Abstract
Rewards are both ‘liked’ and ‘wanted’, and those two words seem almost interchangeable. However, the brain circuitry that mediates the psychological process of ‘wanting’ a particular reward is dissociable from circuitry that mediates the degree to which it is ‘liked’. Incentive salience or ‘wanting’, a form of motivation, is generated by large and robust neural systems that include mesolimbic dopamine. By comparison, ‘liking’, or the actual pleasurable impact of reward consumption, is mediated by smaller and fragile neural systems, and is not dependent on dopamine. The incentive-sensitization theory posits the essence of drug addiction to be excessive amplification specifically of psychological ‘wanting’, especially triggered by cues, without necessarily an amplification of ‘liking’. This is due to long-lasting changes in dopamine-related motivation systems of susceptible individuals, called neural sensitization. A quarter-century after its proposal, evidence has continued to grow in support the incentive-sensitization theory. Further, its scope is now expanding to include diverse behavioral addictions and other psychopathologies.
Keywords: pleasure, desire, reward, addiction, motivation, brain, limbic, dopamine
It is now widely accepted that brain mechanisms that determine how much a reward is ‘wanted’ are dissociable from those that determine how much the same reward is ‘liked’. However, that idea, which we first proposed in 1989 as a post hoc explanation for some negative results on the role of the brain’s mesolimbic dopamine system in pleasure (Berridge et al 1989), originally came as a surprise even to us. At the time, we and most other investigators generally accepted the idea that dopamine mediates reward pleasure: the hedonic impact of tasty food, addictive drugs and many other rewards. Our early experiment was simply intended to provide another bit of evidence for the dopamine-pleasure hypothesis -- but results turned out otherwise.
As background, many studies had found that brain dopamine systems were activated by most rewards, and further that manipulating dopamine altered ‘wanting’ for rewards: for example changing how much animals preferred, pursued, worked for, or consumed the reward (Koob & Le Moal, 1997; Wise, 1985). Changes in ‘wanting’ were naturally interpreted to reflect corresponding changes in ‘liking’, based on the assumption that ‘wanting’ was proportional to ‘liking’. Our approach to measuring pleasure impact was different, and more similar to how for millennia parents have asked their newborn infants whether the taste of a particular food was enjoyable. We used a naturalistic or ethological assay of sweetness pleasure, based on affective facial expressions of ‘liking’ (Steiner, 1973). Sweetness elicits relaxed facial expressions and rhythmic tongue and mouth expressions of ‘liking’, whereas bitterness elicits ‘disgust’ gapes and turning away. Those affective facial expressions to taste are homologous in human infants, apes and monkeys, and even rats (Grill & Norgren, 1978; Steiner, Glaser, Hawilo, & Berridge, 2001).
In our initial experiment we hypothesized that depletion of brain dopamine in rats via a neurochemical lesion would reduce ‘liking’ reactions for pleasant tastes, based on the notion that dopamine mediates ‘liking’. We expected this would be reflected as a reduction of hedonic orofacial expressions elicited by sweetness. But that is not what we found. We were surprised to find that liking’ reactions of rats to sugar taste were completely normal even after depletion of nearly all brain dopamine ‘(Berridge, Venier, & Robinson, 1989). The dopamine lesions did apparently abolish all motivation – the rats were profoundly aphagic and no longer sought or consumed food rewards, confirming what others had described. To make sense of these paradoxical findings, we proposed that mesolimbic dopamine systems mediate ‘wanting’ (in particular, a psychological process called incentive salience), but not ‘liking’ for the same reward (Berridge et al., 1989; T. E. Robinson & Berridge, 1993). A follow-up study using implanted electrodes to stimulate the same mesolimbic systems and raise dopamine levels also failed to enhance pleasure ‘liking’, despite quadrupling a rat’s ‘wanting’ to eat food rewards (Berridge & Valenstein, 1991). In humans, similar brain stimulation by many so-called ‘pleasure electrodes’, upon closer inspection, may also have turned on ‘wanting’ without ‘liking’, and not been so pleasant after all (Berridge & Kringelbach, 2015).
In the 1990s, it was a lonely scientific position to maintain that dopamine didn’t mediate pleasure. But in about decade, studies of dopamine in human pleasure began to catch up. For example, eventually it was reported that suppressing dopamine neurotransmission in people did not reduce their pleasure ratings of drug rewards, such as cocaine or amphetamine, even when it reduced their desire to consume more drug (Brauer & De Wit, 1997; M Leyton, Casey, Delaney, Kolivakis, & Benkelfat, 2005). Similarly, dopamine suppression in ordinary people or in Parkinson’s disease was reported to not reduce pleasure ratings of tasting delicious foods (Hardman, Herbert, Brunstrom, Munafo, & Rogers, 2012; Sienkiewicz-Jarosz et al., 2013). Further, neuroimaging studies began to report that changes in brain dopamine neurotransmission in people was correlated more with their subjective ratings of wantingdrug and food rewards, than with their liking ratings (Evans et al., 2006; Leyton, 2010; C. T. Smith, Dang, Cowan, Kessler, & Zald, 2016; Volkow et al., 2002). In sum, many studies have now accumulated supporting our original conclusion that dopamine mediates desire rather than pleasure (Salamone & Correa, 2012), and it is now rather rare to find an affective neuroscientist studying reward who still asserts that dopamine mediates pleasure ‘liking’.
Psychological Features of Incentive Salience: ‘Wanting’
Note that our use of the word ‘wanting’ above is often in quotation marks, because we use that term to refer to a particular form of desire – namely, mesolimbic incentive salience. This type of ‘wanting’ is often triggered in pulses by reward-related cues or by vivid imagery about the reward (Berridge, 2012). The ordinary sense of wanting (without quotation marks) refers to a cognitive desire with a declarative goal. However, incentive salience ‘wanting’ is less connected to cognitive goals and more tightly linked to reward cues, making those cues attention-grabbing and attractive (Anderson & Yantis, 2013; Hickey & Peelen, 2015). The cues simultaneously become able to trigger urges to obtain and consume their rewards (Ostlund, LeBlanc, Kosheleff, Wassum, & Maidment, 2014; Pecina & Berridge, 2013; Zhou et al., 2011). ‘Wanting’ is mediated largely by brain mesocorticolimbic systems involving midbrain dopamine projections to forebrain targets, such as the nucleus accumbens and other parts of striatum (Figure 1). The intensity of the triggered urge depends both on the cue’s reward association and on the current state of dopamine-related brain systems in an individual. This interaction allows ‘wanting’ peaks to be amplified by brain states that heighten dopamine reactivity, such as stress, emotional excitement, relevant appetites or intoxication (Anselme, 2016; Berridge, 2012; M. J. Robinson & Berridge, 2013). State-dependent amplification of incentive salience is one reason why many addicts find it so hard to stop at ‘just one hit’. In the face of an amplified urge, the one hit may turn into many hits, or even a lost weekend. It is also a reason why stressful states – or even happy life stresses like winning the lottery – can promote vulnerability to relapse in addiction and related disorders (Sinha, 2013). Addiction is not so much about satisfaction, pleasure, need or withdrawal, by this view, as it is about ‘wanting’.
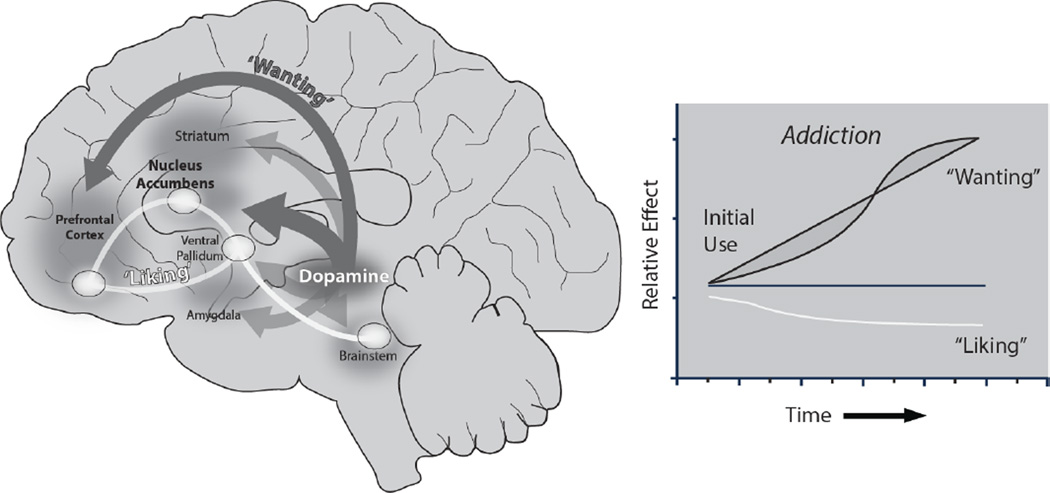
Figure 1.
‘Liking’ and ‘wanting’ in brain and in addiction. ‘Wanting’ is mediated by a robust brain system including dopamine projections (left, dark gray), whereas ‘liking’ is mediated by a restricted brain system of small hedonic hotspots (white) (described in Berridge & Kringelbach, 2015). The incentive-sensitization theory of addiction (right) shows how ‘wanting’ may grow over time independently of ‘liking’ as an individual becomes an addict, due to sensitization of brain mesolimbic systems. (The figure was adapted by Shannon Cole and Daniel Castro from Robinson & Berridge, 1993).
Ordinarily, cognitive wanting and incentive salience ‘wanting’ go together, so that incentive salience can give heightened urgency to feelings of cognitive desire. But the two forms of wanting vs. ‘wanting’ can sometimes dissociate, so that incentive salience can occur either in opposition to a cognitive desire or even unconsciously in absence of any cognitive desire. Incentive salience ‘wanting’ in opposition to cognitive wanting, for example, occurs when a recovering addict has a genuine cognitive desire to abstain from taking drugs, but still ‘wants’ drugs, so relapses anyway when exposed to drug cues or during vivid imagery about them. Nonconscious ‘wants’ can be triggered in some circumstances by subliminal stimuli, even though the person remains unable to report any change in subjective feelings while motivation increases are revealed in their behavior (Childress et al., 2008; Winkielman, Berridge, & Wilbarger, 2005).
Motivational Salience in Desire Versus Dread
For readers interested in the psychology of emotion and motivation, we note another intriguing feature of incentive salience. This is that ‘wanting’ brain mechanisms can also operate in a different neurobiological mode to generate an active coping form of fear (Berridge & Kringelbach, 2015). Although fear seems almost the psychological opposite of desire in valence, fearful salience is generated by the same mesolimbic circuitry as incentive salience. Fearful salience also makes percepts become attention-riveting, but with a negative threatening aspect rather than positive attraction, calling out active coping responses (Richard & Berridge, 2011). This dopamine-related fearful salience has been suggested to contribute to human paranoia symptoms in schizophrenia (Barch, Treadway, & Schoen, 2014; Heinz & Schlagenhauf, 2010; Howes & Kapur, 2009) and in psychostimulant-induced psychosis (Cicero, Docherty, Becker, Martin, & Kerns, 2014).
So Where Does ‘Liking’ Come From in the Brain?
In contrast to the large and robust ‘wanting’ system in the brain, a much smaller and functionally fragile system appears to generate intense pleasure or ‘liking’ reactions. Experiments in the Berridge lab have established that this ‘liking’ system comprises a collection of interactive hedonic hotspots, and this hedonic circuitry may be shared by diverse pleasures ranging from sensory food and drug pleasures to human cultural and social pleasures (Berridge & Kringelbach, 2015). The pleasure-generating hotspots are anatomically tiny, neurochemically restricted, and easily disrupted – perhaps a reason why intense pleasures are relatively few and far between in life compared to intense desires (Castro & Berridge, 2014; Mahler, Smith, & Berridge, 2007; Peciña & Berridge, 2005; K. S. Smith, Berridge, & Aldridge, 2011). Each hedonic hotspot is nestled within its larger limbic structure. For example, a nucleus accumbens hedonic hotspot is only one cubic millimeter in a rat brain, and probably about a cubic centimeter in humans. The hotspot constitutes only 10% of total nucleus accumbens volume: the remaining 90% of the nucleus accumbens lacks any ability to enhance ‘liking’, though still robustly causes intense ‘wanting’.
Hedonic hotspots exist in limbic prefrontal cortex, in orbitofrontal and insula regions, where they may correspond to human sites that code sensory and higher pleasures (Kringelbach, 2010; Kringelbach, O'Doherty, Rolls, & Andrews, 2003; Small, Zatorre, Dagher, Evans, & Jones-Gotman, 2001). Other hotspots are buried deeper in subcortical brain structures. Each hedonic hotspot has the special ability when neurochemically stimulated, such as by opioid or endocannabinoid neurotransmitters (the brain’s natural heroin-like and marijuana-like signals), to amplify ‘liking’ reactions, and so make sweetness appear even more enjoyable. Dopamine stimulations even in hedonic hotspots, by contrast always fail to enhance ‘liking’ (K. C. Berridge & Kringelbach, 2015; K. S. Smith et al., 2011) – the role of dopamine seems restricted to ‘wanting’.
Especially crucial to the normal capacity for pleasure may be a particular hedonic hotspot located in the ventral pallidum, which lies at the base of the subcortical forebrain (K. S. Smith & Berridge, 2007). In addition to enhancing ‘liking’ for intense pleasure, this ventral pallidal hotspot is the only known site in the brain where a small lesion conversely also eliminates normal pleasure, and reverses the hedonic impact of sweet sensation from ‘liked’ to instead ‘disgusting’ (so that afterwards sucrose elicits bitterness-typical gapes and related negative expressions) (Berridge & Kringelbach, 2015; Ho & Berridge, 2014).
Addiction Distorts ‘Wanting’ Versus ‘Liking’
Our discovery that ‘wanting’ and ‘liking’ are mediated by dissociable brain systems took us halfway toward the Incentive-Sensitization theory of addiction (T. E. Robinson & Berridge, 1993, 2008). The other half of the journey came from the discovery around the same time that brain dopamine systems can be enduringly ‘sensitized’ by many drugs of abuse (cocaine, amphetamine, heroin, alcohol, nicotine, etc.), not just stimulated while those drugs are actually on board (T. E. Robinson & Becker, 1986). Mesolimbic sensitization happens especially if the drugs are taken repeatedly, and at high doses spaced apart (for example, in weekend binges) (Kalivas & Stewart, 1991; Post, 1980; T. E. Robinson & Becker, 1986). Once induced, sensitization is very long lasting, and possibly even permanent.
Early research on sensitization in the T.E. Robinson lab focused particularly on dopamine neurons, and increases in release of dopamine, but it is now clear that mesolimbic sensitization changes other neurotransmitters and neurons too. For example, drug sensitization also alters glutamate neurons that project from cortex to nucleus accumbens (Wolf, 2010), which interact with dopamine there, and similarly are receiving attention as potential targets of future addiction therapies (e.g. Creed, Pascoli, & Lüscher, 2015; Douglas & Peter, 2015). Sensitization also changes the physical structure of mesolimbic neurons, such as altering the shape and number of tiny spines on dendrites of neurons in nucleus accumbens, which act as their ‘receiving antennae’ for incoming signals (T. E. Robinson & Kolb, 2004; Singer et al., 2009; Steketee & Kalivas, 2011). Initially, the main experimental evidence for mesolimbic sensitization by drugs came from studies in rodents, but now sensitization is well-documented in humans as well (Boileau et al., 2007; Paulson & Robinson, 1995; M. J. F. Robinson, Fischer, Ahuja, Lesser, & Maniates, 2015; Vezina & Leyton, 2009).
Functionally, mesolimbic sensitization renders brain ‘wanting’ systems hyper-reactive to drug cues and contexts, thus conferring more intense incentive salience on those cues or contexts. Consequently, addicts have stronger cue-triggered urges and intensely ‘want’ to take drugs (Figure 1). ‘Liking’, by contrast, need not increase with sensitization, and may even decrease. Sensitized ‘wanting’ can persist for years, even if the person cognitively doesn’t want to take drugs, doesn’t expect the drugs to be very pleasant, and even long after withdrawal symptoms have subsided (Berridge & Robinson, 2011; T. E. Robinson & Berridge, 2003; T. E. Robinson & Berridge, 2008). Thus, the central tenet of the incentive-sensitization theory is that addiction becomes compulsive when mesolimbic systems become sensitized and hyper-reactive to the incentive motivational properties of drug cues (Childress et al., 2008; Ostlund et al., 2014; Witteman et al., 2015; Zhou et al., 2011). This theory of addiction is specifically meant to explain individuals who have near-compulsive levels of urge to take drugs, and who remain vulnerable to a persisting risk of relapse even after a significant period of drug abstinence.
A sensitized dopamine system is not always hyper-active, but it is hyper-reactive to drug cues and contexts. That hyper-reactivity produces pulses of heightened dopamine release, brain activations and motivation that last seconds or minutes (T. E. Robinson & Berridge, 2008; Tindell, Berridge, Zhang, Peciña, & Aldridge, 2005). Drug contexts powerfully gate the ability of both drugs themselves and of discrete cues to elicit sensitized neural hyper-reactivity (M. Leyton & Vezina, 2013; T. E. Robinson, Browman, Crombag, & Badiani, 1998). This means that surges of intense ‘wanting’ are most likely to be triggered when drug cues are encountered (or imagined) in contexts previously associated with taking drugs.
Sensitization in Human Addiction
Laboratory neuroimaging studies have shown that even the oral administration of relatively low doses of amphetamine can produce mesolimbic sensitization in people without a history of drug use (Boileau et al., 2006; Leyton & Vezina, 2013). Furthermore, in nondependent cocaine users the ability of self-administered cocaine (taken by the intranasal route) to increase dopamine levels in the ventral striatum is positively correlated with amount of lifetime cocaine use, suggesting past use sensitized dopamine systems (Cox et al., 2009). However, there is reason to expect even stronger sensitization from higher street-typical doses, or by intravenous or smoking routes of consumption (which deliver drugs to the brain more rapidly than swallowing or snorting), based on animal studies (Allain, Minogianis, Roberts, & Samaha, 2015). Indeed, addicts tend to prefer to smoke or inject drugs, because those routes deliver drugs to the brain more rapidly. Consequently, real-life addicts may have greater mesolimbic sensitization than so far demonstrated by laboratory studies in nonusers.
Do human addicts actually show the brain hyper-reactivity to drug cues that is posited by incentive-sensitization? The short answer is ‘yes’. There have been many reports over the past 10 years that mesolimbic brain responses to drug cues, such as viewing photos of drug paraphernalia or of other people taking drugs, are enhanced in individuals with addiction (Kühn & Gallinat, 2011). Furthermore, “more years of cocaine use [are] associated with greater activation to cocaine cues in ventral striatum” (Prisciandaro et al., 2014), indicating progressively intense sensitization. Similar findings have been reported with alcohol use (Claus, Ewing, Filbey, Sabbineni, & Hutchison, 2011).
We note as a caveat that most reports of such hyper-reactivity used fMRI measures, which do not directly measure dopamine, but rather oxygenated blood signals (BOLD), which are used to infer neural activity. However, recent research confirms that dopamine release does cause striatal BOLD activations (Ferenczi et al., 2016), supporting the interpretation that fMRI hyper-reactivity to drug cues in addicts reflects a higher dopamine surge, and indicates incentive-sensitization. Further, several studies that have used more direct PET measures of dopamine release in people (i.e., via dopamine displacement of radioactive raclopride from D2 receptors) also confirm that drug cues do trigger higher increases in dopamine release, and in fact, “the greater the cue-induced dopamine release the greater the craving” to take more drug (Leyton & Vezina, 2013, p. 2004). These intense cue-triggered neural signatures are very much what one would expect based on the incentive-sensitization theory of addiction.
Disentangling Reports of Mesolimbic Suppression Versus Sensitization in Addiction
As another caveat, it is only fair to note that some studies have reported nearly the opposite of sensitized brain responses as described above: that is, neural suppression or blunted rise in dopamine displacement elicited when an addict takes a drug. Suppressed brain responses are typically not to the drug cues that trigger urges, but rather to drugs themselves once actually taken, such as amphetamine or methylphenidate (Volkow, Koob, & McLellan, 2016). However, we caution that two points need to be considered before jumping to a conclusion that addicts have too little brain dopamine, as some have suggested. First, suppression of drug-elicited brain activation to drugs is by no means a universal finding. For example, as mentioned above, sensitized or increased dopamine rises elicited by exposure to a drug are also sometimes reported. For instance, alcohol is reported to elicit greater dopamine release in the striatum of alcoholics than in social drinkers (Yoder et al., 2016). Still, suppression of drug-induced dopamine is found often enough in addicts to have led some observers to suggest that the essence of addiction is primarily too-little dopamine in nucleus accumbens and striatum (Volkow et al., 2016). That dopamine-deficit suggestion is quite a contrast to incentive-sensitization, and is often wrapped together implicitly with the older assumption that lower dopamine causes reduced pleasure and that addicts simply seek pleasure (despite the emerged consensus that the dopamine pleasure hypothesis not true). Second, however, partial compensations to excessive dopamine stimulation may occur in the brain after heavy drug use, which at least for a while can mask the expression of neural sensitization. We would agree that compensatory neural suppressions (e.g., receptor downregulation) do accompany heavy drug use, while drug-taking continues. Suppressions produce tolerance to drug highs (and to the aversive effects of some addictive drugs -- which permits the person to take higher doses, inducing even more tolerance). Neural suppressions also produce withdrawal for a while, once the drug is finally stopped.
However, even the same investigators that report suppression of responses to drugs often also report the same addicts show intense neural hyper-activations – not suppressions -- to the drug cues that trigger urges to take drugs. That is, compensatory suppressions of drug-elicited reactions as consequences of over-stimulation need not contradict incentive-sensitization as the primary mechanism for the compulsive craving in addiction, consistent with incentive-sensitization. Further, many tolerance-related neural suppressions are merely temporary. Suppressions are partial compensatory responses to the high levels of mesolimbic stimulation induced by drugs, essentially a temporary cellular effort by neurons to turn down their levels of neurochemical over-stimulation. Sensitization and tolerance can develop simultaneously in the same brain while drug is being taken, because they have parallel mechanisms involving different intra-cellular signalling cascades. But many tolerance/withdrawal suppressions are apt to fade within weeks if drug-taking is stopped. By contrast the neural changes that cause incentive-sensitization do not fade over months of drug abstinence – if anything, sensitization grows for some time during abstinence (Paulson & Robinson, 1995), a phenomenon sometimes called ‘incubation of drug craving’, which is an increase in relapse vulnerability after a month or so of drug abstinence (Pickens et al., 2011). Incubation of craving is impossible to explain by a neural suppression or withdrawal view of addiction, because those fade, not grow, over a month of abstinence, but is entirely plausible in light of incentive-sensitization. Finally, suppression of neural responses to drugs may occur mostly in test situations that are very different from situations in which drugs were usually taken – such while in a neuroimaging scanner in a hospital setting (M. Leyton & Vezina, 2013). By contrast, neural suppression may be converted into sensitized hyper-reactions when neuroimagers take efforts to provide realistic drug-related cues and contexts during the neuroimaging test (Leyton & Vezina, 2013). Early animal studies showed that giving drug in a test environment where it never before was experienced can completely prevent the expression of behavioral and neural sensitization, even when it clearly has been induced, whereas a previously drug-associated context enables the sensitized response to fully reappear again when drug is retaken (T. E. Robinson et al., 1998). That is, sensitized ‘wanting’ urges are much more likely to occur in drug-associated contexts than in biomedical neuroimaging situations. Recent neuroimaging evidence indicates that drug-related contexts gate sensitized brain reactions in humans too (Leyton & Vezina, 2013). Therefore, it may be crucial that PET studies of drug-elicited brain responses take steps to better recreate drug-related contexts and cues in order to reveal sensitized hyper-reactive brain responses to drugs that would occur in real-life drug situations, and which may underlie addictive urges to take more drugs. Of course, how addicts perceive contexts is likely complex, so it might help to let addicts also actively engage in their drug-taking rituals (e.g., preparing lines of cocaine to sniff, or preparing an injection), or to experience diverse drug-related auditory, smell, taste or other sensations in order to unmask sensitized hyper-reactivity in mesolimbic systems (Cox et al., 2009). It might also be useful to test with the same drug an addict most commonly takes rather than with an unfamiliar drug (such as methylphenidate, which has been substituted for an addict’s habitual drug in some neuroimaging studies).
Individual vulnerability
Another important point that any addiction explanation must deal with is that most people who take drugs never become addicts. For example, only about 30% of people who take cocaine actually go on to become long-term addicts. Accordingly, individuals also differ considerably in their susceptibility to mesolimbic sensitization, even when exposed to the same drugs and doses (Becker, Perry, & Westenbroek, 2012; Robinson & Berridge, 2008). Genetic factors are important determinants of susceptibility to sensitization in rodents, and genes also contribute strongly to addiction vulnerability in humans (Franklin et al., 2011; Hoft, Stitzel, Hutchison, & Ehringer, 2011; Hutchison et al., 2008; Moeller et al., 2013). Other determinants of sensitization vulnerability include gender and the presence of sex hormones, and whether the individual has had major stresses in life before taking drugs (Becker, Perry, & Westenbroek, 2012; T. E. Robinson & Berridge, 2008). Individuals with combinations of these several factors may be most at risk to develop incentive-sensitization and addiction. Finally, among those who experiment with drugs in the first place, important situational factors can facilitate incentive-sensitization, or alternatively make it less likely. These include how long drugs have been taken, whether dose has escalated, and whether the person took drugs by routes that resulted in drug rapidly reaching the brain (i.e., inhalation or intravenous use).
Another type of individual difference concerns neuropsychological traits of incentive salience that might predispose toward either drug taking or addiction. For example, even animals differ as individuals in their propensity to attribute incentive salience to discrete predictive reward cues (T. E. Robinson, Yager, Cogan, & Saunders, 2014). For example, some rats come to rapidly approach a discrete Pavlovian cue that predicts delivery of a food or drug reward (such as sudden appearance of a lever), and will work avidly to get the cue (these individuals are called sign-trackers). However, other rats will instead go directly to the location of impending delivery of a food reward when its predictive cue appears (called goal-trackers). Studies in the T.E. Robinson lab have found that discrete cocaine or opioid cues acquire greater incentive salience in sign-trackers than in goal-trackers, and in some situations sign-trackers are also more likely than goal-trackers to show cue-triggered relapse of drug-taking behavior (T. E. Robinson et al., 2014). However, both types of individuals eventually show addictive-type patterns of high drug intake when they have weeks of intermittent access to cocaine, in binge-like periods, and develop robust incentive-sensitization (Kawa, Bentzley, & Robinson, 2016). Thus, being relatively attracted to discrete reward cues may make some individuals more susceptible to develop addiction when they initially start to use drugs (Mahler & de Wit, 2010; van Hemel-Ruiter, de Jong, Ostafin, & Oldehinkel, 2015; Vollstädt-Klein et al., 2012), though most individuals may develop neural incentive-sensitization eventually if they take drugs for long enough and at high enough doses.
Is incentive-sensitization a brain disease?
Addiction has sometimes been called a ‘brain disease’ (Leshner, 1997). Yet recently that label has come under criticism on the grounds that some addicts may not regard themselves as diseased, incentives often still can influence drug use as though it were an ordinary choice, and because believing that one has a ‘brain disease’ could encourage a fatalistic acceptance of the condition (Hall, Carter, & Forlini, 2015; Lewis, 2015). Also, though addiction is accompanied by distinct changes in the brain, some critics also note that related changes in the brain can occur in normal life: indeed, the brain mechanisms of addiction do overlap the mechanisms of ordinary desires such as love or hunger that are shared by everyone.
While these objections have some validity, we do believe that incentive-sensitization can make the temptations faced by addicts harder to resist than those most other people are called upon to face. This is because the underlying neural sensitization can distort psychological incentive salience function to a pathological degree, with deleterious consequences. If success versus failure is probabilistic when temptations are very strong, and if success in escaping addiction requires saying no every time a temptation occurs, then a dopamine-sensitized person who faces a series of hundreds of intense temptations may eventually be expected to fail. Such features may make the ‘brain disease’ label a reasonably fair description.
Other Behavioral Addictions?
Various ‘behavioral addictions’ have emerged in recent years that do not involve drugs at all: eating addiction, gambling addiction, sex or pornography addiction, internet addiction, shopping addiction, and so on (Davis & Carter, 2009; Gearhardt et al., 2011; Hartston, 2012; Linnet et al., 2012; S. S. O'Sullivan et al., 2011; Ray et al., 2012; Voon et al., 2014). Although drugs were central to mechanisms underlying the original incentive-sensitization hypothesis, perhaps surprisingly, neural sensitization of ‘wanting’ mechanisms may in some cases occur without drugs. In support, evidence is emerging that individuals with these behavioral addictions may have some sensitization-like patterns of brain hyper-reactivity to cues related to their own personal addictions (Davis & Carter, 2009; Gearhardt et al., 2011; Hartston, 2012; Linnet et al., 2012; O'Sullivan et al., 2011; Ray et al., 2012; Voon et al., 2014). Conceivably, sensitization-related brain changes arise in some highly susceptible individuals to produce these addictions without need of drugs, through mechanisms not yet understood.
Related proof-of-principle evidence for the idea that diverse compulsive motivations such as gambling can be caused by hyper-stimulation of dopamine-related systems also comes from Parkinson’s patients, about 15% of whom develop what is called dopamine dysregulation syndrome when treated with newer dopamine-stimulating medications that directly activate their brain dopamine receptors (i.e., direct agonist medications) (Callesen, Scheel-Kruger, Kringelbach, & Moller, 2013; Friedman & Chang, 2013; Ondo & Lai, 2008; Politis et al., 2013). These patients can develop intense compulsive motivations to pursue gambling, shopping, sex, internet use, excessive hobbies, or similar activities (O'Sullivan, Evans, & Lees, 2009). Some patients may even take excessive amounts of their medication in a more classic drug-addiction fashion. Usually, the compulsive motivations rapidly fade if the dopamine-stimulating medications are stopped. This pattern demonstrates intense and idiosyncratically diverse ‘wants’ arising from dopamine-stimulating medications, and is also a further contradiction of the notion that dopamine-suppression causes addictions (i.e., since Parkinson’s patients are in dopamine suppression states prior to medication or if taken-off medication, but no longer have compulsive motivations at those times). It provides further support for the sensitization idea that dopamine over-stimulation is the more likely culprit behind addictive compulsions (i.e., while taking high doses of medications that stimulate dopamine receptors). Finally, such patients with intense motivations have virtually never been reported to experience intense pleasure from their medications or compulsions, any more than individuals with spontaneous behavioral addictions – or some drug addicts (Pettorruso et al., 2016). In short, evidence continues to build that dopamine hyper-reactivity produces intense reward ‘wanting’ but not ‘liking’, and can cause addictions.
Treatment Implications
At present, most addiction medications are of quite limited efficacy. Opioid blockers (e.g., naltrexone) conceivably help somewhat blunt ‘wanting’ as well ‘liking’ peaks. Anti-depressants take the edge off negative mood-based reasons to take drugs again, and opioid-substitutes prevent withdrawal symptoms. Immune-based vaccines help reduce drug-induced highs, though can be circumvented by taking higher doses or different drugs. Psychological approaches, such as cognitive and behavioral therapies, 12-step programs, contingency management and mindfulness therapy arguably remain more effective than any medications available today. Still, while those psychological approaches are quite useful in helping some individuals to escape addiction, they are still not sufficient for many others.
We suggest that a more effective neurobiological treatment would need to reverse the neuroadaptations underlying sensitized mesolimbic hyper-reactivity to drug cues, yet not impede normal motivations nor induce adverse side effects. In practice this may be difficult to achieve, though in principle not impossible. Encouragingly, some recent findings from animal studies have suggested it may be possible to rather specifically reverse sensitized mesolimbic hyper-reactivity by new neurobiological techniques, without adverse other effects (Creed et al., 2015). Although human applications are still a long way off, such findings give at least some reason to hope that more effective sensitization-reversing treatments might be developed in future.
Conclusion
In summary, a quarter-century after we proposed the Incentive-Sensitization theory of addiction, we conclude that its key tenets still seem well supported. In addicts mesolimbic circuits are hyper-responsive to drug cues, which may cause strong cue-triggered ‘wanting’ to take drugs, leading to relapse. Our journey into understanding addiction began with basic science investigations of brain systems and their psychological functions in animals. The insights from those studies have proven remarkably applicable to humans. Those insights were that the psychological process of motivationally ‘wanting’ a reward has distinct brain mechanisms from hedonically ‘liking’ the same reward, and that sensitization of dopamine-related hyper-reactivity specifically promotes excessive ‘wanting’. Gradually, substantial evidence from both human and animal studies has emerged to further support and refine those conclusions. Finally, the scope of clinical applications has extended in the past decade beyond drug addiction to other behavioral addictions, schizophrenia, depression, Parkinson’s disease, and other forms of psychopathology. This story is still being written, and it seems likely that future findings will continue to reveal exciting facets of normal reward processes in the brain, how their distortion can impact psychological disorders, and how new therapies might someday overcome distortions to improve function again.
Acknowledgments
We thank Shannon Cole and Daniel Castro for redrawing Figure 1. Our research has been supported by grants from the National Institutes of Health (DA015188 and MH63649 to Kent Berridge and PO1 DA031656 to Terry E. Robinson).
Footnotes
The authors received the 2016 APA Award for Distinguished Scientific Contribution for collaboration. This article is based on an invited presentation at the 124th Annual Convention of the American Psychological Association, held August 4–7, 2016, in Denver, Colorado.
References
- Allain F, Minogianis EA, Roberts DC, Samaha AN. How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev. 2015;56:166–179. doi: 10.1016/j.neubiorev.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Anderson BA, Yantis S. Persistence of value-driven attentional capture. J Exp Psychol Hum Percept Perform. 2013;39(1):6–9. doi: 10.1037/a0030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselme P. Motivational control of sign-tracking behaviour: A theoretical framework. Neuroscience & Biobehavioral Reviews. 2016;65:1–20. doi: 10.1016/j.neubiorev.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: Reduced effort allocation predicts amotivation and functional impairment. Journal of Abnormal Psychology. 2014;123(2):387–397. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3(1):14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. European Journal of Neuroscience. 2012;35(7):1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86(3):646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Drug addiction as incentive sensitization. In: Poland J, Graham G, editors. Addiction and Responsibility. Cambridge, MA: MIT Press; 2011. pp. 21–54. [Google Scholar]
- Berridge KC, Valenstein ES. What psychological process mediates feeding evoked by electrical stimulation of the lateral hypothalamus? Behavioral Neuroscience. 1991;105(1):3–14. doi: 10.1037//0735-7044.105.1.3. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Venier IL, Robinson TE. Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: Implications for arousal and anhedonia hypotheses of dopamine function. Behavioral Neuroscience. 1989;103(1):36–45. doi: 10.1037//0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63(12):1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27(15):3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer LH, De Wit H. High dose pimozide does not block amphetamine-induced euphoria in normal volunteers. Pharmacology Biochemistry and Behavior. 1997;56(2):265–272. doi: 10.1016/s0091-3057(96)00240-7. [DOI] [PubMed] [Google Scholar]
- Callesen MB, Scheel-Kruger J, Kringelbach ML, Moller A. A systematic review of impulse control disorders in Parkinson's disease. J Parkinsons Dis. 2013;3(2):105–138. doi: 10.3233/JPD-120165. [DOI] [PubMed] [Google Scholar]
- Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: Mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. The Journal of Neuroscience. 2014;34(12):4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress A, Ehrman R, Wang Z, Li Y, Sciortino N, Hakun J, O'Brien C. Prelude to passion: limbic activation by "unseen" drug and sexual cues. PLoS ONE. 2008;3(1):e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero DC, Docherty AR, Becker TM, Martin EA, Kerns JG. Aberrant Salience, Self-Concept Clarity, and Interview-Rated Psychotic-Like Experiences. Journal of Personality Disorders. 2014:1–21. doi: 10.1521/pedi_2014_28_150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SWF, Filbey FM, Sabbineni A, Hutchison KE. Identifying Neurobiological Phenotypes Associated with Alcohol Use Disorder Severity. Neuropsychopharmacology. 2011;36(10):2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SML, Benkelfat C, Dagher A, Delaney JS, Durand F, McKenzie SA, Leyton M. Striatal Dopamine Responses to Intranasal Cocaine Self-Administration in Humans. Biological Psychiatry. 2009;65(10):846–850. doi: 10.1016/j.biopsych.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Lüscher C. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347(6222):659–664. doi: 10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53(1):1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Douglas JR-W, Peter WK. Glutamate Transporter GLT-1 as a Therapeutic Target for Substance Use Disorders. CNS & Neurological Disorders - Drug Targets. 2015;14(6):745–756. doi: 10.2174/1871527314666150529144655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, Piccini P. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59(5):852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351(6268):aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Li Y, Suh JJ, Goldman M, Lohoff FW, Childress AR. Dopamine transporter genotype modulation of neural responses to smoking cues: confirmation in a new cohort. Addiction Biology. 2011;16(2):308–322. doi: 10.1111/j.1369-1600.2010.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JH, Chang V. Crack cocaine use due to dopamine agonist therapy in Parkinson disease. Neurology. 2013;80(24):2269–2270. doi: 10.1212/WNL.0b013e318296e9d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural Correlates of Food Addiction. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.32. archgenpsychiatry.2011.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978;143(2):263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Hall W, Carter A, Forlini C. The brain disease model of addiction: is it supported by the evidence and has it delivered on its promises? The Lancet Psychiatry. 2015;2(1):105–110. doi: 10.1016/S2215-0366(14)00126-6. [DOI] [PubMed] [Google Scholar]
- Hardman CA, Herbert VM, Brunstrom JM, Munafo MR, Rogers PJ. Dopamine and food reward: effects of acute tyrosine/phenylalanine depletion on appetite. Physiology & Behavior. 2012;105(5):1202–1207. doi: 10.1016/j.physbeh.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Hartston H. The case for compulsive shopping as an addiction. Journal of Psychoactive Drugs. 2012;44(1):64–67. doi: 10.1080/02791072.2012.660110. [DOI] [PubMed] [Google Scholar]
- Heinz A, Schlagenhauf F. Dopaminergic Dysfunction in Schizophrenia: Salience Attribution Revisited. Schizophrenia Bulletin. 2010;36(3):472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Peelen Marius V. Neural Mechanisms of Incentive Salience in Naturalistic Human Vision. Neuron. 2015;85(3):512–518. doi: 10.1016/j.neuron.2014.12.049. [DOI] [PubMed] [Google Scholar]
- Ho C-Y, Berridge KC. Excessive disgust caused by brain lesions or temporary inactivations: mapping hotspots of the nucleus accumbens and ventral pallidum. European Journal of Neuroscience. 2014 doi: 10.1111/ejn.12720. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft NR, Stitzel JA, Hutchison KE, Ehringer MA. CHRNB2 promoter region: association with subjective effects to nicotine and gene expression differences. Genes Brain Behav. 2011;10(2):176–185. doi: 10.1111/j.1601-183X.2010.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophrenia Bulletin. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, Filbey F. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry. 2008;65(7):841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Research - Brain Research Reviews. 1991;16(3):223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology (Berl) 2016 doi: 10.1007/s00213-016-4393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The hedonic brain: A functional neuroanatomy of human pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford, U.K.: Oxford University Press; 2010. pp. 202–221. [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs – a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience. 2011;33(7):1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Addiction Is a Brain Disease, and It Matters. Science. 1997;278(5335):45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Lewis M. The biology of desire. Philadelphia: Perseus books; 2015. [Google Scholar]
- Leyton M. The neurobiology of desire: Dopamine and the regulation of mood and motivational states in humans. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford, U.K.: Oxford University Press; 2010. pp. 222–243. [Google Scholar]
- Leyton M, Casey K, Delaney J, Kolivakis T, Benkelfat C. Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav Neurosci. 2005;119(6):1619–1627. doi: 10.1037/0735-7044.119.6.1619. [DOI] [PubMed] [Google Scholar]
- Leyton M, Vezina P. Striatal ups and downs: their roles in vulnerability to addictions in humans. Neurosci Biobehav Rev. 2013;37(9 Pt A):1999–2014. doi: 10.1016/j.neubiorev.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet J, Mouridsen K, Peterson E, Møller A, Doudet DJ, Gjedde A. Striatal dopamine release codes uncertainty in pathological gambling. Psychiatry Research: Neuroimaging. 2012 doi: 10.1016/j.pscychresns.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS ONE. 2010;5(11):e15475. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward. Neuropsychopharmacology. 2007;32(11):2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Parvaz MA, Shumay E, Beebe-Wang N, Konova AB, Alia-Klein N, Goldstein RZ. Gene × Abstinence Effects on Drug Cue Reactivity in Addiction: Multimodal Evidence. The Journal of Neuroscience. 2013;33(24):10027–10036. doi: 10.1523/JNEUROSCI.0695-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan S, Evans A, Lees A. Dopamine dysregulation syndrome: an overview of its epidemiology, mechanisms and management. CNS Drugs. 2009;23(2):157–170. doi: 10.2165/00023210-200923020-00005. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SS, Wu K, Politis M, Lawrence AD, Evans AH, Bose SK, Piccini P. Cue-induced striatal dopamine release in Parkinson's disease-associated impulsive-compulsive behaviours. Brain. 2011;134(Pt 4):969–978. doi: 10.1093/brain/awr003. [DOI] [PubMed] [Google Scholar]
- Ondo WG, Lai D. Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism Relat Disord. 2008;14(1):28–32. doi: 10.1016/j.parkreldis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, LeBlanc KH, Kosheleff AR, Wassum KM, Maidment NT. Phasic Mesolimbic Dopamine Signaling Encodes the Facilitation of Incentive Motivation Produced by Repeated Cocaine Exposure. Neuropsychopharmacology. 2014;39(10):2441–2449. doi: 10.1038/npp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19(1):56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered 'wanting' for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. 2013;37(9):1529–1540. doi: 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: Where do mu-opioids cause increased hedonic impact of sweetness? J. Neurosci. 2005;25(50):11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettorruso M, Fasano A, De Risio L, Ricciardi L, Di Nicola M, Martinotti G, Bentivoglio AR. Punding in non-demented Parkinson's disease patients: Relationship with psychiatric and addiction spectrum comorbidity. J Neurol Sci. 2016;362:344–347. doi: 10.1016/j.jns.2016.02.016. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34(8):411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis M, Loane C, Wu K, O'Sullivan SS, Woodhead Z, Kiferle L, Piccini P. Neural response to visual sexual cues in dopamine treatment-linked hypersexuality in Parkinson's disease. Brain. 2013;136(Pt 2):400–411. doi: 10.1093/brain/aws326. [DOI] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: Effect of time interval on the development of sensitization or tolerance. Life Sciences. 1980;26(16):1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Prisciandaro JJ, Joseph JE, Myrick H, McRae-Clark AL, Henderson S, Pfeifer J, Brady KT. The relationship between years of cocaine use and brain activation to cocaine and response inhibition cues. Addiction. 2014;109(12):2062–2070. doi: 10.1111/add.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray N, Miyasaki JM, Zurowski M, Ko JH, Cho SS, Pellecchia G, Strafella AP. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson's patients with medication-induced pathological gambling: A [11C] FLB-457 and PET study. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Nucleus Accumbens Dopamine/Glutamate Interaction Switches Modes to Generate Desire versus Dread: D1 Alone for Appetitive Eating But D1 and D2 Together for Fear. The Journal of Neuroscience. 2011;31(36):12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Berridge KC. Instant transformation of learned repulsion into motivational "wanting". Curr Biol. 2013;23(4):282–289. doi: 10.1016/j.cub.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJF, Fischer AM, Ahuja A, Lesser EN, Maniates H. Roles of “Wanting” and “Liking” in Motivating Behavior: Gambling, Food, and Drug Addictions. Berlin Heidelberg: Springer; 2015. pp. 1–32. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54(1):25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neuroscience and Biobehavioral Reviews. 1998;22(2):347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Pt B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone John D, Correa M. The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron. 2012;76(3):470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienkiewicz-Jarosz H, Scinska A, Swiecicki L, Lipczynska-Lojkowska W, Kuran W, Ryglewicz D, Bienkowski P. Sweet liking in patients with Parkinson's disease. J Neurol Sci. 2013;329(1–2):17–22. doi: 10.1016/j.jns.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Singer BF, Tanabe LM, Gorny G, Jake-Matthews C, Li Y, Kolb B, Vezina P. Amphetamine-Induced Changes in Dendritic Morphology in Rat Forebrain Correspond to Associative Drug Conditioning Rather than Nonassociative Drug Sensitization. Biological Psychiatry. 2009;65(10):835–840. doi: 10.1016/j.biopsych.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The clinical neurobiology of drug craving. Curr Opin Neurobiol. 2013;23(4):649–654. doi: 10.1016/j.conb.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate - From pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Smith CT, Dang LC, Cowan RL, Kessler RM, Zald DH. Variability in paralimbic dopamine signaling correlates with subjective responses to d-amphetamine. Neuropharmacology. 2016;108:394–402. doi: 10.1016/j.neuropharm.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. Journal Of Neuroscience. 2007;27(7):1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci U S A. 2011;108(27):E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept. 1973;4:254–278. [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25(1):53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63(2):348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22(10):2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- van Hemel-Ruiter ME, de Jong PJ, Ostafin BD, Oldehinkel AJ. Reward-Related Attentional Bias and Adolescent Substance Use: A Prognostic Relationship? PLoS ONE. 2015;10(3):e0121058. doi: 10.1371/journal.pone.0121058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Pappas N. "Nonhedonic" food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44(3):175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, Kiefer F. Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addiction Biology. 2012;17(4):807–816. doi: 10.1111/j.1369-1600.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- Voon V, Mole TB, Banca P, Porter L, Morris L, Mitchell S, Irvine M. Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours. PLoS ONE. 2014;9(7):e102419. doi: 10.1371/journal.pone.0102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Personality and Social Psychology Bulletin. 2005;31(1):121–135. doi: 10.1177/0146167204271309. [DOI] [PubMed] [Google Scholar]
- Wise RA. The anhedonia hypothesis: Mark III. Behavioral and Brain Sciences. 1985;8:178–186. [Google Scholar]
- Witteman J, Post H, Tarvainen M, de Bruijn A, Perna Ede S, Ramaekers JG, Wiers RW. Cue reactivity and its relation to craving and relapse in alcohol dependence: a combined laboratory and field study. Psychopharmacology (Berl) 2015;232(20):3685–3696. doi: 10.1007/s00213-015-4027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33(9):391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, Graves T, Kareken DA. Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug Alcohol Depend. 2016;160:163–169. doi: 10.1016/j.drugalcdep.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Li X, Zhang M, Zhang F, Zhu C, Shen M. Behavioural approach tendencies to heroin-related stimuli in abstinent heroin abusers. Psychopharmacology. 2011;221(1):171–176. doi: 10.1007/s00213-011-2557-0. [DOI] [PubMed] [Google Scholar]