Abstract
Background
The liquids (e-liquids) used in an electronic cigarette (e-cigarette) contain myriad chemicals without adequate human inhalation safety data. Furthermore, the absence of e-liquid labeling requirements poses a formidable challenge to understanding how e-liquid constituents may promote nicotine addiction and / or have independent or synergistic biological effects when combined with nicotine. Ethyl alcohol is such a constituent, but has received little scientific interest in this context.
Methods
Using a randomized, double blind, crossover design, acute changes in subjective drug effects, motor performance and biochemical measures of alcohol and nicotine intake were evaluated after directed and ad lib puffing from two commercially available e-liquids containing nicotine (8 mg/ml), vanilla flavor and either 23.5% (high) or 0.4% (trace) alcohol.
Results
While no differences in subjective drug effects were observed between alcohol conditions, performance on the Purdue Pegboard Dexterity Test (PPDT) improved under the trace, but not under the 23.5% alcohol condition. Although plasma alcohol levels remained undetectable during testing, urine ethyl glucuronide (EtG), an alcohol metabolite, became measurable in three participants after puffing from the 23.5% alcohol e-cigarette.
Conclusions
Brief use of a widely available type of e-cigarette containing an e-liquid purchased from an Internet vendor can negatively impact psychomotor performance and in some instances, produce detectable levels of a urine alcohol metabolite. Given the widespread and unregulated use of e-cigarettes, especially by youth and other vulnerable populations, further studies are needed to evaluate both the acute safety and long-term health risks of using alcohol-containing e-cigarettes.
Keywords: nicotine, alcohol, e-cigarettes, e-liquid, vaping, alcohol inhalation
1. Introduction
Identifying the human health consequences from inhaling the complex, poorly characterized, and evolving chemical mixtures associated with unregulated e-cigarette use is a daunting public health problem (Dutra and Glantz, 2014; Bhatnagar et al., 2014; Grana et al., 2014). While most e-liquids contain a ‘base mixture’ of glycerol and propylene glycol to which nicotine and various flavoring ingredients are added, ethanol (ethyl alcohol) is also a variable but frequent ingredient due to its ubiquity and utility as a solvent for e-liquid additives (Cai and Kendall, 2009; Ellicott, 2009; Herrington and Myers, 2015; Tygat 2007; Valance and Ellicott, 2008). For example, in a recent chemical analysis or 42 commercial e-liquids, 30 contained ethyl alcohol (Varlet et al., 2015) and in a separate analysis of four leading brand first generation e-cigarettes, ethyl alcohol was found in all four although it was absent from their ingredient lists (Herrington et al., 2015). Importantly, alcohol was also found in the aerosols produced from these four e-cigarettes and an earlier analysis identified alcohol in an e-cigarette ‘mist’ (Laugesen, 2008).
Compared to the exhaustively studied effects of ingested alcohol, there are only a few studies on the behavioral effects of inhaled alcohol in humans, and these have focused on simulating low-level occupational exposures (Nadeau et al., 2003; Dumas-Campagna et al., 2014). To our knowledge, no previous studies have systematically examined the acute effects of alcohol inhaled from an e-cigarette. Likewise, the prevalence and patterns of alcohol exposure from e-cigarette use are largely unknown. While Internet-based anecdotal evidence indicates that some e-cigarette users ‘spike’ their e-liquids with various types of alcohol, the motivation for such use remains speculative (https://www.e-cigarette-forum.com/forum/trheads/effects-of-adding-liquor-to-e-liquid.57173). In other instances, alcohol may be deliberately added to e-liquids to ‘thin’ viscous solutions as recommended by do-it-yourself (DIY) e-liquid forums (https://vaporizingtimes.com/alcohol-e-cig/) and ‘alcohol e-cigarettes’ may soon be entering the marketplace (U.S.P.T.O., 2015; http://www.clearette.com/blog/alcoholic-e-cigarettes/). Consequently, whether from commercially prepared products or from self-made e-liquids, many e-cigarette users are likely repeatedly inhaling variable levels of alcohol during routine e-cigarette use.
The possibility of widespread exposure to inhaled alcohol as a consequence of e-cigarette use has many significant public health implications, especially for youth and other vulnerable populations. While the amount of alcohol delivered by an e-cigarette may be insufficient to induce typical intoxication, any alcohol that is inhaled is likely to rapidly enter the brain and modulate its functions. For example, if inhaled alcohol has reinforcing properties, then its combination with nicotine may enhance nicotine reinforcement and promote progression to nicotine dependence in non-dependent users (Oliver et al., 2013). Because nicotine is a relatively weak primary reinforcer when compared to other drugs of abuse (e.g., cocaine, alcohol or opiates), the inhalation of combined nicotine and alcohol during e-cigarette use may promote the development and maintenance of nicotine addiction through exposure to this additional reinforcer (Sorge et al., 2009). Likewise, the co-administration of alcohol and nicotine may promote the progression to dependence for both substances given their synergistic effects on reinforcement (McKee, 2006). Finally, because inhalation is a reliable and rapid method for inducing alcohol dependence in rodents, chronic inhalation from alcohol-containing e-cigarettes may promote the development of alcohol dependence in humans (Gilpin et al., 2008), while episodic use may provide ‘priming’ doses that induce craving for alcohol (Duka et al., 1999; O’Malley et al., 2002).
To our knowledge, no previous studies have examined the acute subjective or motor effects of puffing from commercial e-liquids with a specified alcohol content. As a first step in addressing this knowledge gap, we assayed the alcohol concentration in a convenience sample of 31 e-liquids. The effects of the e-liquid with the highest concentration (23.5% alcohol) were then compared to an e-liquid with a trace amount of alcohol (0.4%) in a double blind, within-sujects, crossover study. Subjective drug effects, psychomotor performance, and changes from baseline in blood alcohol levels and urine ethyl glucuronide (EtG) were compared between the ‘nicotine + high alcohol’ and ‘nicotine + trace alcohol’ e-cigarette conditions.
2. Methods
2.1 Participants
Twenty (14 male and 6 female) cigarette smokers between 21 and 35 years old, who reported drinking socially, using an e-cigarette at least once in the past year, and daily or non-daily use of tobacco cigarettes within the past 6 months, were enrolled. Exclusion criteria included unstable medical or psychiatric conditions, use of psychotropic medications and pregnancy. Four participants were excluded from the analysis because they did not complete any test session (two for for exclusionary drug use and two for a failure to return after screening). The final sample size of completers (those completing both test days) was 16. The average (SD) age of participants was 25.7 (2.7) and the range of ‘smoked cigarettes per day’ was 7 to 40 with a mean of 13.6 (8.9). Participants smoked for an average of 8.7 (4.3) years and scored 4.6 (2.4) on the Fagerstrom Test for Nicotine Dependence (FTND) indicating moderate dependence (Fagerstrom, 1978). The median lifetime duration of e-cigarette use was 2 months with a range 0 to 16 months. Twenty-seven percent of participants reported preferring e-cigarettes, 47% cigarettes, 13% having no preference and 13% that their preference was dependent on the context of use. The cumulative percentage of responses to four different intensity intervals (in number days) of any e-cigarette in the past month was 20% no use, 93% 1–10 days, 93% 11–20 days and 100% over 21 days. Written consent was provided before participation. This study was approved by the VA Connecticut Healthcare System, Human Subjects Subcommittee. Participants were paid for their participation.
2.2 Procedures
Using a randomized, within-subjects, counterbalanced design, the effects of an e-liquid containing 23.5% alcohol (Organic French Vanilla) were compared to a trace (0.4%) alcohol e-liquid (Organic Naked Vanilla, both from Virgin Vapors, Lower Lake, CA). The e-liquids also contained nicotine (8 mg/ml) in a based of 50% propylene glycol and 50% vegetable glycerin. Therefore, as verified by our measurements of alcohol and nicotine levels, the primary difference between the e-liquids was alcohol content. We used e-liquids selected from our convenience sample (n=31) in which 35% were without measurable levels of alcohol, 39% contained 0.1 to 0.75%, 23% contained 1.0 to 3.0% and one sample contained 23.5% (Supplementary Table).
We used a popular type of e-cigarette, the Joyetech eGo-C™, with a single coil atomizer (2.2 ohm), 2 ml tank, and a 650 mAH battery operating at 3.7 volts (6.2 W). To reduce variability in aerosol delivery during test sessions, subjects practiced using the e-cigarette in an adaptation session, inhaling more softly, but for a longer duration (3–4 seconds) than is customary for a tobacco cigarette. Participants were instructed to notify research staff immediately if any undesirable flavors developed that indicated overheating of the e-liquid (i.e. ‘dry puffs’). Subjects were told the purpose of the study was “to measure the effects of alcohol contained in a commercially available e-cigarette refill liquid”. They were not informed about the study hypothesis. Both participants and study personnel were blind to the randomization.
Test sessions were conducted in the early afternoon and participants were instructed to abstain from alcohol for 48 hours, and from all tobacco and nicotine products for 12 hours, before the sessions. Urine drug screens and exhaled carbon monoxide (Breath CO, Vitalograph, Inc., Lenexa, KS) identified exclusionary drug use and recent smoking (defined as a breath CO > 10 ppm). Breathalyzer measurements (Alco-Sensor IV, Intoximeters, Inc., St. Louis, MO) identified recent alcohol use. Prior to each test session, an indwelling 20-gauge, flexible catheter was inserted into an antecubital vein for blood sample collection. Each test session consisted of a 5 minute directed puffing session (10 puffs total) followed by two, 20 minute ad lib sessions separated by 20 minutes. The sessions were conducted at least 48 hours apart to minimize carryover effects.
After each puffing session, an 11-item Drug Effects Questionnaire (DEQ, 100 mm visual analogue scale ranging from “not at all” to “extremely”) and the Biphasic Alcohol Effects Scale (BAES, seven stimulant and seven sedative adjectives rated on a Likert-type scale ranging from 0 - “not at all” to 10 - “extremely”) were administered (Blomberg et al., 2009). The Purdue Pegboard Dexterity Test (PPDT, Lafayette Instruments, Lafayette, IN) measures two types of motor performance: fine finger dexterity and gross movements of the fingers, hands, and arms. The PPDT is simple to administer, subject to practice effects, and sensitive to the acute effects of alcohol. (Breckenridge and Berger, 1990; Buddenberg and Davis, 2000; Marczinski et al., 2012; Tarter et al.,1971). At baseline, then just after directed puffing and the first ad lib puffing sessions, participants picked up metal pins, one at a time, and filled as many sequential holes as possible, from top to bottom, within 30 seconds using the dominant hand, then the non-dominant hand, and then both hands.
Serial plasma nicotine concentrations were determined by tandem mass spectrometry (LC/MS/MS) (Sofuoglu et al., 2012). Plasma alcohol was measured just after directed puffing (high alcohol condition) using head-space gas chromatography. Urine EtG levels collected at baseline and after the last ad lib session (high alcohol condition) were measured using high-performance liquid chromatography coupled to tandem mass spectrometry (Jatlow et al., 2014; Weinmann et al., 2004).
2.3 Data Analysis
Descriptive statistics were calculated for each dependent measure and all dependent measures were assessed for normality prior to analysis. Log-transformation was applied to skewed data but when data could not be transformed to normality, non-parametric analyses were used. Each dependent variable was analyzed using a mixed effects model (Brown and Prescott, 2009), or the nonparametric alternative (Domhof and Langer, 2002), with fixed effects of condition (high alcohol, trace alcohol), time-point and their interaction. The best fitting variance-covariance structure was selected using Schwartz’ Bayesian information criterion. Post-hoc tests were used to evaluate significant condition or condition-by-time effects in the models. Hommel’s multiple testing procedure was used to adjust for analysis of multiple outcome measures within domain (Hommel, 1988).
3. Results
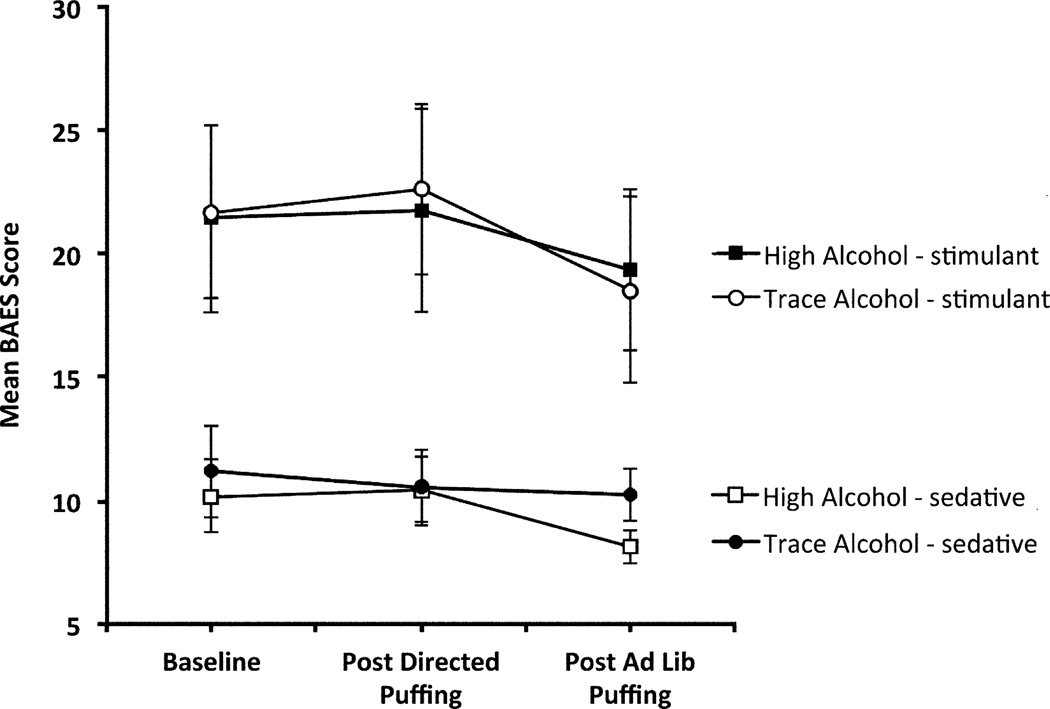
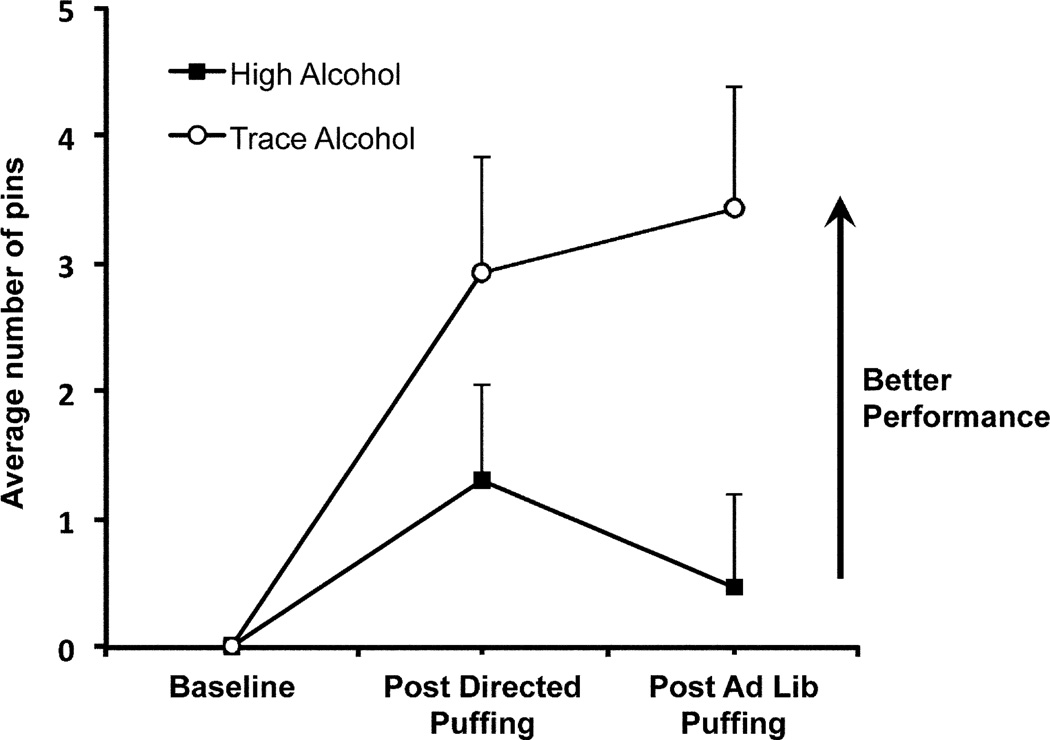
Subjective drug effects, as assessed by the DEQ and BAES, did not show any condition differences (condition or condition by time effects p-values>0.05, Fig.1). As expected, group mean PPDT performance scores in descending order were for the dominant hand, the non-dominant hand and then both hands (main effect for hand: F (2,251)=58.59, p<.0001). Furthermore, composite performance scores (the sum of scores for the right, left and both hands) improved under the trace (F (2,251)=9.10, p=0.0002), but not under the high alcohol condition (F (2,251)=1.18, p=.31; significant condition-by-time effect: F (2,251)=3.06, p=0.05, Fig. 2).
Fig. 1.
The subjective ratings of stimulant effects and sedating effects of the BAES under high (23.5%) and trace (0.4%) alcohol e-liquid conditions. Assessments were obtained at baseline, after directed puffing and at the end of the first ad lib session. No significant treatment differences were observed (p>0.05). Error bars = SEM
Fig. 2.
Change in composite Purdue Pegboard Dexterity Test (PPDT) performance scores under high and trace alcohol conditions. Assessments were obtained at baseline, after directed puffing and at the end of the first ad lib session. With repeated administration, PPDT performance improved (more pins completed in allotted time) in the trace alcohol condition, but not under the high alcohol condition. Error bars = SEM.
All plasma alcohol levels remained below the detection threshold after directed puffing in the high alcohol condition, therefore, plasma from the trace alcohol condition was not analyzed. In eight subjects, baseline urine EtG levels were below cut-off limits (<100 ng/ml) with three becoming detectable after puffing in the high alcohol condition (average concentration of 371 (43) ng/ml). The other eight subjects had detectable EtG at baseline suggesting alcohol use during the last 24–48 hours or possible recent exposure to mouthwash or hand sanitizers (Costantino et al., 2006). However, all participants had negative breathalyzer readings at baseline ruling out alcohol intake on the morning of test sessions. The number of subjects with positive EtG levels at baseline was not significantly different between the two conditions (8 in the high alcohol, 7 in the trace alcohol condition).
Plasma nicotine levels increased under both conditions (main effect of time [F (6,188) = 15.68, p<.0001) without condition or condition by time effects (p-values > 0.42, Supplementary Fig. 1). Therefore, differences in PPDT performance cannot be attributed to group differences in plasma nicotine levels. The amount of e-liquid consumed under the high [0.53 (0.30)] and trace alcohol conditions [0.48 (0.35)], as indexed by changes in weight (milligrams) of the e-liquid tank, were not significantly different (p=0.15).
4. Discussion
In young adult smokers, puffing from a commercial e-liquid containing 23.5% alcohol, as compared to use of a matched trace alcohol e-liquid, did not alter ratings of subjective drug effects. As expected, performance on the PPDT improved with repeated testing under trace alcohol, but no such improvement was observed under the 23.5% alcohol condition. Although the differences in the PPDT composite change scores between the two conditions, 2.9 after directed puffing and 3.4 after ad lib puffing, were less than the score of 5.3 observed 30 minutes after subjects drank alcohol (with a mean peak plasma alcohol level of 0.09%, Tarter et al., 1971), they reflect an ecologically valid alteration in psychomotor performance. Because alcohol disrupts many psychomotor functions, including those impacting driving performance, dose-dependently with blood alcohol concentrations just above zero, individuals using e-liquids with high alcohol content under ordinary circumstances may be at increased risk of accidents (Blomberg et al., 2009). Furthermore, as indicated by the absence of significant changes in subjective ratings across conditions, this increased risk may occur without awareness of impairment.
In addition, in three of our subjects, acute alcohol delivery from the e-cigarette was confirmed by the emergence of detectable urine EtG while plasma alcohol levels remained undetectable. EtG is known for its high sensitivity to low levels of alcohol intake and the urine collected at the end of session reflect cumulative alcohol intake during the session (Jatlow and O'Malley, 2010; Wurst et al., 2015). Collectively, our findings suggest that impairment from inhaled alcohol may occur under conditions that are undetectable with commonly used methods of assessing alcohol exposure.
In our convenience sample of e-liquids, 62% contained detectable levels of alcohol at or below 3%, consistent with the relatively low alcohol levels observed in previous analyses of e-liquid samples (Varlet et al., 2015). However, among the thousands of commercially available e-liquids, we identified a product that contained 23.5% alcohol from a sample of only 31. Furthermore, while 23.5% alcohol may represent an upper bound on the range of alcohol content found in commercial e-liquids, individuals drawn to the novelty and experimentation within ‘DIY vaping’ subculture may use e-liquid recipes that result in alcohol concentrations approaching the 23.5% used in this experiment (https://www.cigaretteforum.com /forum/threads/guide-to-diy-flavoring.74109/page-6). Future experiments should examine whether e-liquids with lower concentrations of alcohol can still impact psychomotor functioning when longer puffing bouts and / or more powerful e-cigarettes are used.
Finally, although we did not find evidence for synergistic effects of nicotine and alcohol based upon changes in subjective measures, a trend toward higher plasma nicotine levels after the second ad lib puffing in the high alcohol condition suggests the potential for interactive effects on reinforcement with more prolonged e-cigarette use. An improved understanding of the differential risk profiles associated with using alcohol-containing e-cigarettes will require data on the prevalence and patterns of alcohol exposure during e-cigarette use. Furthermore, studies using an expanded set of behavioral and subjective measures with various combinations of nicotine and alcohol, including an alcohol-only condition, and different dosing schedules will be needed to more fully characterize the acute safety and long-term health risks from using e-cigarettes that contain alcohol.
Supplementary Material
References
- Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, Robertson RM, McAuley T, Goff D, Benowitz N. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130:1418–1436. doi: 10.1161/CIR.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg RD, Peck RC, Moskowitz H, Burns M, Fiorentino D. The Long Beach/Fort Lauderdale relative risk study. J. Safety Res. 2009;40:285–292. doi: 10.1016/j.jsr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Breckenridge RL, Berger RS. Locus of control and perceived alcohol ingestion in performance of a fine motor skill. Psychol. Rep. 1990;66:179–185. doi: 10.2466/pr0.1990.66.1.179. [DOI] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied mixed models in medicine. John Wiley & Sons; 2009. [Google Scholar]
- Buddenberg LA, Davis C. Test-retest reliability of the Purdue Pegboard Test. The Am. J. Occup. Ther. 2000;54:555–558. doi: 10.5014/ajot.54.5.555. [DOI] [PubMed] [Google Scholar]
- Cai X, Kendall M. Gas chromatography mass spectrometry (GC-MS) analysis report. [accessed 11.14.15];Job Number C09Y8961. Evans Analytical Group, Sunnyvale, CA., USA. 2009 http://truthaboutecigs.com/science/14.pdf.
- Costantino A, Digregorio EJ, Korn W, Spayd S, Rieders F. The effect of the use of mouthwash on ethylglucuronide concentrations in urine. J. Anal. Toxicol. 2006;30:659–662. doi: 10.1093/jat/30.9.659. [DOI] [PubMed] [Google Scholar]
- Domhof S, Langer F. Nonparametric analysis of longitudinal data in factorial experiments. Wiley-Interscience; 2002. [Google Scholar]
- Dumas-Campagna J, Tardif R, Charest-Tardif G, Haddad S. Ethanol toxicokinetics resulting from inhalation exposure in human volunteers and toxicokinetic modeling. Inhal. Toxicol. 2014;26:59–69. doi: 10.3109/08958378.2013.853714. [DOI] [PubMed] [Google Scholar]
- Duka T, Jackson A, Smith DC, Stephens DN. Relationship of components of an alcohol interoceptive stimulus to induction of desire for alcohol in social drinkers. Pharmacol. Biochem. Behav. 1999;64:301–309. doi: 10.1016/s0091-3057(99)00080-5. [DOI] [PubMed] [Google Scholar]
- Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among U.S. adolescents: a cross-sectional study. JAMA Pediatr. 2014;168:610–617. doi: 10.1001/jamapediatrics.2013.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellicott M. Analysis of components from "e-juice XX high 36mg/ml rated nicotine solution” ref S 55434. Report number: E249A. Lancashire, UK: LPD Lab Services; 2009. [accessed 11.14.15]. http://truthaboutecigs.com/science/11.pdf. [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict. Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al] 2008. Vapor inhalation of alcohol in rats. Chapter 9, Unit 9.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J. Chromatogr. A. 2015;1418:192–199. doi: 10.1016/j.chroma.2015.09.034. [DOI] [PubMed] [Google Scholar]
- Herrington JS, Myers C, Rigdon A. Restek ChromaBLOGraphy Technical Resource Document. Bellefonte, PA., USA: Restek; 2015. [accessed 14.11.15]. Analysis of Nicotine and Impurities in Electronic Cigarette Solutions and Vapor. http://www.restek.com/Technical-Resources/Technical Library/Foods-Flavors-Fragrances/fff_FFAN2127-UNV. [Google Scholar]
- Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75:383–386. [Google Scholar]
- Jatlow P, O'Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin. Exp. Res. 2010;34:968–975. doi: 10.1111/j.1530-0277.2010.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatlow PI, Agro A, Wu R, Nadim H, Toll BA, Ralevski E, Nogueira C, Shi J, Dziura JD, Petrakis IL, O'Malley SS. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin. Exp. Res. 2014;38:2056–2065. doi: 10.1111/acer.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugesen M. Second safety report on the Ruyan® e-cigarette. Christchurch, New Zealand: Health New Zealand Ltd; 2008. [accessed 14.11.15]. www.healthnz.co.nz/2ndSafetyReport_9Apr08.pdf. [Google Scholar]
- Marczinski CA, Fillmore MT, Henges AL, Ramsey MA, Young CR. Effects of energy drinks mixed with alcohol on information processing, motor coordination and subjective reports of intoxication. Exp. Clin. Psychopharmacol. 2012;20:129–138. doi: 10.1037/a0026136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau V, Lamoureux D, Beuter A, Charbonneau M, Tardif R. Neuromotor effects of acute ethanol inhalation exposure in humans: a preliminary study. J. Occup. Health. 2003;45:215–222. doi: 10.1539/joh.45.215. [DOI] [PubMed] [Google Scholar]
- Oliver JA, Blank MD, Van Rensburg KJ, MacQueen DA, Brandon TH, Drobes DJ. Nicotine interactions with low-dose alcohol: pharmacological influences on smoking and drinking motivation. J. Abnorm. Psychol. 2013;122:1154–1165. doi: 10.1037/a0034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology. 2012;37:1509–1516. doi: 10.1038/npp.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Jones BM, Simpson CD, Vega A. Effects of task complexity and practice on performance during acute alcohol intoxication. Percept. Mot. Skills. 1971;33:307–318. doi: 10.2466/pms.1971.33.1.307. [DOI] [PubMed] [Google Scholar]
- Tygat J. “Super Smoker” Expert Report. Final Report. Leuven, Belgium: Toxicology Laboratory, Catholic University; 2007. [accessed 14.11.15]. http://truthaboutecigs.com/science/15.pdf. [Google Scholar]
- Valance C, Ellicott M. Analysis of chemical components from high, med & low nicotine cartridges. Report number: D318. Lancashire, UK: LPD Lab Service; 2008. [accessed 14.11.15]. http://www.tobaccoharmreduction.org/tox1.pdf. [Google Scholar]
- United States Patent and Trademark Office. Electronic vaping device. Application number US 14/587,117. Publication number US 20150181945 A1. 2015 [Google Scholar]
- Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter J-F. Toxicity assessment of refill liquids for electronic cigarettes. Int. J. Environ. Res. Public Health. 2015;12:4796–4815. doi: 10.3390/ijerph120504796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann W, Schaefer P, Thierauf A, Schreiber A, Wurst FM. Confirmatory analysis of ethylglucuronide in urine by liquid-chromatography/electrospray ionization/tandem mass spectrometry according to forensic guidelines. J. Am. Soc. Mass Spectrom. 2004;15:188–193. doi: 10.1016/j.jasms.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Thon N, Yegles M, Schruck A, Preuss UW, Weinmann W. Ethanol Metabolites: Their Role in the Assessment of Alcohol Intake. Alcohol Clin. Exp. Res. 2015;39:2060–2072. doi: 10.1111/acer.12851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.