Abstract
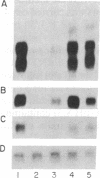
The Zea mays P gene has been postulated to regulate the biosynthetic pathway of a flavonoid-derived pigment in certain floral tissues [Styles, E. D. & Ceska, O. (1977) Can. J. Genet. Cytol. 19, 289-302]. We have characterized two P transcripts that are alternatively spliced at their 3' ends. One message of 1802 nucleotides encodes a 43.7-kDa protein with an N-terminal region showing approximately 40% homology to the DNA-binding domain of several members of the myb family of protooncogene proteins. A second message of 945 nucleotides encodes a 17.3-kDa protein that contains most of the myb-homologous domain but differs from the first protein at the C terminus. The deduced P-encoded proteins show an even higher homology (70%) in the myb-homologous domain to the maize regulatory gene C1. Additionally, the P and C1 genes are structurally similar in the sizes and positions of the first and second exons and first intron. We show that P is required for accumulation in the pericarp of transcripts of two genes (A1 and C2) encoding enzymes for flavonoid biosynthesis--genes also regulated by C1 in the aleurone.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay P. C., Brink R. A. The Relation between Modulator and Activator in Maize. Proc Natl Acad Sci U S A. 1954 Dec;40(12):1118–1126. doi: 10.1073/pnas.40.12.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V. L., Radicella J. P., Robbins T. P., Chen J., Turks D. Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences. Plant Cell. 1989 Dec;1(12):1175–1183. doi: 10.1105/tpc.1.12.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cone K. C., Burr F. A., Burr B. Molecular analysis of the maize anthocyanin regulatory locus C1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9631–9635. doi: 10.1073/pnas.83.24.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta P., Saikumar P., Reddy C. D., Reddy E. P. Myb protein binds to human immunodeficiency virus 1 long terminal repeat (LTR) sequences and transactivates LTR-mediated transcription. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8090–8094. doi: 10.1073/pnas.87.20.8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R A. Genetical Studies of Variegated Pericarp in Maize. Genetics. 1917 Jan;2(1):1–35. doi: 10.1093/genetics/2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Klein T. M., Roth B. A., Fromm M. E., Cone K. C., Radicella J. P., Chandler V. L. Transactivation of anthocyanin biosynthetic genes following transfer of B regulatory genes into maize tissues. EMBO J. 1990 Aug;9(8):2517–2522. doi: 10.1002/j.1460-2075.1990.tb07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt I. M. A chromosome replication pattern deduced from pericarp phenotypes resulting from movements of the transposable element, modulator, in maize. Genetics. 1984 Oct;108(2):471–485. doi: 10.1093/genetics/108.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechelt C., Peterson T., Laird A., Chen J., Dellaporta S. L., Dennis E., Peacock W. J., Starlinger P. Isolation and molecular analysis of the maize P locus. Mol Gen Genet. 1989 Oct;219(1-2):225–234. doi: 10.1007/BF00261181. [DOI] [PubMed] [Google Scholar]
- Ludwig S. R., Wessler S. R. Maize R gene family: tissue-specific helix-loop-helix proteins. Cell. 1990 Sep 7;62(5):849–851. doi: 10.1016/0092-8674(90)90259-h. [DOI] [PubMed] [Google Scholar]
- Paz-Ares J., Ghosal D., Wienand U., Peterson P. A., Saedler H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987 Dec 1;6(12):3553–3558. doi: 10.1002/j.1460-2075.1987.tb02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C. W., Sippel A. E., Vingron M., Klempnauer K. H. Drosophila and vertebrate myb proteins share two conserved regions, one of which functions as a DNA-binding domain. EMBO J. 1987 Oct;6(10):3085–3090. doi: 10.1002/j.1460-2075.1987.tb02616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson T. Intragenic transposition of Ac generates a new allele of the maize P gene. Genetics. 1990 Oct;126(2):469–476. doi: 10.1093/genetics/126.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Shepherd N., Tacke E., Gierl A., Rohde W., Leclercq L., Mattes M., Berndtgen R., Peterson P. A., Saedler H. Influence of transposable elements on the structure and function of the A1 gene of Zea mays. EMBO J. 1987 Feb;6(2):287–294. doi: 10.1002/j.1460-2075.1987.tb04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Genes encoding actin in higher plants: intron positions are highly conserved but the coding sequences are not. J Mol Appl Genet. 1983;2(1):111–126. [PubMed] [Google Scholar]
- Slamon D. J., Boone T. C., Murdock D. C., Keith D. E., Press M. F., Larson R. A., Souza L. M. Studies of the human c-myb gene and its product in human acute leukemias. Science. 1986 Jul 18;233(4761):347–351. doi: 10.1126/science.3014652. [DOI] [PubMed] [Google Scholar]
- Weber B. L., Westin E. H., Clarke M. F. Differentiation of mouse erythroleukemia cells enhanced by alternatively spliced c-myb mRNA. Science. 1990 Sep 14;249(4974):1291–1293. doi: 10.1126/science.2205003. [DOI] [PubMed] [Google Scholar]