Abstract
By inducing DNA damage, radiotherapy both reduces tumor burden and enhances anti-tumor immunity. Here, we will review the mechanisms by which radiation induces anti-tumor immune responses that can be augmented using immunotherapies to facilitate tumor regression. Radiotherapy increases inflammation in tumors by activating the NF-κB and the Type I interferon response pathways to induce expression of pro-inflammatory cytokines. This inflammation coupled with antigen release from irradiated cells facilitates dendritic cell maturation and cross-presentation of tumor antigens to prime tumor-specific T cell responses. Radiation also sensitizes tumors to these T cell responses by enhancing T cell infiltration into tumors and the recognition of both malignant cancer cells and non-malignant stroma that present cognate antigen. Yet, these anti-tumor immune responses may be blunted by several mechanisms including regulatory T cells and checkpoint molecules that promote T cell tolerance and exhaustion. Consequently, the combination of immunotherapy using vaccines and/or checkpoint inhibitors with radiation is demonstrating early clinical potential. Overall, this review will provide a global view for how radiation and the immune system converge to target cancers and the early attempts to exploit this synergy in clinical practice.
Keywords: Radiotherapy, Immunotherapy, Checkpoint blockade
Introduction
The radiation dose necessary to cause complete tumor regression is often less than the dose expected to kill all of the cancer cells, suggesting that radiation activates other tumoricidal mechanisms. Stone et al. provided early evidence that the immune system facilitated the regression of irradiated tumors because the radiation dose necessary for tumor control was 1.67-fold higher in immunodeficient mice compared to immunocompetent mice (1). Cameron et al. provided additional evidence that radiation sensitized cancers to anti-tumor immune responses by showing that mice treated with tumor infiltrating lymphocytes (TILs) and focused radiotherapy developed fewer metastatic colonies compared to either treatment individually (2). Subsequent advances have now provided greater insight into radiation-induced immune responses that are now being employed for therapeutic gain.
Irradiated cancer cells likely activate immunological pathways that had initially evolved to defend the body against intracellular pathogens. When a pathogen such as a virus infects a cell, the cell recognizes the foreign intruder using pattern recognition receptors (PRRs) to induce inflammatory responses. Inflammatory signals mature dendritic cells (DCs) that acquire foreign peptides at infected sites and then migrate to the draining lymph node (DLN) to present antigen to naïve T cells, a process called antigen presentation. Recognizing the cognate antigen presented by mature DCs, naïve T cells activate into effector T cells that return to the site of infection to clear virally-infected cells. Since excessive stimulation of immune responses can also damage non-infected tissues, the immune system has also evolved mechanisms that prevent or suppress unintended immune responses. For cancers, radiation induces innate and adaptive immune responses against antigenic cancer cells that had pirated immunosuppressive mechanisms to escape destruction. Recent findings regarding this process are detailed below.
Modulation of tumor inflammation by radiation
Although inflammation is an inherent aspect of cancer development, radiotherapy alters the inflammatory milieu leading to the maturation of antigen presenting cells and the activation of anti-tumor T cells. To this end, Hallahan et al. first showed that radiation induced the expression of TNFα (3). Subsequently, other pro-inflammatory cytokines including interferon-α, interferon-β and interferon-γ were also shown to be induced in irradiated cancer cells (4, 5). Recent work has demonstrated that radiation activates two main pathways, the NF-κB pathway and the Type I Interferon (IFN) response pathway, which are detailed below and in Figure 1.
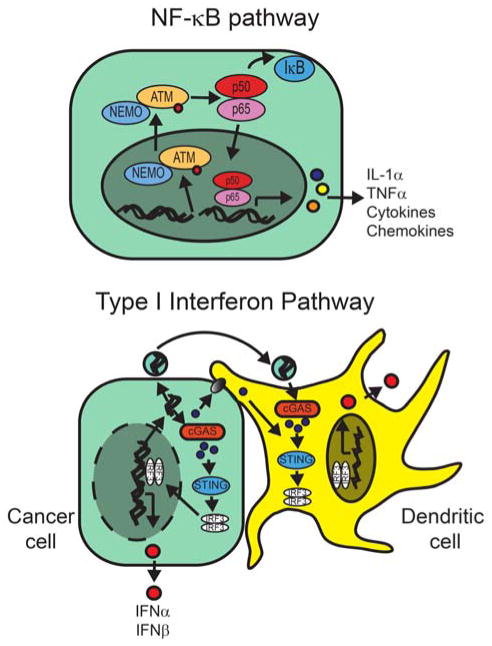
Figure 1. Radiation activates inflammatory cytokine pathways.
(Upper panel) Radiation-induced DSBs activate the alternative pathway for NF-kB activation by inducing the nuclear translocation of ATM. (Lower panel) Irradiated cells release nucleic acids into the cytoplasm that are transferred via gap junctions or endocytosis to DCs and are recognized by cGAS that activates the STING pathway.
The role of NF-κB in radiation-induced inflammation
The NF-κB pathway is a master regulator of both innate and adaptive immunity. NF-κB is a transcription factor composed of homo- or heterodimers of the NF-κB/Rel family: RelA (p65), RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2). Although NF-κB is activated through both canonical and non-canonical pathways, the end result is the translocation of the NF-κB complex to the nucleus to induce pro-inflammatory gene expression including TNFα, IL-6, IL-1α and IL-1β among others. Radiation activates the genotoxic stress arm of the NF-κB pathway via recognition of double strand DNA breaks (DSBs) by ataxia telangiectasia mutated protein (ATM) (6, 7). After recruitment to DSBs in the nucleus, the ATM-complex then translocates to the cytoplasm to activate the p50/p65 complex of NF-κB.
Although radiation induces many pro-inflammatory cytokines through NF-κB, the overall impact of NF-κB activation in cancer cells remains nebulous due to its paradoxical radioprotective and radiosensitizing effects. Activation of NF-κB may directly prevent cell death by inducing anti-apoptotic genes including the inhibitor of apoptosis protein (IAP) family members and the TNFα-induced protein 3 (TNFAIP3/A20) that inhibits cell death (8). NF-κB activation may also induce inflammatory stimuli that promote tumor growth such as matrix metalloproteinases and vascular endothelial growth factor (VEGF). Although NF-κB activates anti-tumor immune responses, the complex role of NF-κB in irradiated cancers requires further investigation before this pathway is manipulated for therapeutic gain.
The role of the IFN response pathway in radiation-induced inflammation
Radiation also induces interferon-related genes including IFNα, IFNβ, STAT1 and IFNγ (4, 5). Type I and Type II interferon responses play predominant roles during distinct phases of anti-tumor immunity. In cancers, secretion of the Type I IFNs, IFNα and IFNβ, facilitates DC maturation that is necessary for the generation of effector T cells (9), which return to the tumor to secrete the Type II IFN, IFNγ, to cause vascular destruction and to sensitize tumors to cytolytic T cells. Demonstrating a role for Type I IFN in irradiated tumor models, Burnett et al. showed that the Type I IFN was essential for the rejection of antigenic melanoma cells after a single fraction of 25 Gy (4). Furthermore, radiation induced IFNβ causing DC maturation that led to the induction of effector CD8+ T cell responses against antigenic tumors. Demonstrating a role for Type II IFN in irradiated tumors, Lugade et al. showed neutralization of IFNγ potentiated the growth of B16 melanoma tumors after 15 Gy (5). Here, loss of IFNγ sensitivity led to decreased recognition of antigenic tumor cells by effector T cells and to decreased vasculature destruction.
In order to induce Type I IFN in cancer cells, radiation likely co-opts intracellular viral sensing pathways involving cytosolic sensors such as STING, RIG1, MDA5 and LGP-2. Initially described in innate anti-viral responses, several groups have shown that the stimulator of interferon genes (STING) responds to cytosolic DNA and possibly RNA that is present during viral infections (10, 11). cGAMP synthase recognizes cytostolic DNA and produces cGAMP(2′-5′) that in turn activates STING. STING induces IFN gene expression via activation of IRF3 as well as the induction of other STAT6-dependent pro-inflammatory genes. Radiation also likely induces type I IFN responses by inducing the accumulation of cytosolic DNA. Deng et al. reported that STING was necessary for radiation-induced IFN responses; radiation failed to stimulate the maturation of intratumoral DCs in mice with STING loss (12). Furthermore, exogenous cGAMP promoted anti-tumor immune responses against irradiated tumors and enhanced tumor regression. In addition, RIG-1, MDA5 and LGP2 are RNA sensors that act through IRF3 and NF-κB to induce pro-inflammatory cytokines. Radiation also activates these RNA sensors, which have been shown to be necessary for the induction of Type I IFN after DNA damage (13). In addition to being directly produced by irradiated cells, it is likely secondary messengers such as cGAMP are transferred via gap junctions to stimulate the STING pathway in non-irradiated cells (14).
Similar to the NF-κB pathway, the activation of the interferon response pathway after radiation may play paradoxical pro-tumorigenic and anti-tumorigenic roles. Although acute activation of the IFN pathway by radiation is cytotoxic, chronic activation of the IFN/STAT1 pathway makes cancers radioresistant (15, 16) and predicts for worse outcomes after radiotherapy and/or chemotherapy (17).
Radiation modulates the priming of adaptive immune response against tumors
Radiation induces immunologic cell death
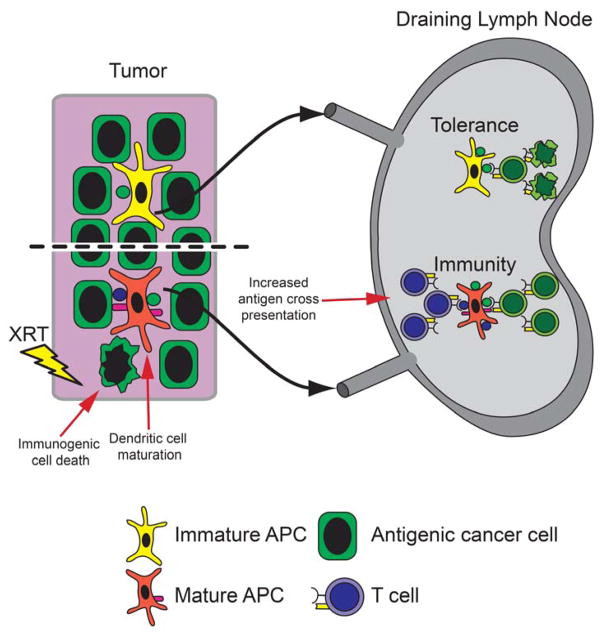
DNA damage often leads to cell death either through apoptosis or necrosis. Apoptosis and necrosis differently impact the generation of immune responses against tumors (Figure 2). Antigens from apoptotic cells were more efficiently cross-presented by DCs likely due to the membrane translocation of calreticulin and phosphatidylserine and other ligands which facilitate DC phagocytosis (18). However, phagocytosis of apoptotic cells is not sufficient for DC maturation and T cell priming. White et al. demonstrated that the activation of caspase-3/7, Apaf-1 and caspase-9 during apoptosis prevented the induction of a STING-dependent Type I IFN response (19). Sauter et al. demonstrated that necrotic tumor cells were necessary to mature DCs that then primed CD8+ T cells (20). Necrotic cells release a high-mobility group B1 protein (HMGB1) that induces DC maturation via an NF-kB pathway. Since radiation induces cell death via apoptosis and/or necrosis, radiation likely helps to prime anti-tumor immunity via increased antigen release by cancer cells, increased antigen uptake by DCs and increased DC maturation.
Figure 2. Radiation induces T cell priming.
In non-irradiated tumors, immature DCs cross-present certain antigens to induce immune tolerance in the DLN. Radiation induces T cell priming by facilitating immunogenic cell death resulting in antigen release and inflammatory signals. DCs cross-present neo-antigens (blue) or increase the presentation of antigens already cross-presented (green). DCs mature under inflammatory signals and migrate to the DLN to induce anti-tumor immune responses.
Radiation enhances cross-presentation of antigen
Compared to direct presentation, cross-presentation requires a higher concentration of antigen in order to be acquired and processed by DCs (Figure 2) (21). Radiation-induced cell death likely causes the release of antigens that is sufficient to exceed the threshold necessary for cross-presentation by DCs (22). For antigens already expressed at levels sufficient for cross-presentation, Lugade et al. demonstrated that radiation further increased the number of DCs presenting antigen, likely due to the release of antigen from dying cells (23). The release of antigen at sufficient levels for cross-presentation likely depended on the fraction size and total dose. Lee et al. demonstrated that a single dose of 20 Gy induced T cell proliferation in the DLN and tumor regression, whereas 20 Gy given in four fractions was significantly less effective in inhibiting tumor growth (24). Although there is little systematic assessment for how different fractionation schemes enhance antigen presentation, several groups suggest that fraction sizes of 7.5 Gy or higher were necessary to facilitate antigen cross-presentation (25, 26). However, many of these studies have used peptide antigens with Major Histocompatibility Complex (MHC) affinities less than 10 nM that may overestimate the capacity of radiation to facilitate the cross-presentation of physiologically relevant antigens that have substantially lower peptide-MHC affinities. Thus, the ability of radiation to stimulate immune responses to immunologically-ignored cancer antigens likely depends on antigen expression level and its MHC affinity.
In addition to increasing antigen release from dying cells, radiation may also alter the peptide repertoire available for anti-tumor immune responses. First, radiation may induce the expression of normally silent genes that serve as neo-antigens. Using HLPC to identify MHC binding proteins eluted from irradiated cells, Reits et al. demonstrated that radiation induced the expression of proteins not normally presented by MHC molecules (27). Sharma et al. also demonstrated that a single dose of 20 Gy induced the expression of normally silent cancer testis antigens in various cancer cell lines and in sarcoma biopsies (28). In addition, radiation-induced inflammatory responses may alter the processing and presentation of peptide epitopes by the formation of an IFN-induced immunoproteasome. Morel et al. demonstrated that the immunoproteosome processes cellular antigens in mature DCs differently compared to the standard proteasome (29). These radiation inducible genes may also serve as antigens for antibody targeted therapy. Yan et al. has developed a monoclonal antibody, 2C6F3, that recognizes radiation inducible Tax interacting protein-1 (TIP-1) (30). Binding of 2C6F3 to irradiated cancer cells promoted antibody dependent cell-mediated cytotoxicity and phagocytosis and a radiolabeled 2C6F3 inhibited tumor growth in vivo. Thus, radiation may facilitate antigen presentation by increasing the quantity and quality of antigen available for cross-presentation.
Radiation induces DC maturation
Once DCs acquire antigen, these cells must mature in order to traffic to the DLN to initiate an immune response (Figure 2). Gupta et al. demonstrated that high-dose irradiation of B16 melanoma caused DC maturation that was necessary for CD8+ T cell priming (31). Antigens released by cell death were not sufficient to stimulate T cell priming because melanoma cells engineered to die under non-inflammatory conditions failed to induce anti-tumor immune responses. Rather, local irradiation caused DC maturation as measured by the increased expression of the co-stimulatory molecules CD70 and CD86 in DCs. In both mouse models and human tumors, CD70 expression in mature DCs was necessary for effective T cell priming against irradiated tumors because CD70 blockade inhibited T cell priming and regression of irradiated tumors (31, 32).
Although many cancers are prone to lymphatic spread, the radiation dose used in elective nodal irradiation to sterilize microscopic disease also likely suppresses effective anti-tumor immune responses. Pelvic irradiation to 39.6 Gy likely induced anti-tumor immune responses as CD4+ and CD8+ T cells demonstrated increased Th1 and Tc1 phenotypes (33). By contrast, DLN irradiated to a higher dose of 50 Gy demonstrated suppressed effector T cell responses indicating that elective nodal irradiation impaired the generation of anti-tumor immune responses in a dose-dependent manner. Consequently, the immunological benefits of radiotherapy more likely occur in scenarios where only the primary tumor is irradiated such as stereotactic body radiotherapy (SBRT).
Radiation modulates the effector T cell responses
Radiation increases T cell infiltration
During the effector phase of the immune response, radiation may facilitate the immunological rejection of tumors by inducing chemokine expression that recruits cytotoxic CD8+ T cells into tumors (Figure 3). Matsumura et al. demonstrated that radiation upregulated the chemokine CXCL16 in murine cancer cells that facilitated T cell recruitment into irradiated tumors in order to enhance tumor regression in combination with immunotherapy (34). In addition, Meng et al. demonstrated that radiation also induced the expression of CCL3, CCL4, CCL5, CXCL9, CXCL10, and CXCL11 (35).
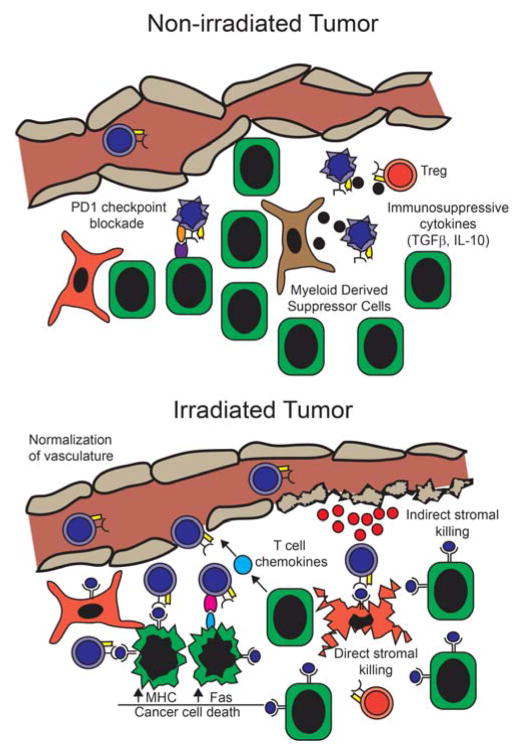
Figure 3. Radiation facilitates effector T cell responses.
(Upper panel) Tumors inhibit immune responses via checkpoint blockade, Tregs, immunosuppressive cytokines, tumor-associated macrophages, myeloid derived suppressor cells and a disorganized vasculature. (Lower panel) Radiation induces chemokine release and vascular normalization that increases T cell infiltration. In addition, irradiated cancer cells induce peptide-MHC and Fas expression increasing their susceptibility to T cell recognition and killing. Finally, radiation induces the stromal presentation of antigen facilitating their direct and indirect killing by T cells.
Radiation therapy may also facilitate T cell infiltration by altering the vascular phenotype (Figure 3). The ability of radiotherapy to facilitate T cell infiltration is due to vascular normalization and the induction of inflammatory cytokines including IFNγ and/or TNFα. Tumors often display a disorganized vasculature that normalizes after treatment with anti-angiogenic agents such as VEGF inhibitors. This normalization of the vasculature by anti-angiogenic agents facilitates lymphocyte infiltration in order to enhance tumor regression (36). Similarly, Ganss et al. demonstrated that a single dose of 10 Gy facilitated the infiltration of antigen-specific T cells into primary insulinomas (37). Of note, the normalization of the tumor vasculature may persist even after completing treatment because antigen-specific T cells displayed increased tumor infiltration even 3 weeks after tumor irradiation.
The ability of radiotherapy to recruit T cells to tumors may be particularly important for tumors with a non-T cell inflamed phenotype. Recent work in melanoma has prompted the classification of cancers by a T-cell inflamed or non-T-cell inflamed phenotypes based on the presence or absence of tumor infiltrating lymphocytes (TILs), respectively (38). The T-cell inflamed phenotype predicts improved responses to PD1-PDL1 checkpoint blockade. Since checkpoint blockade fails in the majority of patients, increasing T cell infiltration into non-T-cell inflamed tumors may improve outcomes. To this end, Tang et al. demonstrated that targeting the TNF superfamily member LIGHT increased T cell infiltration and sensitized non-T-cell inflamed tumors to PDL-1 therapy (39). Similarly, other pro-inflammatory agents, such as CpG oligonucleotides also enhanced the extravasation of T cells into tumors suggesting that inflammatory environments may facilitate a T-cell inflamed phenotype. As an extension, the ability of radiotherapy to promote inflammation and recruit T cells into tumors may help sensitize cancers that are otherwise resistant to PDL-1 therapy.
Radiation facilitates effector T cell recognition of antigenic tumors
Radiation also facilitates the recognition and killing of tumor cells by effector CD8+ T cells by increasing the recognition of irradiated cancer cells that have a lower threshold for being killed by cognate T cells. (Figure 3). In both in vivo and in vivo models, radiation induced MHC expression on cancer cells that facilitated the recognition of antigenic cancer cells by T cells (26, 28, 40). The upregulation of MHC by radiation was likely due to the induction of Type I IFN after radiation. Furthermore, radiation doses as low as 2 Gy induced MHC upregulation on cancer cells indicating that conventional fractionation likely facilitates the immunological recognition of antigenic cancer cells. To sensitize cancer cells to killing by cytotoxic T cells, radiation upregulated the expression of the cell death ligand Fas in multiple murine and human cancer cell lines (41, 42). Of note, Fas expression persisted for longer than 11 days after radiation, suggesting that effector T cells may recognize irradiated tumors for several weeks after completing treatment. Yet, it remains unclear whether conventional fraction sizes (approximately 2 Gy) are sufficient to induce Fas expression as previous studies used only radiation doses of 8 Gy or higher.
In addition to increasing direct recognition and killing of antigenic cancer cells, radiation may also enhance the immunologic recognition and destruction of the tumor stroma that is essential for tumor growth (Figure 3). Stromal cells cross-presenting tumor antigens also become targets for cytotoxic T cells, resulting in the indirect killing of antigenic cancer cells, a process that can be facilitated by radiation (22, 43). Similarly, Wu et al. loaded the stroma with intratumoral injection of exogenous antigen to facilitate the immunological rejection of irradiated tumors (44). Thus, radiation may facilitate the immunological destruction of tumors by sensitizing both malignant cancer cells and non-malignant stromal cells to killing by cytotoxic T cells.
Radiation facilitates Natural Killer (NK) cell recognition of tumors
Although radiation facilitates the specific recognition of antigenic cancer cells by cytotoxic CD8+ T cells, cancer cells that have lost MHC may escape radiation induced immune-mediated rejection. As an immunosurveillance mechanism against the loss of antigen presentation or “missing self”, Karre et al. demonstrated that RMA-S cancer cells that have lost the MHC expression were recognized and eliminated by NK cells. By contrast, NK cells were ineffective against wild-type RMA cells that retained MHC expression (45). Subsequent work identified activating receptors that enable NK cells to non-specifically recognize and kill of cancer cells. When ligands for the NK activating receptor NKG2D is upregulated on cancer cells, NK cells lysed these target cells even in the presence of MHC expression (46). The DNA damage pathway upregulated ligands on target cells that bind to activating NK receptors in order to enhance the recognition of irradiated cancer cells. Gasser et al. demonstrated that radiation and other genotoxic stress induced expression of ligands binding NKG2D that was dependent of ATM, ATR or CHK1 (47). Similarly, DNA damage also upregulated cellular ligands for the activating DNAM-1 NK immunoreceptor (48). By contrast, Fine et al. demonstrated that radiation also downregulated Clr-b a ligand for the inhibitory NK receptor NKR-P1B that mitigates NK cell cytolytic activity (49). Thus, radiation also sensitizes cancer cells to recognition by NK cells via upregulating or downregulating ligands for activating or inhibitory NK receptors, respectively.
Modulators of radiation-induced tumor immunity
Tumor-associated myeloid cells
Tumor-associated macrophages (TAMs) and myeloid derived suppressor cells (MDSCs) are two components of the myeloid compartment that negatively regulate anti-tumor immune responses. TAMs have generally been associated with promoting tumor growth by enhancing cancer cell survival, angiogenesis, invasion and metastasis (50). Although TAMs can display a tumoricidal M1 phenotype, the tumorigenic M2 phenotype secretes immunosuppressive cytokines that inhibit anti-tumor CD8+ T cell responses. In an autochthonous breast cancer model, Ruffell et al. demonstrated that TAMs secrete IL-10 to suppress DC maturation and the generation of anti-tumor immune responses (51). Similarly, MDSCs inhibit effector CD8+ T cell responses in the tumor microenvironment as well as promote angiogenesis and tumor growth directly via cytokine production. MDSCs represent either an immature myeloid cell or a separate myeloid lineage. Although markers for human MDSCs are poorly defined, murine MDSCs express both the macrophage marker CD11b as well as the granulocyte marker Gr1. The increased number of TAMs have been associated with worse survival in patients with lymphoma (52), breast (53), ovarian and lung cancers, among others. Furthermore, in transplant tumor models, increasing TAM content correlated with worse tumor control after radiation (54). Similarly, increases in MDSCs have been associated with worse prognosis in breast cancers (55) and liver cancers, among others.
Several groups have recently shown that radiation directly eliminates or alters the phenotype of TAMs and/or MDSCs to impact anti-tumor immune responses. Filatenkov et al. demonstrated that a single fraction of 30 Gy caused the loss of MDSCs and increased CD8+ T cell infiltration in tumors (56). However, it remains unclear if radiotherapy directly caused the loss of MDSCs or if radiation induced T cell infiltration leading to the MDSC elimination. By contrast, irradiated tumors displayed increased macrophage infiltration likely via the upregulations of the chemoattractants SDF-1 and CSF-1 as well as the SDF-1 receptor CXCR4 (57–59). Increased TAM influx promotes tumor growth because macrophage neutralization using CD11b blocking antibodies protected irradiated tumors and was associated with increased vasculogenesis and/or matrix metalloproteinase expression (57, 60, 61). Radiation may also promote an M1 macrophage phenotype because multiple fractions of 1–2 Gy induced nitric oxide synthase in TAMs resulting in vascular remodeling and the recruitment of effector CD8+ T cells (62). However, the ability of radiation to promote M1 or M2 TAM phenotypes is likely influenced by the host’s genotype (63). Thus, myeloid cells protect irradiated tumors both directly by fostering a pro-growth environment and indirectly by inhibiting anti-tumor immune responses.
Regulatory T cells
Previously known as suppressor T cells, regulatory T cells (Tregs) are a subpopulation of T cells that abrogates anti-tumor immune responses to self-antigens. Unlike immune responses that were often specific to unique tumor antigens, Tregs likely react against common self-antigens because a single population of “suppressor” cells inhibited immune responses against multiple tumor types (64). Tregs have been defined by the cellular markers CD4, CD25 and Foxp3. Since many lymphocytes at various stages of activation express these markers, it is difficult to specifically identify regulatory T cells without functional assays.
Radiation increases the numbers of Tregs in tumors that likely depends on the radiation dose (26, 65). Consequently, efforts to deplete Tregs may enhance the effectiveness of radiotherapy by increasing anti-tumor immunity. Depletion of Tregs using an anti-CD25 antibody, PC61, augmented radiation-induced antigen-specific immune responses, enhanced tumor regression of irradiated tumors and improved survival in murine models (26, 65). Since activated T cells also express CD25, the widespread use of this approach may also negate the benefit of radiation-induced anti-tumor immune responses. In humans, the most suppressive T cells, CD45RA−FOXP3hiCD4+ T cells, specifically express CCR4. Sugiyama et al. demonstrated that anti-CCR4 antibody treatment reduced Tregs and potentiated NY-ESO1 specific CD8+ T cell responses in cancer patients (66). Consequently, it is interesting to speculate whether anti-CCR4 antibody may also enhance radiation-induced immune responses in humans.
Immunological checkpoints
Many preclinical insights into augmenting radiation-induced anti-tumor immune responses have focused on combining checkpoint agonists and antagonists with radiation (Figure 4). Immune checkpoints are molecules that enhance (i.e., co-stimulatory molecules) or inhibit immune responses. The two most widely-studied checkpoints are Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) and Programmed Death 1(PD1). Expressed on helper CD4+ T cells, CTLA-4 binds to CD80 (B7-1) or CD86 (B7-2) on antigen presenting cells to inactivate helper T cells and mitigate the expansion of effector CD8+ T cells (67, 68). PD1 is expressed on effector T cells and binds to the ligands PDL-1 or PDL-2 expressed on DCs, stromal cells and some cancer cells to induce apoptosis in effector T cells and exhaust anti-tumor immune responses.
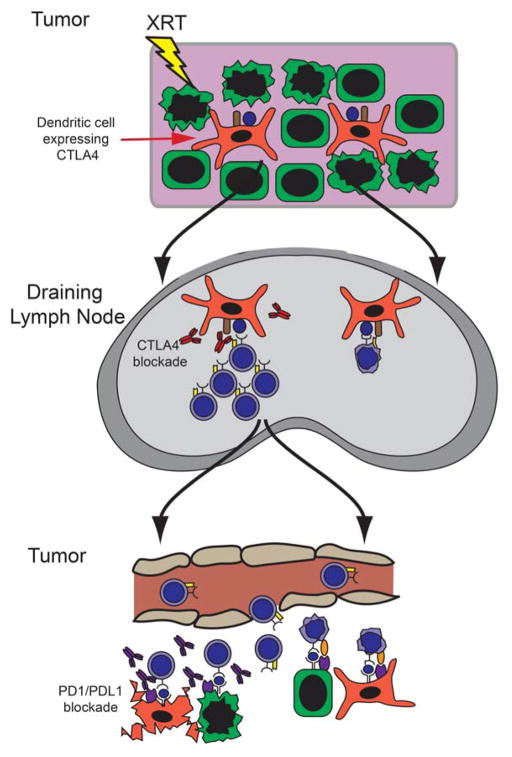
Figure 4. Checkpoint blockade synergizes with radiotherapy to facilitate tumor regression.
Radiation induces cross-presentation of antigen by DCs in the DLN. Since some DCs express CTLA-4 that mitigates the expansion of cytotoxic T cells, ipilimumab blockade of CTLA-4 promotes T cell expansion. These effector T cells then return to the tumor where cancer cells and stromal cells expressing PD-L1 or PD-L2 induce T cell apoptosis. Blockade of PD-1 using nivolumab or pembrolizumab prevents T cell apoptosis to facilitate tumor cell killing.
The use of radiation with PDL-1 and CTLA4 inhibitors has been extensively studied. Demaria et al. demonstrated that CTLA-4 combined with radiation improved survival in tumor-bearing mice due to reduced lung metastases but had minimal effect on the growth of irradiated tumors (69). In this model, invariant natural killer (iNK) T cells likely inhibited the regression of irradiated tumors because tumors regressed in 50% of mice deficient in iNK T cells (70). In other models, radiation and CTLA-4 blockade also inhibited the growth of irradiated tumors by arresting TIL motility and increasing TIL accumulation in order to facilitate tumor cell killing via NKG2D upregulation (71). Similarly, Deng et al. demonstrated that PD-1 inhibition synergized with radiation by blocking PD-L1 on both cancer cells and myeloid stromal cells causing a reduction of MDSCs in the tumor microenvironment (72). Sharabi et al. demonstrated that high-dose radiotherapy synergized with PD-1 inhibition by blocking local suppression of anti-tumor immune responses that were stimulated by radiation (26). PD-1 inhibition may reverse the upregulation of PD-L1 expression on cancer cells induced by radiation (73). Several additional tumor models including gliomas, colon cancers and breast cancers have been used to confirm the synergism between PD-1 blockade and radiation (73, 74). Finally, radiation may benefit from combinatorial checkpoint blockade where radiation improved antigen presentation, CTLA-4 blockade promoted T cell expansion, and PD-1 blockade prevented T cell exhaustion (75).
In addition, there are other checkpoints that may be targeted with radiation to enhance anti-tumor immune responses in order to achieve improved cancer control.
Radiation combined with checkpoint activators including OX40 and 4-1BB prevented T cell exhaustion and facilitated the regression of irradiated tumor (76, 77). Similarly, targeting inhibitors of T cell function, such as TGFβ and IDO, also facilitated the regression of irradiated tumors (77–80).
Organ-induced immune tolerance
The spleen likely regulates anti-tumor immunity because splenectomy enhanced tumor regression that was associated with loss of intratumoral CCR2+ monocytes and activation of CD8+ CTLs (81). Consistent with this observation, Ugel et al. demonstrated that the peripheral tolerance to tumor antigens in the spleen was mediated by CCR2+ monocytes that cross-presented antigen into order to tolerize memory CD8+ T cells (82). Given that splenic irradiation is essentially non-toxic, it is interesting to speculate whether irradiating the spleen would augment immunotherapy. To this end, Albers et al. has demonstrated that 6 Gy local splenic irradiation caused an abscopal effect in four patients with CML (83).
In addition, the presence of metastasis in the central nervous system may also adversely impact the ability of radiation to stimulate anti-tumor immune responses. Using a B16 melanoma model, Jackson et al. demonstrated that melanoma brain metastases induced greater CD8+ T cell tolerance compared to peripheral tumors (84). Radiation of brain metastases coupled with vaccination against tumor antigens reversed immune tolerance and improved survival.
Other factors that potentially modulate radiation-induced anti-tumor immunity
Other variables including the cancerzs genetic landscape, the patient’s age and the enteric flora can impact immune responses and modulate the effectiveness of radiotherapy. Since the genomic landscape of cancer cells correlates with cytolytic T cell signatures, it is likely the genetic profiles of tumors also dictate radiation-induced immune responses and, therefore, could be used to guide the selection of patients for combined radiotherapy and immunotherapy protocols. (85). Age is associated with both an increasing cancer incidence and with impaired immunity (86). Consequently, T cells from young mice but not aged mice rejected established tumors expressing immunodominant antigens (87). Therefore, attempts to rejuvenate aged immune responses may rescue a senile immune system and potentially benefit older patients undergoing radiotherapy. Finally, the gut flora can regulate local and systemic inflammation and, consequently, immune responses against tumors (88, 89). Since radiation also alters the inflammatory tumor microenvironment, the impact of oral and gastrointestinal flora may influence radiation-induced immune responses against tumors.
Clinical applications of immunotherapy with radiation
The clinical implementation of immunotherapy with radiation is currently limited to patients with metastatic disease given the potential of minimally toxic radiation doses to induce an abscopal response. The abscopal response is systemic and causes the regression of non-irradiated lesions with localized radiotherapy (reviewed by Siva et al. (90)) and, consequently, may lead to improved disease-free and overall survival in cancer patients.
Radiation combined with tumor vaccines and adjuvants
Vaccines include peptide-based vaccines, recombinant viral-based vaccines and antigen-loaded DC-based vaccines. In prostate cancer patients, a recombinant TRICOM vaccine against PSA combined with localized prostate radiotherapy induced antigen-specific T cell responses in irradiated patients (91). Recently, a DC-based vaccine, Spuleucel-T, has been approved in patients with metastatic prostate cancer (92), and the combination of this vaccine with radiotherapy is being tested in clinical trials.
In addition, combining Toll-like receptor (TLR) agonists with radiation may potentiate anti-tumor immune responses. TLRs are PRRs on antigen presenting cells that bind ligands present in pathogenic organisms in order to induce DC maturation. In 15 patients with low grade Non-Hodgkin’s Lymphoma, Brody et al. combined radiotherapy with the TLR-9 agonist PF-351676 resulting in one complete response and three partial responses (93).
Radiotherapy has also been combined with systemic or intratumoral cytokines that promote T cell priming and/or T cell survival. In a Phase I trial, Seung et al. treated patients with renal cell carcinoma or melanoma metastases with SBRT followed by high-dose IL-2 resulting in response rates that were significantly better than historical controls (94). However, the widespread use of IL-2 is limited given the toxic vascular effects that often require hospitalization. In addition, Golden et al. combined radiotherapy with intratumoral injection of GM-CSF, a cytokine that promotes DC maturation (95). Of the 41 patients treated, 26.8% had responses at non-irradiated lesions suggesting a potential abscopal effect; however this treatment did not impact outcomes. Table 1 lists ongoing trials combining various vaccines with radiotherapy.
Table 1. Selected ongoing clinical studies of vaccines in combination with radiation.
A search of ClinicalTrials.gov was performed in April 2016 for “Interventional Studies”, “vaccine and radiation”, “open studies”, and “immunotherapy and radiation” retrieving 271 records. Selected studies were reviewed for inclusion in this summary Table based on the authors’ perception of the size and overall impact of the trial.
| ClinicalTrials.gov Identifier; Year Opened; Sponsor | Drug | Type of Cancer | Radiation Dose and schedule | Any additional intervention | Comments |
|---|---|---|---|---|---|
| NCT02287428; 2014; Dana Farber Cancer Institute | Personalized NeoAntigen Cancer Vaccine derived from tumor specific mutations. | Glioblastoma | EBRT to 60 Gy. | none | Phase 1: Assesses T cell specific responses against unique tumor antigens that are less likely cause tolerance compared to tumor associated antigens. |
| NCT02501278; 2016; EORTC, with Inovio Pharmaceuticals | IL-12 DNA plasmid vaccine against HPV 16 and HPV18 E6–E7 viral proteins. | Locally Advanced Cervical Cancer | EBRT and brachytherapy | Concurrent cisplatin chemotherapy. | Phase II: Assesses immunization against non-self viral antigens. Efficacy previously shown in pre-invasive disease. |
| NCT02405585; 2015; NewLink Genetics Corporation | Algenpantucel-L: Human pancreatic cancer cells modified with murine alpha-1,3-galactosyltransferase to induce ADCC. | Pancreatic Cancer | 50.4 Gy EBRT in 28 fractions | mFOLFIRINOX during radiation and Gemcitabine: after radiation | Phase 2: Tests efficacy of immunization against undefined pancreatic antigens. ADCC is not often associated w/tumor regression. |
| NCT02648282; 2016; Sidney Kimmel Comprehensive Cancer Center | GM-CSF-secreting Allogeneic Pancreatic Cancer Vaccine (GVAX) | Locally Advanced Pancreatic Cancer | SBRT (6.6 Gy for 5 days) | Cyclophosphamide and Pembrolizumab | Phase 2: 2 previous phase 3 trials in prostate cancer were terminated due to lack of efficacy. |
| NCT01436968; 2011; Advantagene, Inc. | ProstAtak: Adenoviral delivered thymidine kinase followed by valacyclovir to induce immunogenic cell death. | Localized Prostate Cancer | Curative EBRT | Androgen deprivation therapy up to 6 months. | Phase 3: Phase 2 suggested improved 5y biochemical control for ProstAtak compared to a historical control arm (5y bPFS: 71% vs. 56%) |
| NCT01807065; 2013; City of Hope Medical Center, with NCI | sipuleucel-T: Cell based dendritic cell vaccine exposed to GM-CSF and Prostatic Acid Phosphatase. | Metastatic Prostate Cancer | Palliative EBRT | none | Phase 2: Phase 3 trials using sipuleucel-T alone demonstrated improved survival without associated improvement in symptoms, PSA or tumor size. |
| NCT01973322; 2013; Istituto Scientifico Romagnolo | Autologous DC vaccine loaded with autologous tumor lysate or homogenate. | Metastatic Melanoma | Palliative EBRT to a single metastasis | IFNα | Phase 2. |
| NCT01421017; 2011; New York University School of Medicine, with NCI | Imiquimod : TLR7 agonist. | Breast cancer skin metastases | Palliative EBRT | Cyclophosphamide to deplete Tregs. | Phase1/2: Use of TLR agonist with radiotherapy. |
Abbreviations: EBRT: External beam radiotherapy; NCI (USA): National Cancer Institute, United States of America; IFN: Interferon; IMRT: Intensity-Modulated Radiation Therapy; NIHR (UK): National Institute for Health Research, United Kingdom; NSCLC: Non-small cell lung cancer; RT: radiotherapy; SBRT: Stereotactic body radiotherapy.
Checkpoint blockade synergizes with radiotherapy
Given the pre-clinical observations using checkpoint inhibitors with radiotherapy, the initial clinical translation of these approaches has recently been described. The clinically-approved checkpoint inhibitors include ipilimumab that targets CTLA-4, nivolumab and pembrolizumab that target PD-1, and atezolizumab that targets PD-L1. Postow et al. described a patient with metastatic melanoma treated with ipilimumab and SBRT to a single lesion resulting in regression at both irradiated and non-irradiated lesions which persisted for at least 10 months (96), although it remains unclear if this therapy stimulated antigen-specific CD8+ T cell responses. Similarly, Hiniker et al. described a patient with multiple metastases treated with ipilimumab and SBRT that also displayed a complete response which persisted for at least one year (97). Finally, the benefits of radiotherapy and checkpoint blockade also extended to other cancer types including non-small-cell lung cancers (NSCLCs) (98).
The addition of radiotherapy to checkpoint blockade likely improves outcomes in patients having specific favorable factors. In a randomized trial, one fraction of 8 Gy to a single bone metastasis enhanced the activity of ipilimumab in patients with castrate-resistant metastatic prostate cancer (99). Although there was no difference in overall survival between ipilimumab and placebo, an unplanned analysis identified three favorable risk factors: alkaline phosphatase < 1.5× normal, hemoglobin concentration > 11 g/dL and the absence of visceral metastases associated with improved survival. Yet, the efficacy of the ipilimumab with radiotherapy is tempered by increased toxicity and early deaths that were attributed to immune-mediated events.
Clinical trials testing the combination of radiation and PD-1/PD-L1 inhibition have been initiated but no results have been reported. Extrapolating from trials comparing PD-1 blockade to CTLA-4 blockade suggest that the combination of radiotherapy with PD-1 blockade will a have similar if not better therapeutic potential compared to radiation and CTLA-4 blockade. In advanced melanoma, the KEYNOTE-006 trial comparing PD-1 blockade to CTLA-4 blockade found that patients treated with PD-1 had better clinical responses (33.7% vs. 11.9%), improved progression-free survival (47.3% vs. 26.5% at 6 months) and overall survival (74.1% vs. 58.2% at 6 months) (100). Furthermore, PD-1 blockade was associated with less toxicity (13.3% vs. 19.9%).
Yet, much study remains to define the role of radiation with checkpoint blockade. First, the immune-mediated toxicities of combined radiotherapy and checkpoint blockade remain unclear. The more common toxicities associated with checkpoint inhibition include increased dermatitis, pneumonitis, gastrointestinal and endocrine toxicities (101). Second, the impact of radiation fractionation on the activity of checkpoint inhibitors remains unclear. Silk et al. suggested that a single high-dose fraction using SRS and ipilimumab improved the median survival of patients with brain metastases compared to patients treated with conventionally fractionated radiotherapy (102). Finally, the optimal strategy to incorporate radiation with one or multiple checkpoint inhibitors has not yet been determined. Table 2 lists ongoing trials combining various checkpoint inhibitors with radiotherapy.
Table 2. Selected ongoing clinical studies of immune checkpoint blockade with radiation.
An advanced search of ClinicalTrials.gov was performed in April 2016 for “Interventional Studies” and “PD-1 and Radiation” (retrieved 34 records), “PD-L1 and Radiation” (retrieved 29 records) and “immunotherapy and radiation” (retrieved 207 records). These were reviewed for inclusion in this summary Table and were selected based on the authors’ perception of the size and overall impact of the trial.
| ClinicalTrials.gov Identifier; Year Opened; Sponsor | Drug | Type of Cancer | Radiation Dose and schedule | Additional interventions | Comments |
|---|---|---|---|---|---|
| NCT02406183; 2015; University Hospital, Ghent | Ipilimumab | Metastatic Melanoma | SBRT: Arm 1: 8 Gy × 3 Arm 2: 10 Gy × 3 Arm 3: 12 Gy × 3 |
None. | Phase I. Will assess immune responses by measuring absolute lymphocytes, Tregs and T cell phenotype. |
| NCT01711515; 2012; NCI | Ipilimumab | Cervical Cancer | EBRT & brachytherapy | Concurrent cisplatin. | Phase I. Will assess anti-HPV T cell responses for immunoprofiling. |
| NCT01935921; 2013; NCI | Concurrent weekly cetuximab Ipilimumab starting at week 4 of radiotherapy every 3 weeks × 3 | Stage III–IVB Head and Neck Cancer | EBRT. | None. | Phase I. Will assess T cell phenotypes, MDSCs and Tregs. |
| NCT02296684; 2015; Washington University School of Medicine | Pre-operative and post-operative pembrolizumab | Head and Neck Cancer undergoing surgery | EBRT | Risk-based concurrent cisplatin | Phase 2. Will directly assess immune responses in resected cancers after checkpoint blockade. |
| NCT02621151; 2016; New York University School of Medicine | Concurrent pembrolizumab × 3. | Muscle-Invasive Bladder Cancer | Concurrent Hypofractionated EBRT: 52 Gy in 20 fractions. | Concurrent gemcitabine. | Phase 2. |
| NCT02303366; 2014; Peter MacCallum Cancer Centre | Adjuvant pembrolizumab every 3 weeks × 8 | Oligometastatic breast cancer | SBRT: 20 Gy × 1 to a maximum of 5 metastatic lesions. | None. | Phase 1. |
| NCT02621398; 2015; Rutgers, with NCI, and Rutgers Cancer Institute of New Jersey | Concurrent pembrolizumab (up to 18 cycles) testing drug schedule at start of RT, mid-treatment or 2 weeks before completing RT. | NSCLC | EBRT to 60 Gy. | Concurrent paclitaxel and carboplatin | Phase 1. |
| NCT02400814; 2015; University of California, Davis, with NCI (USA). | Neoadjuvant, concurrent and adjuvant MPDL3280A (anti-PD-L1 antibody) | Stage IV NSCLC | SBRT. | None. | Phase 1. |
| NCT02525757; 2015; M.D. Anderson Cancer Center. | Concurrent or adjuvant MPDL3280A. | Lung cancer | EBRT to 60–66 Gy. | Concurrent carboplatin and paclitaxel. | Phase 2. |
| NCT02336165; 2015; Ludwig Institute for Cancer Research, with Cancer Research Institute and Cure Brain Cancer Foundation. | Durvalumab (anti-PD-1 mAb) + RT. | Glioblastoma | EBRT | None. | Phase 2. |
| NCT02735239; 2016; Ludwig Institute for Cancer Research, with AstraZeneca | Neoadjuvant and concurrent Durvalumab ± Tremelimumab (anti-CTLA4) | Esophageal Cancer | EBRT | Concurent Oxaliplatin; Capecitabine | Phase 1/2. Phase I will assess PD-1 inhibition alone while Phase 2 will assess combination of PD-1 and CTLA-4 inhibition. |
Abbreviations: EBRT: External Beam Radiation Therapy; NCI (USA): National Cancer Institute, United States of America; NIHR (UK): National Institute for Health Research, United Kingdom; NSCLC: Non-small cell
Radiation enhances adoptive T cell transfer
Radiotherapy also augments the effectiveness of adoptive T cell therapy. The response rates for adoptive transfer of patient-derived TILs range between 20–40%. However, the expansion of these adoptively transferred T cells are regulated by homeostatic mechanisms to maintain the total size of the T cell pool at near constant levels (103). Since radiation causes lymphodepletion, TBI has been employed to augment the expansion of adoptively transferred CD8+ T cells to facilitate tumor rejection (104). In patients with metastatic melanoma, TBI and adoptive transfer of TILs resulted in response rates as high as 50–72%, a substantial improvement over previous TIL strategies (105).
Conclusions and Future Directions
The immune system and cellular responses to radiation intersect at multiple points to facilitate tumor eradication. Radiation activates inflammatory pathways, facilitates DC maturation, increases T cell priming and sensitizes tumor cells to immune recognition. Yet, cancers have also coopted self-protective mechanisms to suppress or to prevent anti-tumor immune responses that may also intersect with radiation resistance pathways. In the future, tailoring immunotherapy with radiotherapy may involve subverting immune tolerance and/or immune suppression by targeting immunological checkpoints, immune suppressive cells such as Tregs and/or MDSC and organ that facilitate immune tolerance. Furthermore, identification of antigens important for immune rejection as well as neo-antigens that arise after radiation may serve as additional targets for the immunotherapy of irradiated tumors. In addition, incorporation of small molecules such as histone deactylase inhibitors that are currently in clinical trials and stimulate anti-tumor immune responses by both sensitizing irradiated cancer cells and protecting irradiated T cells by facilitating DNA repair (106, 107). Thus, the role of the immune system in irradiated tumors is being increasingly understood and exploited for therapeutic gain.
Summary sentence.
This review addresses mechanism by which radiotherapy and immunotherapy cooperate to generate antitumor immune responses that are currently being applied for therapeutic gain.
Acknowledgments
Given the depth of this radiotherapy and immunotherapy review, we regret any oversight in failing to cite all works. The authors wish to thank Amy K. Huser for her thoughtful research, writing, and editing assistance. This work was supported in part by the Ludwig Center for Metastasis Research Grant and National Institutes of Health Grants R21 CA195075A (R.R.W.), Burroughs Wellcome Career Award for Medical Scientists (1010964, M.T.S.) and the Fanconi Anemia Research Fund (M.T.S.). The authors report no conflicts of interest.
References
- 1.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63:1229–1235. [PubMed] [Google Scholar]
- 2.Cameron RB, Spiess PJ, Rosenberg SA. Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin 2, and local tumor irradiation. Studies on the mechanism of action. J Exp Med. 1990;171:249–263. doi: 10.1084/jem.171.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer research. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. Journal of immunology. 2008;180:3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 6.Piret B, Schoonbroodt S, Piette J. The ATM protein is required for sustained activation of NF-kappaB following DNA damage. Oncogene. 1999;18:2261–2271. doi: 10.1038/sj.onc.1202541. [DOI] [PubMed] [Google Scholar]
- 7.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. The Journal of clinical investigation. 1991;88:691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 9.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, Shu HB. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranoa DR, Parekh AD, Pitroda SP, Huang X, Darga T, Wong AC, Huang L, Andrade J, Staley JP, Satoh T, Akira S, Weichselbaum RR, Khodarev NN. Cancer therapies activate RIG-I-like receptor pathway through endogenous non-coding RNAs. Oncotarget. 2016 doi: 10.18632/oncotarget.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, Hornung V. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503:530–534. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khodarev NN, Minn AJ, Efimova EV, Darga TE, Labay E, Beckett M, Mauceri HJ, Roizman B, Weichselbaum RR. Signal transducer and activator of transcription 1 regulates both cytotoxic and prosurvival functions in tumor cells. Cancer research. 2007;67:9214–9220. doi: 10.1158/0008-5472.CAN-07-1019. [DOI] [PubMed] [Google Scholar]
- 16.Cheon H, Holvey-Bates EG, Schoggins JW, Forster S, Hertzog P, Imanaka N, Rice CM, Jackson MW, Junk DJ, Stark GR. IFNbeta-dependent increases in STAT1, STAT2, and IRF9 mediate resistance to viruses and DNA damage. EMBO J. 2013;32:2751–2763. doi: 10.1038/emboj.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, Su AW, Shaikh AY, Roach P, Kreike B, Roizman B, Bergh J, Pawitan Y, van de Vijver MJ, Minn AJ. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 19.White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie ME, Huang DC, Kile BT. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, Kranz DM, Schreiber H. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. Journal of immunology. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. International journal of radiation oncology, biology, physics. 2012;83:1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma A, Bode B, Wenger RH, Lehmann K, Sartori AA, Moch H, Knuth A, Boehmer L, Broek M. gamma-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PloS one. 2011;6:e28217. doi: 10.1371/journal.pone.0028217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morel S, Levy F, Burlet-Schiltz O, Brasseur F, Probst-Kepper M, Peitrequin AL, Monsarrat B, Van Velthoven R, Cerottini JC, Boon T, Gairin JE, Van den Eynde BJ. Processing of some antigens by the standard proteasome but not by the immunoproteasome results in poor presentation by dendritic cells. Immunity. 2000;12:107–117. doi: 10.1016/s1074-7613(00)80163-6. [DOI] [PubMed] [Google Scholar]
- 30.Yan H, Kapoor V, Nguyen K, Akers WJ, Li H, Scott J, Laforest R, Rogers B, Thotala D, Hallahan D. Anti-tax interacting protein-1 (TIP-1) monoclonal antibody targets human cancers. Oncotarget. 2016 doi: 10.18632/oncotarget.9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. Journal of immunology. 2012;189:558–566. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 32.Huang J, Wang QJ, Yang S, Li YF, El-Gamil M, Rosenberg SA, Robbins PF. Irradiation enhances human T-cell function by upregulating CD70 expression on antigen-presenting cells in vitro. J Immunother. 2011;34:327–335. doi: 10.1097/CJI.0b013e318216983d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battaglia A, Buzzonetti A, Martinelli E, Fanelli M, Petrillo M, Ferrandina G, Scambia G, Fattorossi A. Selective changes in the immune profile of tumor-draining lymph nodes after different neoadjuvant chemoradiation regimens for locally advanced cervical cancer. International journal of radiation oncology, biology, physics. 2010;76:1546–1553. doi: 10.1016/j.ijrobp.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, Demaria S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. Journal of immunology. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng Y, Efimova EV, Hamzeh KW, Darga TE, Mauceri HJ, Fu YX, Kron SJ, Weichselbaum RR. Radiation-inducible immunotherapy for cancer: senescent tumor cells as a cancer vaccine. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:1046–1055. doi: 10.1038/mt.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer research. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer research. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 38.Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin Oncol. 2015;42:663–671. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, Wang J, Wang X, Fu YX. Facilitating T Cell Infiltration in Tumor Microenvironment Overcomes Resistance to PD-L1 Blockade. Cancer Cell. 2016;29:285–296. doi: 10.1016/j.ccell.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PloS one. 2012;7:e32542. doi: 10.1371/journal.pone.0032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer research. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 42.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer research. 2004;64:7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 43.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 44.Wu CY, Yang LH, Yang HY, Knoff J, Peng S, Lin YH, Wang C, Alvarez RD, Pai SI, Roden RB, Hung CF, Wu TC. Enhanced cancer radiotherapy through immunosuppressive stromal cell destruction in tumors. Clin Cancer Res. 2014;20:644–657. doi: 10.1158/1078-0432.CCR-13-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 46.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foa R, Santoni A. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–3511. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 49.Fine JH, Chen P, Mesci A, Allan DS, Gasser S, Raulet DH, Carlyle JR. Chemotherapy-induced genotoxic stress promotes sensitivity to natural killer cell cytotoxicity by enabling missing-self recognition. Cancer research. 2010;70:7102–7113. doi: 10.1158/0008-5472.CAN-10-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milas L, Wike J, Hunter N, Volpe J, Basic I. Macrophage content of murine sarcomas and carcinomas: associations with tumor growth parameters and tumor radiocurability. Cancer research. 1987;47:1069–1075. [PubMed] [Google Scholar]
- 55.Bergenfelz C, Larsson AM, von Stedingk K, Gruvberger-Saal S, Aaltonen K, Jansson S, Jernstrom H, Janols H, Wullt M, Bredberg A, Ryden L, Leandersson K. Systemic Monocytic-MDSCs Are Generated from Monocytes and Correlate with Disease Progression in Breast Cancer Patients. PloS one. 2015;10:e0127028. doi: 10.1371/journal.pone.0127028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S, Shizuru JA, Negrin RN, Engleman EG, Strober S. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res. 2015;21:3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. The Journal of clinical investigation. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang SC, Yu CF, Hong JH, Tsai CS, Chiang CS. Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis. PloS one. 2013;8:e69182. doi: 10.1371/journal.pone.0069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer research. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8363–8368. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schakel K, Garbi N, Jager D, Weitz J, Schmitz-Winnenthal H, Hammerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 63.Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer research. 2008;68:450–456. doi: 10.1158/0008-5472.CAN-07-3050. [DOI] [PubMed] [Google Scholar]
- 64.North RJ. Radiation-induced, immunologically mediated regression of an established tumor as an example of successful therapeutic immunomanipulation. Preferential elimination of suppressor T cells allows sustained production of effector T cells. J Exp Med. 1986;164:1652–1666. doi: 10.1084/jem.164.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, McBride WH, Schaue D. Radiation enhances regulatory T cell representation. International journal of radiation oncology, biology, physics. 2011;81:1128–1135. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, Karbach J, Jager E, Sakaguchi S. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 69.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 70.Pilones KA, Kawashima N, Yang AM, Babb JS, Formenti SC, Demaria S. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res. 2009;15:597–606. doi: 10.1158/1078-0432.CCR-08-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, Liu M, Formenti SC, Dustin ML, Demaria S. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. The Journal of clinical investigation. 2012;122:3718–3730. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. The Journal of clinical investigation. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer research. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 74.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, Ford E, Wong J, Hammers HJ, Mathios D, Tyler B, Brem H, Tran PT, Pardoll D, Drake CG, Lim M. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. International journal of radiation oncology, biology, physics. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gough MJ, Crittenden MR, Sarff M, Pang P, Seung SK, Vetto JT, Hu HM, Redmond WL, Holland J, Weinberg AD. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother. 2010;33:798–809. doi: 10.1097/CJI.0b013e3181ee7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, Nicholas S, Kellett M, Ruzevick J, Jackson C, Albesiano E, Durham NM, Ye X, Tran PT, Tyler B, Wong JW, Brem H, Pardoll DM, Drake CG, Lim M. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PloS one. 2014;9:e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maleki Vareki S, Rytelewski M, Figueredo R, Chen D, Ferguson PJ, Vincent M, Min W, Zheng X, Koropatnick J. Indoleamine 2,3-dioxygenase mediates immune-independent human tumor cell resistance to olaparib, gamma radiation, and cisplatin. Oncotarget. 2014;5:2778–2791. doi: 10.18632/oncotarget.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouquet F, Pal A, Pilones KA, Demaria S, Hann B, Akhurst RJ, Babb JS, Lonning SM, DeWyngaert JK, Formenti SC, Barcellos-Hoff MH. TGFbeta1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res. 2011;17:6754–6765. doi: 10.1158/1078-0432.CCR-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, Demaria S. TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer research. 2015;75:2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levy L, Mishalian I, Bayuch R, Zolotarov L, Michaeli J, Fridlender ZG. Splenectomy inhibits non-small cell lung cancer growth by modulating anti-tumor adaptive and innate immune response. Oncoimmunology. 2015;4:e998469. doi: 10.1080/2162402X.2014.998469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2012;2:628–639. doi: 10.1016/j.celrep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 83.Aalbers AM, Aarts MJ, Krol AD, Marijnen CA, Posthuma EF. The beneficial local and abscopal effect of splenic irradiation in frail patients with chronic lymphocytic leukaemia. Neth J Med. 2016;74:122–129. [PubMed] [Google Scholar]
- 84.Jackson CM, Kochel CM, Nirschl CJ, Durham NM, Ruzevick J, Alme A, Francica BJ, Elias J, Daniels A, Dubensky TW, Jr, Lauer P, Brockstedt DG, Baxi EG, Calabresi PA, Taube JM, Pardo CA, Brem H, Pardoll DM, Lim M, Drake CG. Systemic Tolerance Mediated by Melanoma Brain Tumors Is Reversible by Radiotherapy and Vaccination. Clin Cancer Res. 2016;22:1161–1172. doi: 10.1158/1078-0432.CCR-15-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. The Journal of clinical investigation. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schreiber K, Arina A, Engels B, Spiotto MT, Sidney J, Sette A, Karrison TG, Weichselbaum RR, Rowley DA, Schreiber H. Spleen cells from young but not old immunized mice eradicate large established cancers. Clin Cancer Res. 2012;18:2526–2533. doi: 10.1158/1078-0432.CCR-12-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Berard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer letters. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 91.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, Camphausen K, Coleman CN, Sullivan F, Steinberg SM, Schlom J, Dahut W. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 92.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF Investigators IS. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 93.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, Wapnir I, Tibshirani RJ, Levy R. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, Miller W, Payne R, Glenn L, Bageac A, Urba WJ. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Sci Transl Med. 2012;4:137ra174. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 95.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg J, Demaria S, Formenti SC. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16:795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 96.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, Swetter SM, Knox SJ. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5:404–407. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengelov L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR Investigators CA. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A K- investigators. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 101.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2:899–906. doi: 10.1002/cam4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krauze AV, Myrehaug SD, Chang MG, Holdford DJ, Smith S, Shih J, Tofilon PJ, Fine HA, Camphausen K. A Phase 2 Study of Concurrent Radiation Therapy, Temozolomide, and the Histone Deacetylase Inhibitor Valproic Acid for Patients With Glioblastoma. International journal of radiation oncology, biology, physics. 2015;92:986–992. doi: 10.1016/j.ijrobp.2015.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pugh JL, Sukhina AS, Seed TM, Manley NR, Sempowski GD, van den Brink MR, Smithey MJ, Nikolich-Zugich J. Histone deacetylation critically determines T cell subset radiosensitivity. Journal of immunology. 2014;193:1451–1458. doi: 10.4049/jimmunol.1400434. [DOI] [PMC free article] [PubMed] [Google Scholar]