treated with OBCS in two centers within the publicly funded NHS Greater Glasgow and Clyde health trust between November 2010 and October 2015, namely, the Victoria Infirmary and Western Infirmary, were prospectively recorded in a standardized institutionalized database. The following characteristics were recorded prospectively in the oncoplastic dataset: demographic data (age, body mass index [BMI], brassiere size, risk factors for breast cancer, and breast surgery), preoperative tumor size, pre- and postoperative pathology, surgical and oncological management, surgical complications, and time and site of recurrence. Patients who had undergone volume replacement OBCS were identified. The clinical records included in the oncoplastic dataset were analyzed for demographics, tumor, treatment characteristics, and recurrences. Missing data were retrospectively searched via case records and included in the analysis. Preoperative tumor size was determined as the largest diameter given on any preoperative imaging. Patients with previous ipsilateral or contralateral ductal carcinoma in situ (DCIS) or breast cancer were excluded.
Patients in whom breast cancers were detected either on screening or after a symptomatic presentation were included. The confirmation of cancer diagnosis was done with radiological and pathological evidence (core biopsy, axillary biopsy, etc.). Treatment plans were decided in a local multidisciplinary meeting consisting of radiologists, pathologists, oncologists, breast surgeons, and breast specialist nurses. Oncoplastic technique was mutually decided between the patient and the oncoplastic breast surgeon or breast surgeon, with or without consultation and surgical co-intervention of a plastic surgeon. Radiotherapy, chemotherapy, and hormone therapy were administered according to evidence-based guidelines of the Beatson West of Scotland Cancer Centre in the given time period (Fig. 1).21
Figure 1.
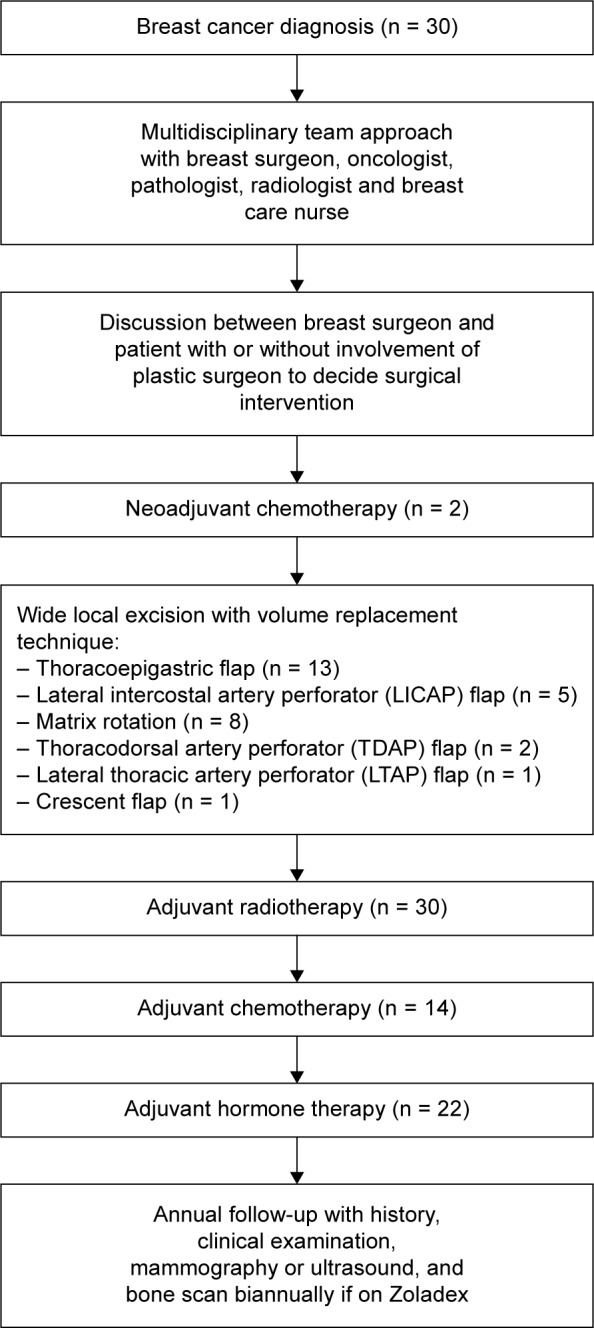
A flowchart showing management of breast cancer with a multidisciplinary team approach.
Surgical, oncological, radiological, and pathological reports were analyzed for follow-up to determine the pattern and timing of recurrence up to April 2016. Length of followup was determined as time elapsed from first treatment. Patients were followed up every 12 months by surveillance mammogram and clinical examination, and abnormal clinical findings were further investigated as appropriate. Recurrences were documented by clinical examination, radiological tests, and/or pathological assessment (Fig. 1).
The primary outcome of this study was tumor-free margin resection rates, and the secondary outcomes were locoregional and distant recurrence rates as these correlate with the overall oncological safety of volume replacement OPBS. We defined tumor-free margins as a distance of at least 1 mm between cut edge of the specimen and the outer limit of the tumor when the pathology was invasive cancer, and 2 mm for DCIS. This is based on findings that greater distances are not associated with improved outcomes.25–27 Surgical complication rates were the secondary outcome of interest in this study.
Results
Baseline characteristics and risk factors
A total of 208 oncoplastic breast conservation procedures have been carried out in this time period. A total of 30 of 208 (15.9%) patients underwent volume replacement surgery, and the remaining underwent volume displacement surgery. The mean age of the former group was 51 years (range 24–69 years). Three patients had A-cup breasts, four patients had B-cup breasts, four patients had C-cup breasts, three patients had D-cup breasts, two patients had E-cup breasts, and two patients had F-cup breasts. The mean BMI was 28 (range 21–37). Six patients were current smokers and two patients were ex-smokers. Comorbidities were diabetes in one patient (3.3%), immunosuppression in four patients (13.3%), and anticoagulation in one patient (3.3%). Baseline characteristics are outlined in Table 1.
Table 1.
Baseline characteristics and risk factors.
| VARIABLE | (n, %) |
|---|---|
| Age (mean, range) | 51, 24–69 |
| BMI (mean, range) | 27.8, 23.6–36.2 |
| Diabetes | |
| Yes | 1 |
| No | 24 |
| No data | 1 |
| Family history | |
| Yes | 5 |
| No | 21 |
| Smoking status | |
| Current smoker | 6 |
| Ex-smoker | ??2 |
| Non-smoker | 20 |
| HRT | |
| Yes | 4 |
| No | 20 |
| No data | 2 |
| Immunosuppression | |
| Yes | 0 |
| No | 30 |
| Breast cup size | |
| A | 3 |
| B | 4 |
| C | 4 |
| D | 3 |
| E | 2 |
| F | 2 |
| Larger than F | 3 |
| No data | 7 |
Tumor characteristics
Twice as many patients presented symptomatically than had tumors detected on screening—20 (66.7%) versus 10 (33.3%). Of these, 11 patients (36.7%) had tumors found in the upper outer quadrant, 4 (13.3%) in the upper inner quadrant, 12 (40.0%) in the lower outer quadrant, and 3 (10.0%) in the lower inner quadrant. The mean preoperative tumor size on radiology was 25.4 mm.
Pathological tumor subtypes were ductal in 23 patients (76.7%), lobular in 5 (16.7%), mixed in 1 (3.3%), and ductal carcinoma in situ in 1 (3.3%). A total of 16 patients (53.3%) had grade 3 tumors, 13 patients (43.3%) had grade 2 tumors, and 1 patient (3.3%) had grade 1 tumor. Mean whole tumor size was 25 mm (range 9–45 mm). Four patients (13.3%) had multifocal tumors. Estrogen receptor was expressed in 23 tumors (79.3%), progesterone receptor was expressed in 21 tumors (72.4%), and HER-2 receptor was expressed in 4 tumors (13.8%). Eight patients had node-positive tumors (27.6%; Table 2).
Table 2.
Tumour characteristics.
| PATIENTS | NO. | INCOMPLETE EXCISIONS |
RECURRENCES |
|---|---|---|---|
| LOCOREGIONAL | |||
| NO. | NO. | ||
| All patients | 26 | 7 | 1 |
| Presentation | |||
| Screening | |||
| Symptomatic | |||
| Laterality | |||
| Left | |||
| Right | |||
| Quadrant | |||
| Upper outer | |||
| Upper inner | |||
| Lower outer | |||
| Lower inner | |||
| Invasive cancer | 25 | 7 | 1 |
| T1 | 8 | 2 | 0 |
| T2 | 21 | 5 | 1 |
| T3 | 0 | 0 | 0 |
| Tumour grade | |||
| G1 | 1 | 0 | 0 |
| G2 | 13 | 4 | 0 |
| G3 | 16 | 3 | 1 |
| Pathological subtype | |||
| Ductal | 23 | 3 | 1 |
| Lobular | 5 | 4 | 0 |
| Mixed | 1 | 0 | 0 |
| Oestrogen receptor status | |||
| Positive | 23 | 7 | 0 |
| Negative | 7 | 0 | 1 |
| Progesterone receptor status | |||
| Positive | 21 | 5 | 0 |
| Negative | 9 | 2 | 1 |
| Her-2 receptor status | |||
| Positive | 4 | 1 | 0 |
| Negative | 26 | 6 | 1 |
| Nodal status | |||
| Positive | 8 | 3 | 1 |
| Negative | 22 | 4 | 0 |
| DCIS | 1 | 0 | 0 |
| Stage of disease | |||
| 0 | 1 | 0 | 0 |
| IA | 8 | 1 | 0 |
| IB | 1 | 0 | 0 |
| IIA | 16 | 5 | 0 |
| IIB | 0 | 0 | 0 |
| IIIA | 1 | 0 | 1 |
| IIIB | 0 | 0 | 0 |
| IIIC | 1 | 1 | 0 |
Surgical techniques
The majority of patients (13 of 30) underwent oncoplastic breast conservation using a thoracoepigastric flap. A total of eight patients underwent pedicled flap reconstructions—five patients received lateral intercostal artery perforator (LICAP) flaps, two patients had thoracodorsal artery perforator (TDAP) flaps, and one patient had a lateral thoracic artery perforator (LTAP) flap. One patient underwent crescent flap volume replacement surgery. Of the eight patients who underwent matrix rotation, five were inferior, one was superomedial, and two were superior matrix rotation.
Synchronously, 24 patients underwent sentinel node biopsy, 5 patients underwent axillary node clearance, and 1 patient underwent symmetrizing contralateral breast reduction (Table 3).
Table 3.
Summary of surgical techniques.
| PATIENTS | NO. (%) | INCOMPLETE EXCISIONS |
RECURRENCES | RECURRENCES |
|---|---|---|---|---|
| OVERALL | LOCAL | |||
| NO. | NO. | NO. | ||
| All patients | 3 | 0 | 0 | |
| Thoracoepigastric flap | 13 (43.3) | 0 | 0 | 0 |
| Matrix rotation | 8 (26.7) | 2 | 1 | 1 |
| Inferior | 5 (16.7) | 1 | 1 | 1 |
| Supero-medial | 1 (3.3) | 0 | 0 | 0 |
| Superior | 1 (3.3) | 1 | 0 | 0 |
| Lateral intercostals artery perforator (LICAP) flap | 5 (16.7) | 1 | 0 | 0 |
| Thoracodorsal artery perforator (TDAP) flap | 2 (6.7) | 0 | 0 | 0 |
| Lateral thoracic artery perforator (LTAP) flap | 1 (3.3) | 0 | 0 | 0 |
| Crescent flap | 1 (3.3) | 0 | 0 | 0 |
Adjuvant and neoadjuvant therapies
Two of 30 patients (6.7%) had neoadjuvant chemotherapy. Postoperatively, 14 patients (48.3%) underwent adjuvant chemotherapy, and all 30 patients were treated with adjuvant radiotherapy. In all, 22 patients (82.7%) were treated with hormonal therapy and 4 patients (13.8%) were treated with Herceptin (Table 4).
Table 4.
Summary of (neo)adjuvant therapies.
| PATIENTS | NO. (%) |
|---|---|
| All patients | 30 (100) |
| Neoadjuvant chemotherapy | 2 (6.7) |
| Radiotherapy | 30 (100) |
| Chemotherapy | 14 (46.7) |
| Hormone therapy | 22 (73.3) |
| Herceptin | 4 (13.3) |
Margins, recurrences, and complications
The rate of incomplete excision was 10% (three patients), which were all subsequently re-excised successfully. From a median follow-up time of 48.5 months (range 6–66 months), we have detected no local recurrences, one regional recurrence involving the brachial plexus, and no distant metastases.
Overall, eight patients (26.7%) encountered some form of complication. Of these, two patients had seromas, two patients had partial flap failure, one patient had a hematoma, two patients had fat necrosis, and one patient had cellulitis. Of these, only two patients (6.7%) required surgical intervention. Specifically, the patient with fat necrosis was returned to theater for a washout, and one of the patients with flap failure required debridement followed by secondary closure.
Discussion
Oncoplastic breast surgery is becoming the preferred option in suitable patients due to its focus on esthetic results without compromising oncological safety. Volume replacement can maintain the original shape and size of the breast and achieve a balanced esthetic result without any contralateral surgery.2
Oncological safety
The safety of OBCS is becoming increasingly established. However, the evidence for long-term outcomes of volume replacement oncoplastic surgery is lacking. The main concern with breast conserving surgery compared with mastectomy is the plausible increased risk of margin-positive resections. Volume replacement OPBS circumvents the problem of replacing volume loss by obtaining volume from autologous non-breast tissue in the combination of skin, fat, fascia, and/or muscle to match the volume resected. However, compared to volume displacement techniques, there is some concern over the relationship between increased volume and decreased efficacy of adjuvant radiotherapy and that distortion of tissue planes complicates re-excision in the case of margin-positive resections and follow-up imaging. Several studies, including ours, have already addressed the issue of follow-up mammography after volume replacement surgery to not be a major factor due to the radiolucent nature of the tissues.28,29
Our data indicate a margin-free resection rate of 83.3%. This is comparable to a recent systematic review focusing on volume displacement surgery by Haloua et al on oncoplastic breast surgery, which found margin-free resection rates to vary between 78% and 93%.30,31 It should be noted that definitions of margin-free resection varied between publications. Reviews focusing on volume replacement OBCS have found margin-positive resection rates to range between 0% and 26.6%.32 Nevertheless, all four patients with margin-positive resections underwent re-resection successfully and have no evidence of recurrence.
In this study, the incidence of locoregional recurrence is 3.3%. We found no incidents of postoperative distant metastasis throughout our follow-up period in our patient population. The patient who had the regional recurrence was one of six to have a triple-negative tumor and had the highest American Joint Committee on Cancer (AJCC) stage of our study population, which was IIIA. This is consistent with findings of several previously published studies and reviews on volume replacement OBCS, which report a range of 0%–8.1% throughout a large variation of follow-up periods.1,32–34 In comparison, reviews focused on volume displacement OBCS found a locoregional recurrence rate to range from 0% to 9.4%.30,31
With a median of 48 months of follow-up, no distant recurrences have been found in our study. Multiple previously published studies on volume replacement OBCS have also found a range of distant metastasis or recurrence rates ranging from 0% to 14.6%.1,32 Haloua et al,30 whose review focused on volume displacement OBCS, found distant metastasis rates to be as high as 13%.
As we had previously reported, our centers have implemented taking cavity shavings as a routine part of our tumor resections, which resulted in a significantly lower incomplete excision rate compared to other centers.22 This may explain the relatively low local and distant recurrence rates in this study.
Complications
Concerns that arise regarding complications of the donor site are unique to the volume displacement techniques in OCBS, but throughout our period of follow-up, we have not found any reported cases of donor site morbidities. However, partial flap failure was reported in two patients. Both patients underwent wide local excisions with immediate thoracoepigastric flap reconstruction, which subsequently had debridement. Five other complications that did not require surgical intervention were recorded. A study by Lee et al1 found acute complication rates (infection and wound dehiscence) of 5.6% and chronic complication rates (fat necrosis) of 12.5%. In a systematic review by Haloua et al30 on volume displacement OBCS, complication rates were found to be low for delayed wound healing (2%–16%), abscess (2%), axillary seroma (4%), hematoma (2%–7%), partial skin necrosis (1%–68%), fat necrosis (3%), and dehiscence (3%–4%). In this review, complications requiring surgical intervention ranged from 4% to 9%.
Limitations
Baseline characteristics and tumor characteristics were not disclosed in many of the studies and reviews referenced in this study. As such, we were unable to make comparisons of the aforementioned characteristics of our patients and relate them with the outcomes in this study.
This study was not designed to evaluate patient perspectives or cosmetic outcomes, which are important considerations in oncoplastic breast conservations.
Conclusion
Our study continues to show the relative oncological safety of volume replacement oncoplastic conservations as an option for reconstruction in breast cancer patients.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 445 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: WH, JD, SS, LR. Analyzed the data: WH, LR. Wrote the first draft of the manuscript: WH, LR. Contributed to the writing of the manuscript: WH, LR. Agree with manuscript results and conclusions: EM, JD, SS. Jointly developed the structure and arguments for the paper: LR. Made critical revisions and approved final version: LR, SS, JD, EM. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Lee J, Jung JH, Kim WW, et al. Oncologic outcomes of volume replacement technique after partial mastectomy for breast cancer: a single center analysis. Surg Oncol. 2015;24:35–40. doi: 10.1016/j.suronc.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi M, Yokoi-Noguchi M, Ohno Y, et al. Oncoplastic breast conserving surgery: Volume replacement vs. volume displacement. Eur J Surg Oncol. 2016;42(7):926–934. doi: 10.1016/j.ejso.2016.02.248. [DOI] [PubMed] [Google Scholar]
- 3.Hernanz F, Sanchez S, Cerdeira MP, Figuero CR. Long-term results of breast conservation and immediate volume replacement with myocutaneous latissimus dorsi flap. World J Surg Oncol. 2011;9:159. doi: 10.1186/1477-7819-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naguib SF. Expanding the role of breast conservation surgery by immediate volume replacement with the latissimus dorsi flap. J Egypt Natl Canc Inst. 2006;18:216–226. [PubMed] [Google Scholar]
- 5.Kat CC, Darcy CM, O’Donoghue JM, Taylor AR, Regan PJ. The use of the latissimus dorsi musculocutaneous flap for immediate correction of the deformity resulting from breast conservation surgery. Br J Plast Surg. 1999;52:99–103. doi: 10.1054/bjps.1997.3035. [DOI] [PubMed] [Google Scholar]
- 6.Dixon JM, Venizelos B, Chan P. Latissimus dorsi mini-flap: a technique for extending breast conservation. Breast. 2002;11:58–65. doi: 10.1054/brst.2001.0312. [DOI] [PubMed] [Google Scholar]
- 7.Rusby JE, Paramanathan N, Laws SA, Rainsbury RM. Immediate latissimus dorsi miniflap volume replacement for partial mastectomy: use of intra-operative frozen sections to confirm negative margins. Am J Surg. 2008;196:512–518. doi: 10.1016/j.amjsurg.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 8.Nano MT, Gill PG, Kollias J, Bochner MA. Breast volume replacement using the latissimus dorsi miniflap. ANZ J Surg. 2004;74:98–104. doi: 10.1046/j.1445-2197.2003.02917.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamdi M, Van Landuyt K, Monstrey S, Blondeel P. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg. 2004;57(6):531–539. doi: 10.1016/j.bjps.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Hamdi M, Van Landuyt K, de Frene B, Roche N, Blondeel P, Monstrey S. The versatility of the inter-costal artery perforator (ICAP) flaps. J Plast Reconstr Aesthet Surg. 2006;59(6):644–652. doi: 10.1016/j.bjps.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Minabe T, Harii K. Dorsal intercostal artery perforator flap: anatomical study and clinical applications. Plast Reconstr Surg. 2007;120(3):681–689. doi: 10.1097/01.prs.0000270309.33069.e5. [DOI] [PubMed] [Google Scholar]
- 12.Munhoz AM, Montag E, Arruda E, et al. Immediate conservative breast surgery reconstruction with perforator flaps: new challenges in the era of partial mastectomy reconstruction? Breast. 2011;20(3):233–240. doi: 10.1016/j.breast.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Wong C, Maia M, Saint-Cyr M. Lateral intercostal artery perforator flap in combination with thoracoabdominal advancement flap for correction of contour deformities following autologous breast reconstruction. Plast Reconstr Surg. 2011;127(6):156e–158e. doi: 10.1097/PRS.0b013e318213a1d4. [DOI] [PubMed] [Google Scholar]
- 14.Cronin TD, Upton J, McDonough JM. Reconstruction of the breast after mastectomy. Plast Reconstr Surg. 1977;59(1):1–14. doi: 10.1097/00006534-197701000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Dakin R, Jobe RP. Toward a more natural breast: the interpolated thoracoepigastric pedicle in breast reconstruction. Plast Reconstr Surg. 1991;88(3):510–513. doi: 10.1097/00006534-199109000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Yang JD, Lee JW, Cho YK, et al. Surgical techniques for personalized oncoplastic surgery in breast cancer patients with small- to moderatesized breasts (part 1): volume displacement. J Breast Cancer. 2012;1:1–6. doi: 10.4048/jbc.2012.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry MG, Fitoussi AD, Curnier A, Couturaud B, Salmon RJ. Oncoplastic breast surgery: a review and systematic approach. J Plast Reconstr Aesthet Surg. 2010;63:1233–1243. doi: 10.1016/j.bjps.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 18.McIntosh J, O’Donoghue JM. Therapeutic mammoplasty—a systematic review of the evidence. Eur J Surg Oncol. 2012;38(3):196–202. doi: 10.1016/j.ejso.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Rietjens M, Urban CA, Rey PC, et al. Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast. 2007;16(4):387–395. doi: 10.1016/j.breast.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Kabir SA, Stallard S, Weiler-Mithoff E, et al. Six-year follow-up of patients treated with oncoplastic reduction mammoplasty: A cohort study. Int J Surg. 2016;26:38–42. doi: 10.1016/j.ijsu.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Mansell J, Weiler-Mithoff E, Martin J, et al. How to compare the oncological safety of oncoplastic breast conservation surgery—to wide local exicision or mastectomy? Breast. 2015;24:497–501. doi: 10.1016/j.breast.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Kahn J, Barrett S, Forte C, et al. Oncoplastic breast conservation does not lead to a delay in the commencement of adjuvant chemotherapy in breast cancer patients. Eur J Surg Oncol. 2013;39:887–891. doi: 10.1016/j.ejso.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Martin J, Mansell J, Weiler-Mithoff E, Stallard S, Doughty JC, Romics L., Jr Oncoplastic breast conservation surgical techniques and postoperative complication rates—the Glasgow experience. Eur J Surg Oncol. 2013;39:461–514. [Google Scholar]
- 25.Kaufmann M, Morrow M, von Minckwitz G, Harris JR. Biedenkopf Expert Panel Members. Locoregional treatment of primary breast cancer: consensus recommendations from an International Expert Panel. Cancer. 2010;116:1184–1191. doi: 10.1002/cncr.24874. [DOI] [PubMed] [Google Scholar]
- 26.Meric F, Mirza NQ, Vlastos G, et al. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast conserving therapy. Cancer. 2003;97:926–933. doi: 10.1002/cncr.11222. [DOI] [PubMed] [Google Scholar]
- 27.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–393. doi: 10.1016/s0002-9610(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 28.Monticciolo DL, Ross D, Bostwick J, III, Eaves F, Styblo T. Autologous breast reconstruction with endoscopic latissimus dorsi musclosubcutaneous flaps in patients choosing breast-conserving therapy: mammographic appearance. Am J Roentgenol. 1996;167:385–389. doi: 10.2214/ajr.167.2.8686611. [DOI] [PubMed] [Google Scholar]
- 29.Noguchi M. Comments on “breast deformity, its correction and assessment of breast conserving surgery”. Breast Cancer Res Treat. 1992;22:181–182. doi: 10.1007/BF01833349. [DOI] [PubMed] [Google Scholar]
- 30.Haloua MH, Krekel NMA, Winters HAH, et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann Surg. 2013;257(4):609–620. doi: 10.1097/SLA.0b013e3182888782. [DOI] [PubMed] [Google Scholar]
- 31.Asgeirsson KS, Rasheed T, McCulley SJ, Macmillan RD. Oncological and cosmetic outcomes of oncoplastic breast conserving surgery. Eur J Surg Oncol. 2005;31:817–823. doi: 10.1016/j.ejso.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Yiannakopoulou EC, Mathelin C. Oncoplastic breast conserving surgery and oncological outcome: Systematic review. Eur J Surg Oncol. 2016;42:625–630. doi: 10.1016/j.ejso.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Losken A, Schaefer TG, Carlson GW, Jones GE, Styblo TM, Bostwick IIIJ. Immediate endoscopic latissimus dorsi flap. Risk or benefit in reconstructing partial mastectomy defects. Ann Plast Surg. 2004;53(1):1–5. doi: 10.1097/01.sap.0000106425.18380.28. [DOI] [PubMed] [Google Scholar]
- 34.Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast conserving therapy of breast carcinoma. Ann Surg. 2003;237(1):26–34. doi: 10.1097/00000658-200301000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]


