Abstract
Bone marrow aspirates (BMAs), due to their innate osteogenic potential, are well-documented supplements to osteoconductive and/or osteoinductive materials. The calcaneal body provides foot and ankle surgeons a convenient harvest site with low morbidity and minimal cost. In the current study, we attempt to identify and characterize multipotent mesenchymal stromal cells (MSCs) in BMAs harvested from human calcaneal body. Ten healthy patients between the age of 18 and 65 were enrolled in this study. BMAs were harvested from the patients without any reported post-operative complications related to the harvest. Cells isolated from all the aspirates were adherent to culture plates and expressed positive MSC surface markers CD105, CD90 and CD73 as well as low level of negative MSC markers CD34 and CD45. The cells maintained the ability to proliferate and differentiate into cells of mesenchymal lineages. The BMAs from the human calcaneal body offer a healthy source of multipotent MSCs.
Keywords: foot and ankle surgery, orthobiologic materials, osteoconduction, osteoinduction, surface markers
Introduction
As podiatric surgery has continued to evolve and advance in complexity, so has the understanding of factors affecting healing - not only of soft tissue but also of bone. Many surgeons have looked towards orthobiologic approaches as a way of supplementing their surgical necessities. Recently the use of bone marrow aspirates (BMA) containing MSCs is becoming increasingly popular in foot and ankle surgery (1-3). In the continually evolving field, BMAs have arisen as a well-documented method to locally introduce endogenous growth factors such as Platelet Derived Growth Factor (PDGF) and Transforming Growth Factor β (TGFβ) into an area to initiate healing of fusion sites, fresh fractures and areas of devascularization (4, 5). They have also been used to enhance healing at graft sites (6, 7). BMAs have been shown to have higher levels of both endogenous Bone Morphogenic Protein 2 (BMP2) and Vascular Endothelial Growth Factor α (VEGF-α), thus providing superior quality as compared to the harvest of peripheral blood (8).
Most orthobiologic materials, in comparison with the current “gold standard” of autologous bone graft, contain not only osteoconductive (scaffold) and osteoinductive (signal protein) properties, but also osteogenic qualities (MSCs) (1). The osteogenic qualities of BMAs (MSCs) have been well documented and used to supplement orthobiologic substances containing only osteoinductive and/or osteoconductive properties (9-11). Moreover, MSCs secrete a multitude of cytokines and growth factors, affecting surrounding cells and matrix to regulate tissue regeneration (12). Several studies have reported the successful aspiration of BMAs at sites with a high concentration of red marrow, such as vertebral body, the iliac crest, and the proximal tibia (13). Reproducible concentrations of hMSCs were also obtained from both the proximal humerus and the distal femur during arthroscopy (14, 15). However, many podiatric surgeons are limited with regard to being able to harvest aspirates from these sites due to factors such as state laws, hospital restrictions, or other inhibitory factors. Although many podiatric surgeons may choose to harvest autografts from the calcaneus or even tibia, this is not a benign procedure and may be associated with surgical morbidity (16). Replacement of red bone marrow by yellow bone marrow during the normal aging process further diminishes the options for the podiatric surgeons (18).
The current study is an attempt to identify the presence or absence of multipotent MSCs in the calcaneal body using a currently accepted method of sampling. As per the guidelines of the International Society for Cellular Therapy (ISCT) (19), minimum criteria for documenting the presence of hMSCs must include the following: a) Plastic adherence when maintained in standard culture conditions, b) Presence or enhanced expression (+) of cell surface markers such as CD105, CD73, and CD90 and absence or suppressed expression (−) of CD45, CD34, CD14 or CD11b, CD79 or CD19 and HLA class II, and c) Ability to differentiate into osteoblasts, adipocytes, and chondrocytes in vitro. To our knowledge, no such study exists in this regard. Our hypothesis is that the calcaneus contains a viable population of multipotent MSCs capable of differentiating into osteoblasts, chondrocytes, and adipocytes. As this study was an evaluation of presence of MSCs, patients were excluded for a variety of factors in an attempt to sample a “healthy” patient population.
Patients and Methods
Study Population
The study was conducted under approved protocol #10-51 at Memorial Hospital of Rhode Island. Ten patients were selected for this prospective, case controlled study. Patients were excluded in an attempt to sample a healthy patient population. Criteria for selection included several factors, such as age, surgical and disease history, smoking and substance abuse. Patients selected were between the ages of 18 and 65. All surgeries were elective in nature and no history of recent trauma to the lower extremity was present in any of the cases. Patients with a history of systemic diseases such as Diabetes Mellitus (DM), Rheumatoid Arthritis (RA), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), end stage renal disease (ESRD), and cardiovascular diseases were all excluded from the study. Patients with documented presence of peripheral artery disease (PAD) and/or peripheral neuropathy were also excluded from the study. Patients were required to have quit smoking at least a year to be eligible for the study as well. Chronic use of corticosteroids or other immunosuppressant therapy was also a criterion for exclusion from the study. Smoking, alcohol abuse, and substance abuse were also considered disqualifying factors from the study.
Surgical Technique
Patients were placed in a supine position. An ipsilateral bump was placed under the hip to ensure adequate exposure of the lateral wall of the calcaneus. While carefully identifying and avoiding the sural nerve and peroneal tendons, 52 cc of BMA was collected along with 8 cc of heparin using an 8G Jamshidi needle inserted percutaneously through the lateral wall of the calcaneal body with continuous retrograde force. The skin wound was then closed with a single 4-0 nylon simple suture. Patients were followed for up to 10 months.
Isolation of human mesenchymal stromal cells (hMSCs)
hMSCs were isolated by Percoll density centrifugation followed by plastic adherence as previously described (20, 21). The entire aspirate volume was mixed 1:1 with phosphate buffered saline (PBS), The diluted bone marrow was subsequently layered over a Ficoll-paque density gradient medium (1.073 g/mL) and centrifuged at 400x g for 30 min to isolate the human mononuclear cells (hMNCs). The mononuclear cells were then washed twice with PBS and resuspended in hMSC growth medium. The growth medium consisted of minimum essential medium alpha (MEM-α) as basal medium supplemented with 10% fetal bovine serum (FBS), antibiotics-antimycotics (100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL fungizone), and 0.1 mM nonessential amino acids (Invitrogen). Cells were counted using a hemocytometer and trypan blue exclusion method and finally seeded at a density of 10×103 cells/cm2 on tissue culture treated plastic (TCP). The cells were kept in an incubator at 37 °C, 5% CO2 and 95% humidity for at least 24 h before testing plastic adherence by observation of the culture specimens under a microscope. Observation of adherent cells deemed the specimen positive for the first minimal criterion of MSCs.
Flow Cytometry
Cells seeded on TCP were washed with PBS to remove non-adherent cells present in the cell culture. The adherent cells were then detached from TCP using 0.25% trypsin-EDTA. Cells were resuspended in Pharmingen Stain Buffer (BD Biosciences) and subsequently stained with the respective fluorescently conjugated antibody. Flow cytometric analysis was performed on a FACSCalibur (Becton Dickinson) while a minimum of 20,000 events was recorded for each sample. Analysis was performed using FlowJo (Tree Star) on dot plots to obtain a more accurate quantification as compared to histogram analysis. Each specimen was tested for the presence of both positive (CD105 (SH2/Endoglin), CD73 (SH3/5′ nucleotidase), and CD90 (Thymocyte Differentiation Antigen 1, Thy-1)) and negative (CD34, CD45) hMSC markers.
Differentiation assays
The potential of isolated cells to differentiate into mesenchymal lineages was evaluated using StemPro Osteogenesis Differentiation Kit, StemPro Chondrogenesis Differentiation Kit, and StemPro Adipogenesis Differentiation Kit (Invitrogen) according to the manufacturer’s instructions. Control cultures were run in parallel during all differentiation assays where cells were cultured in growth media without supplemental differentiation factors.
For osteogenic differentiation, cells were seeded into 12-well plates at a density of 5,000 cells/cm2 and incubated in growth medium for up to 4 days until cells reached confluency. The cells were then maintained in osteogenic medium for 21 days and medium was refreshed every 2 to 3 days. Cells that underwent osteogenesis were fixed with 4% formaldehyde and stained with 2% Alizarin red S solution (pH 4.2) for the detection of calcium accumulation.
Chondrogenic differentiation was induced in micromass culture. Micromass cultures were generated by seeding 5 μL droplets of cell suspension (1.6×107 cells/mL) in the center of 12-well plate wells. Cells were then cultivated for 2 hours and medium switched to chondrogenic medium. After 14 days of culture, cells were fixed with 4% formaldehyde and stained with 1% Alcian Blue for the detection of glycosaminoglycan in cartilage.
For adipogenic differentiation, cells were seeded into 12-well plates at a density of 10,000 cells/cm2 and cultivated in growth medium until the cells reached confluency. The medium was then replaced with adipogenic medium and the cultures continued for 14 days. Cells that underwent adipogenesis were fixed with 4% formaldehyde and stained with oil red O to visualize lipid droplet formation.
Results
Bone marrow aspirates were harvested from the calcaneus of the ten patients enrolled in the study. All patients were followed for an average of 4 months with a range of 2 months to 10 months of follow up. The suture was removed at approximately 2 weeks after bone marrow aspiration. All 10 patients remained non weight bearing (NWB) for at least 6 weeks with an average period of NWB being 12 weeks. A return to weight bearing (WB) status was determined by healing factors in the primary procedure performed and was not influenced by the bone marrow aspirate. No evidence of any post-operative complications related to the harvest were noted including, but not limited to sural nerve injury, peroneal tendon injury, or calcaneal fracture. The described technique for harvesting BMAs from human calcaneus in the current study was safe, as demonstrated in other studies (17, 22, 23).
We next sought to identify the presence of hMSCs in BMAs from human calcaneus. We followed the guidelines set by the International Society for Cellular Therapy (ISCT) (19), to document the presence of hMSCs. To isolate hMSCs from calcaneus BMA, hMNCs were first isolated from BMA by density gradient centrifugation. The collected fraction containing hMNCs were then subjected to plastic adherence and attached cells grew into a confluent cell layer. All 10 specimens were observed to adhere to plastic under standard culture conditions. Figure 1 shows plastic adherence of a representative specimen 110211a at 16 days in culture. By this standard the requirement of plastic adherence was met, as all 10 were reported as positive.
Figure 1.
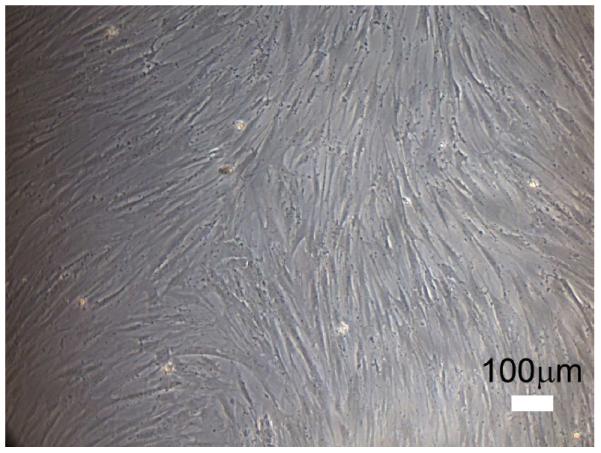
Stem cell morphology at day 16 (110211A). Cells attached to plastic and grew into a confluent cell layer.
Flow cytometric analysis was performed to study the expression of MSC surface markers. A subpopulation in the scatter plot was selected from the total population that was over 90% CD105 positive in all populations to exclude the dominant hematopoietic cell population and therefore also reducing variability between specimens (Fig. 2). This population was analyzed and percentages of cells were quantified using both the hMSC positive (CD73, CD90, CD105) as well as negative (CD34, and CD45) markers (Table 1). The exclusion marker CD34 was noted to be low in general and the CD45 marker substantially decreased over time. The inclusion markers CD73 and CD90, while initially only measured at about 10% of short-term cultures, were all found to be present in 100% of cells in long-term cultures. The positive marker CD105 was noted to be approximately 90% in all specimens taken, which was expected due to the subset being based on CD105+ cells.
Figure 2.
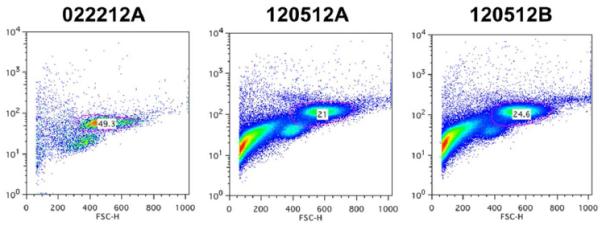
Representative flow cytometry analysis of stem cell marker CD105. The gated sub-polulations (pink circles, 49.3% in 022212A, 21% in 120512A, and 24.6% in 120512B) were selected for further evaluation of the expression of CD90, CD73, CD34 and CD45.
Table.
Flow cytometric analysis of surface marker expression on isolated hMSCs
| Flow Cytometry | ||||||
|---|---|---|---|---|---|---|
| ID | Culture time (in days) |
CD34 (%) |
CD45 (%) |
CD73 (%) |
CD90 (%) |
CD105 (%) |
| 110211A* | 1 | 7.20 | 4.06 | 6.57 | 85.30 | 95.20 |
| 120512A | 1 | 0.34 | 47.80 | 6.48 | 8.22 | 91.90 |
| 120512B | 1 | 0.33 | 39.70 | 26.30 | 5.90 | 95.20 |
| 022212A | 2 | 0.72 | 11.60 | 38.10 | 94.90 | 94.10 |
| 022212B | 2 | 5.44 | 11.90 | 13.60 | 62.60 | 82.60 |
| 032112A | 2 | 1.63 | 22.10 | 4.79 | 9.26 | 84.90 |
| 032013A | 2 | 4.87 | 0.00 | 7.33 | 2.53 | 90.30 |
| 111711A | 21 | 0.13 | 0.81 | 99.90 | 99.20 | 99.70 |
| 111711B | 21 | 0.09 | 0.28 | 100.0 | 98.10 | 97.80 |
| 110211A* | 28 | 1.02 | 0.40 | 99.60 | 100.00 | 99.60 |
Flow Cytometry performed before culturing (day 1) and on day 28 of culture
Finally, the potential of isolated hMSCs to differentiate into cells of mesenchymal lineages was evaluated. hMSCs isolated from all specimens had the ability to proliferate extensively and were capable of differentiating into osteoblasts, chondrocytes, and adipocytes with respective differentiation cues. Osteogenic differentiation was indicated by the alizarin red staining of calcium deposition at day 21 (Fig. 3A and D). Chondrogenic differentiation was indicated by the alcian blue staining of the sulfated proteoglycans deposits at day 14 (Fig. 3B and E). Oil red O staining at day 14 indicated the accumulation of lipid droplets during adipogenic differentiation (Fig. 3C and F). The multilineage differentiation potential varied among patients.
Figure 3.
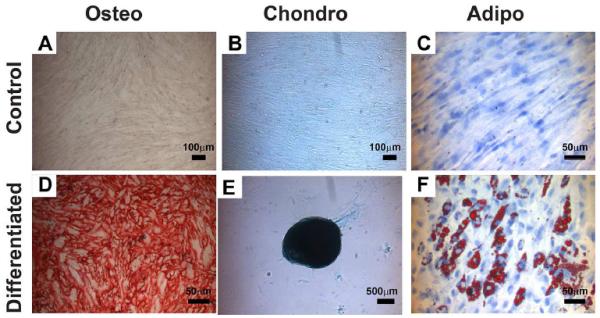
Differentiation of isolated hMSCs into mesenchymal lineages (032013A). Cells were shown to differentiate to osteogenic (Osteo), Chondro (chondrogenic) and adipo (adipogenic) lineages. Alizarin red staining indicated the calcium deposition during osteogeninc differentiation (A and D). Alcian blue staining indicated the sulfated proteoglycans deposits during chondrogenic differentiation (B and E). Oil red-O staining indicated the accumulation of lipid droplets during adipogenic differentiation (C and F).
Discussion
The purpose of this study was to identify the presence of multipotent hMSCs in BMAs harvested from human calcaneus. In the continually evolving field of orthobiologics, the use of autologous BMA on its own or coupled with a carrier is emerging as a viable alternative to the use of autologous bone graft in the treatment of a variety of lower extremity pathologies (24). A variety of factors currently limit the podiatric foot and ankle surgeon in terms of ideal locations for harvest. This study was an attempt to present a reasonable alternative to the previously documented harvest sites including the iliac crest, vertebral body, proximal tibia, and distal femur using a defined method of determining the presence of hMSCs. While there is no such thing as a “benign” surgical procedure, harvest of BMA from the calcaneus has been reported to have a relatively low risk of associated morbidity (17). Moreover, calcaneal bone marrow aspirate used in conjunction with carriers demonstrated enhanced success (25). Previous study identified the presence of osteoblastic progenitor cells in BMA from human calcaneus (26). However, definitive proof of the actual presence of multipotent hMSCs in BMA from human calcaneus has been lacking. To our knowledge, this study represents the first isolation, characterization and in vitro differentiation of hMSCs in BMAs harvested from human calcaneus. All 10 specimens collected from healthy donors were noted to be what is defined as “positive” for hMSCs according to the currently accepted standard of identification defined by the ISCT (19). Since the study was limited to two results (positive or negative for hMSCs), a score of 10/10 “positives” is statistically significant, indicating that BMA from calcaneus is a reliable source for hMSCs.
In the current study, we showed that hMSCs isolated from human calcaneus have high capacity of self-renewal and osteogenic differentiation, suggesting their potential use in bone regeneration. Due to the limited number of MSCs in unprocessed BMA, in order to gain the maximum potential the BMA can be further processed to concentrate MSCs or the isolated hMSCs be expanded ex vivo before implantation (7, 27). The ex vivo expanded MSCs and bone marrow aspirate concentrate (BMAC) can provide additional benefits as they both are rich sources of growth factors/cytokine/chemokines (28, 29). Further studies to evaluate the secretome of the ex vivo expanded calcaneal hMSCs and the growth factor/cytokine/chemokine profile in BMAC from human calcaneus will be needed.
Whether the BMA harvested from human calcaneus is as potent as that from the iliac crest and vertebral body is yet to be determined. Advanced analysis would be needed to identify the concentration of hMSCs in each aspirate and then compared to previously reported concentrations from other sites. One would expect a lower hMSC concentration in calcaneal BMA and this would require a higher volume for application. Previous reports suggest that BMA collected from the iliac crest had a higher mean concentration of osteoblastic progenitor cells compared with the calcaneus (26). Moreover, it is necessary to compare the osteogenic potential of BMAs from calcaneus to that of aspirates from other sites.
One limitation to this study is the relatively homogenous population studied. As a preliminary attempt to identify presence of hMSCs in BMAs from human calcaneus, patients with factors known to suppress regeneration were excluded. Future study is warranted to assess those factors as potential predictors of BMA quality.
In conclusion, the aspirates collected from the calcaneus contain a healthy population of cells which are able to adhere to plastic under standard culture conditions, showed the presence or enhanced expression (+) of cell surface markers such as CD105, CD73, and CD90 and absence or suppressed expression (−) of CD45, CD34; and showed the ability to differentiate into osteoblasts, adipocytes, and chondrocytes in vitro. Based on the guidelines set by the ISCT, we infer that multipotent hMSCs were successfully isolated from bone marrow aspirate collected from human calcaneus. They maintained the ability to proliferate and differentiate into cells of mesenchymal lineage. The bone marrow aspirate from the calcaneal body offers a reliable, healthy source of multipotent mesenchymal stromal cells.
Acknowledgement
We thank ETEX Corporation and the NIH P41 (P41 EB002520) Tissue Engineering Resource Center (TERC) for support of this study.
Footnotes
Financial Disclosure: Study financially supported by ETEX Corporation.
Conflict of Interest: Michael Strunk, Jerry Chang and Siddhesh Angle are paid employees of Etex Corporation and hold stock options at Etex Corporation. David Kaplan has received research funds as a principal investigator from Etex Corporation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnett MDJ, Pomeroy GC. Use of Platelet-Rich Plasma and Bone Marrow-Derived Mesenchymal Stem Cells in Foot and Ankle Surgery. Techniques in Foot & Ankle Surgery. 2007;6:89–94. [Google Scholar]
- 2.Hatzokos I, Stavridis SI, Iosifidou E, Karataglis D, Christodoulou A. Autologous Bone Marrow Grafting Combined with Demineralized Bone Matrix Improves Consolidation of Docking Site After Distraction Osteogenesis. 2011 doi: 10.2106/JBJS.J.00514. [DOI] [PubMed] [Google Scholar]
- 3.Rush SM, Hamilton GA, Ackerson LM. Mesenchymal Stem Cell Allograft in Revision Foot and Ankle Surgery: A Clinical and Radiographic Analysis. The Journal of Foot and Ankle Surgery. 2009;48:163–169. doi: 10.1053/j.jfas.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 4.McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of Osteoprogenitor Cells for Augmenting Spinal Fusion: Comparison of Progenitor Cell Concentrations from the Vertebral Body and Iliac Crest. 2005 doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinzur MS. Use of Platelet-Rich Concentrate and Bone Marrow Aspirate in High-Risk Patients with Charcot Arthropathy of the Foot. Foot & Ankle International. 2009;30:124–127. doi: 10.3113/FAI-2009-0124. [DOI] [PubMed] [Google Scholar]
- 6.Dallari D, Savarino L, Stagni C, Cenni E, Cenacchi A, Fornasari PM, Albisinni U, Rimondi E, Baldini N, Giunti A. Enhanced Tibial Osteotomy Healing with Use of Bone Grafts Supplemented with Platelet Gel or Platelet Gel and Bone Marrow Stromal Cells. 2007 doi: 10.2106/JBJS.F.01026. [DOI] [PubMed] [Google Scholar]
- 7.Jäger M, Herten M, Fochtmann U, Fischer J, Hernigou P, Zilkens C, Hendrich C, Krauspe R. Bridging the gap: Bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. Journal of Orthopaedic Research. 2011;29:173–180. doi: 10.1002/jor.21230. [DOI] [PubMed] [Google Scholar]
- 8.Smiler D, Soltan M, Soltan C, Matthews C. Growth Factors and Gene Expression of Stem Cells: Bone Marrow Compared With Peripheral Blood. Implant Dentistry. 2010;19:229–240. doi: 10.1097/ID.0b013e3181dc24a9. [DOI] [PubMed] [Google Scholar]
- 9.Connolly J, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clinical Orthopaedics and Related Research. 1991;266:259–270. [PubMed] [Google Scholar]
- 10.Guyton GP, Miller SD. Stem Cells in Bone Grafting: Trinity Allograft with Stem Cells and Collagen/Beta-Tricalcium Phosphate with Concentrated Bone Marrow Aspirate. Foot and ankle clinics. 2010;15:611–619. doi: 10.1016/j.fcl.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Tiedeman J, Garvin K, Kile T, Connolly J. The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics. 1995;18:1153–1158. doi: 10.3928/0147-7447-19951201-05. [DOI] [PubMed] [Google Scholar]
- 12.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of Cellular Biochemistry. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 13.Muschler GF, Boehm C, Easley K. Aspiration to Obtain Osteoblast Progenitor Cells from Human Bone Marrow: The Influence of Aspiration Volume. 1997;79:1699–1709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Beitzel K, McCarthy MBR, Cote MP, Durant TJS, Chowaniec DM, Solovyova O, Russell RP, Arciero RA, Mazzocca AD. Comparison of Mesenchymal Stem Cells (Osteoprogenitors) Harvested From Proximal Humerus and Distal Femur During Arthroscopic Surgery. Arthroscopy. 2013;29:301–308. doi: 10.1016/j.arthro.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Fennema EM, Renard AJS, Leusink A, van Blitterswijk CA, de Boer J. The effect of bone marrow aspiration strategy on the yield and quality of human mesenchymal stem cells. Acta Orthopaedica. 2009;80:618–621. doi: 10.3109/17453670903278241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raikin S, Brislin K. Local bone graft harvested from the distal tibia or calcaneus for surgery of the foot and ankle. Foot and ankle international. 2005;26:449–453. doi: 10.1177/107110070502600604. [DOI] [PubMed] [Google Scholar]
- 17.Roukis TS, Hyer CF, Philbin TM, Berlet GC, Lee TH. Complications Associated with Autogenous Bone Marrow Aspirate Harvest from the Lower Extremity: An Observational Cohort Study. The Journal of Foot and Ankle Surgery. 2009;48:668–671. doi: 10.1053/j.jfas.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. Journal of Orthopaedic Research. 2001;19:117–125. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 19.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 20.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 21.Glettig DL, Kaplan DL. Long-term phenotypic characterization of human bone marrow and adipose tissue derived mesenchymal stromal cells. Stem Cell Discovery. 2013;3:99–116. [Google Scholar]
- 22.Schade VL, Roukis TS. Percutaneous Bone Marrow Aspirate and Bone Graft Harvesting Techniques in the Lower Extremity. Clinics in Podiatric Medicine and Surgery. 2008;25:733–742. doi: 10.1016/j.cpm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Schweinberger MH, Roukis TS. Percutaneous Autologous Bone Marrow Harvest from the Calcaneus and Proximal Tibia: Surgical Technique. The Journal of Foot and Ankle Surgery. 2007;46:411–414. doi: 10.1053/j.jfas.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 24.McGlamry MC. BONE MARROW ASPIRATE: Science and Application in Foot and Ankle Surgery. In: Southerland J, Alder D, Boberg J, Downey M, Nakra A, Rabjohn L, Rabjohn LV, editors. McGlamry's Comprehensive Textbook of Foot and Ankle Surgery. The Podiatry Institute; 2012. [Google Scholar]
- 25.Jia X, Peters PG, Schon L. The Use of Platelet-Rich Plasma in the Management of Foot and Ankle Conditions. Operative Techniques in Sports Medicine. 2011;19:177–184. [Google Scholar]
- 26.Hyer CF, Berlet GC, Bussewitz BW, Hankins T, Ziegler HL, Philbin TM. Quantitative Assessment of the Yield of Osteoblastic Connective Tissue Progenitors in Bone Marrow Aspirate from the Iliac Crest, Tibia, and Calcaneus. 2013;95:1312–1316. doi: 10.2106/JBJS.L.01529. [DOI] [PubMed] [Google Scholar]
- 27.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. New England Journal of Medicine. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 28.King W, Tan M, Ponticeillo M, Woodell-May J. Anti-Inflammatory Properties of the Output of an Autologous Bone Marrow Concentrating Device; ORS meeting; 2016. [Google Scholar]
- 29.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the Mesenchymal Stem Cell Secretome for the Treatment of Cardiovascular Disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


