Abstract
Blood gas and tissue pH regulation depend on the ability of the brain to sense CO2 and/or H+ and alter breathing appropriately, a homeostatic process called central respiratory chemosensitivity. We show that selective expression of the proton-activated receptor GPR4 in chemosensory neurons of the mouse retrotrapezoid nucleus (RTN) is required for CO2-stimulated breathing. Genetic deletion of GPR4 disrupted acidosis-dependent activation of RTN neurons, increased apnea frequency and blunted ventilatory responses to CO2. Reintroduction of GPR4 into RTN neurons restored CO2-dependent RTN neuronal activation and rescued the ventilatory phenotype. Additional elimination of TASK-2, a pH-sensitive K+ channel expressed in RTN neurons, essentially abolished the ventilatory response to CO2. The data identify GPR4 and TASK-2 as distinct, parallel and essential central mediators of respiratory chemosensitivity.
Central respiratory chemosensitivity refers to the homeostatic reflex by which brainstem circuits regulate breathing in response to changes in CO2 or its proxy, H+ (1, 2). This requires sensory cells that detect CO2 and/or H+, and promote adjustments in ventilation that regulate CO2 excretion and acid-base balance. A cluster of Phox2b (Paired-like homeobox 2b)-expressing excitatory neurons in the retrotrapezoid nucleus (RTN) subserve this chemosensory role (3–7). RTN neurons do not develop in Phox2b27Ala/+ mouse models carrying a human Phox2b mutation that causes congenital central hypoventilation syndrome (CCHS) (6, 7); like CCHS patients, these mice display disrupted central respiratory chemosensitivity, increased apneas, and impaired survival. Alkaline-activated TASK-2 (K2P5) background K+ channels mediate pH sensitivity in a subset of RTN neurons, but other molecular pH sensors appear to be required (8, 9).
In insects, receptors that couple via heterotrimeric guanine nucleotide binding proteins (GPCRs) are sufficient for CO2 chemosensation (10). We examined ventilatory responses to CO2 in conscious, unrestrained mice deleted for each of the mammalian proton-activated GPCRs – GPR4, GPR65 and GPR68 (11, 12). In mice lacking GPR65 and GPR68, the ventilatory effect of 5% CO2 was identical to that in wild-type mice (fig. S1). By contrast, in mice lacking GPR4 (in two genetic backgrounds), ventilation was decreased over a range of CO2 concentrations (by ~65% at 6% and 8% CO2; Fig. 1A, fig. S1 and S2). GPR4 is expressed in the carotid body (fig. S1), but the blunted ventilatory response to raised CO2 persisted in mice after bilateral transection of the carotid sinus nerve (fig. S1), and was observed under high O2 conditions that minimize activation of carotid body chemoreceptors (Fig. 1A). Mice lacking GPR4 retained a normal ventilatory response to hypoxia (Fig. 1B, fig. S1). There were no effects of GPR4 deletion on spontaneous activity or exploratory behavior (fig. S3) and mice lacking GPR4 had only a mild renal acidotic phenotype (table S1) (13), without any of the vascular abnormalities previously associated with inactivation of this receptor (12, 14). Finally, the incidence of spontaneous apneic events was ~6-fold higher in GPR4−/− mice (Fig. 1C, fig. S2). Thus, GPR4 deletion in mice approximates breathing deficits caused by selective genetic loss of RTN neurons (6, 7) and recapitulates two cardinal neurological features of CCHS: blunted central respiratory CO2 chemosensitivity and increased apneic episodes (6, 7, 15).
Figure 1. GPR4 deletion disrupts CO2-evoked ventilatory stimulation and RTN neuronal activation in vivo, and increases incidence of spontaneous apneas.
(A) Left, Respiratory flow recording from Jx-GPR4+/+ and Jx-GPR4−/− mice (cross between Jx-Phox2b-eGFP and GPR4 lines) with increased CO2 concentrations in the inspired air (balance O2). Right, Minute ventilation (VE) during incremental CO2 challenge in Jx-GPR4+/+ and Jx-GPR4−/− mice (n=16 & 52; †, P<0.0001 between genotypes by 2-way repeated measures (RM)-ANOVA; ****, P<0.0001 for pairwise comparisons).
(B) Jx-GPR4+/+ and Jx-GPR4−/− mice exhibited similar increases in peak VE during exposure to 10% O2 (n=16 & 52; ****, P<0.0001 hypoxia vs. normoxia, by 2-way RM-ANOVA).
(C) The frequency of spontaneous apneic events during quiet hyperoxic (100% O2) breathing was greater in Jx-GPR4−/− than in Jx-GPR4+/+ mice (n=23 & 8; *, P<0.05 by unpaired t-test).
(D) GPR4 expression by in situ hybridization in coronal brainstem section from an adult Jx-GPR4+/+ mouse. Inset is enlarged in a merged image; GPR4 mRNA is observed in 68.1 ± 5.1% of GFP+ neurons, n=6; counts do not exclude C1 neurons); no labeling was detected in sections from Jx-GPR4−/− mice. pyr: pyramid. Scale bar = 25 μm.
(E) Jx-GPR4+/+ and Jx-GPR4−/− mice were exposed to CO2 in vivo and activated RTN (GFP+:TH-) neurons (asterisks) identified by immunoreactivity for cFos (left, black reaction product in cell nuclei denoted by arrows) and GFP (right,). Scale bars = 50 and 25 μm.
(F) Upper: Parasagittal schematic of mouse brainstem depicting the RTN and surrounding neuronal populations, with areas containing Phox2b-expressing cells denoted in green. Lower: The number of RTN neurons activated by 8% CO2 challenge (cFos+:Phox2b+:TH-) was strongly reduced throughout the RTN in Jx-GPR4−/− mice, as compared to Jx-GPR4+/+ mice (n=5 & 6).
(G) The total number of RTN neurons counted (every 3rd section) from Jx-GPR4+/+ and Jx-GPR4−/− mice activated in response to 0% (n=4 & 4), 6% (n=5 & 5) or 8% CO2 (n=6 & 5). (P<0.0001, between genotypes by 2-way RM-ANOVA; **, P<0.01 and ****, P<0.0001 for pairwise comparisons).
We examined expression of GPR4 in the RTN neurons that are implicated in CCHS and central respiratory CO2 chemosensitivity (6, 7, 9). Multiplex in situ hybridization revealed strong GPR4 expression in a large fraction of Phox2b-expressing neurons throughout the rostrocaudal extent of the RTN (~68%, Fig. 1D); GPR4 expression was evident, but low, in some raphe neurons and undetectable elsewhere in the brainstem (Fig. 1D). We used a more sensitive multiplex single cell PCR assay in dissociated green fluorescent protein (GFP)-expressing RTN neurons (from Jx-Phox2b-eGFP transgenic mice) and found that nearly all RTN neurons expressed GPR4 (91%) and very few expressed other members of the receptor family (fig. S4). Expression of GPR4 was eliminated in GFP-positive RTN neurons from Jx-GPR4−/− mice (crossed with Jx-Phox2b-eGFP line) with no apparent compensatory up-regulation of other proton-activated GPCRs (fig. S4). Acute exposure of Jx-GPR4+/+ mice to CO2 (in hyperoxia) caused a concentration-dependent increase in cFos-immunoreactivity in RTN neurons (Fig. 1, E to G), indicative of neuronal activation in vivo (16, 17). By contrast, very few CO2-activated RTN neurons were found in Jx-GPR4−/− mice (Fig. 1, E to G). GPR4 expression was detected in C1 adrenergic and serotonergic raphe neurons (fig. S4), but there were no genotype-dependent differences in the small fraction of those neurons activated following CO2 exposure (fig. S5).
Activation of RTN neurons by CO2 and/or H+ can also be observed in brainstem slice preparations in vitro (18). Most GFP-expressing RTN neurons from wild-type mice (~90%) increased firing rate during extracellular acidification and decreased firing rate with bath alkalization (Fig. 2, A, C and D). In contrast to the nearly universal pH sensitivity of wild-type neurons (defined as >30% decrease in firing from pH 7.0 to pH 7.8; fig. S6), we found that ~40% of RTN neurons from Jx-GPR4−/− mice were pH insensitive; the remaining ~60% of GPR4-deleted neurons were pH sensitive, but they displayed lower firing rates across the pH range (Fig. 2, B to D). Under voltage clamp, a pH-dependent background K+ current was observed in pH-sensitive RTN neurons from both wild-type and Jx-GPR4−/− mice (3, 4) but this current was absent in the pH-insensitive population from Jx-GPR4−/− mice (Fig. 2, E and F). The residual pH-sensitive background K+ current is likely mediated by TASK-2, an intrinsically pH-sensitive K2P channel that is expressed in ~60–70% of RTN neurons from wild-type and GPR4-deleted mice (fig. S7) (8). However, it is unlikely that TASK-2 is the downstream effector for GPR4 because pH-dependent modulation of TASK-2 was unaffected by GPR4 signaling (fig. S7).
Figure 2. GPR4 deletion ablates pH sensitivity and a pH-sensitive background K+ current in a subset of RTN neurons in vitro.
(A, B) Effects of bath pH on firing activity (cell-attached mode) in representative GFP-expressing RTN neurons. A typical pH-sensitive from a GPR4+/+ mouse is depicted (A); in GPR4−/− mice (B), RTN neurons that were either pH-sensitive (upper) or pH-insensitive (lower).
(C) The percentage of pH-sensitive and pH-insensitive RTN neurons was significantly different between GPR4+/+ and GPR4−/− mice (**, P<0.0001 by χ2; n provided for each group).
(D) Averaged firing rates at different bath pH for RTN neurons from GPR4+/+ mice (n=71) and for RTN neurons from GPR4−/− mice that were identified as pH-sensitive (n=45) or pH-insensitive (n=32). *, P<0.05 for GPR4+/+ vs GPR4−/−:pH-sensitive; and †, P<0.05 for GPR4+/+ vs GPR4−/−: pH-insensitive, by 2-way RM-ANOVA with Dunnett’s test.
(E) Effects of bath acidification and alkalization on holding current (at −60 mV) and conductance during whole cell voltage clamp recordings in a pH-sensitive neuron from Jx-GPR4+/+ mouse (left) and a pH-insensitive neuron from a Jx-GPR4−/− mouse (right).
(F) I-V relationship of pH-sensitive difference current (pH 8 minus pH 7) for RTN neurons from GPR4+/+ mice (n=16) and from GPR4−/− mice that were identified in cell-attached recordings as pH-sensitive (n=11) or pH-insensitive (n=5). Data overlaid with fits using the Goldman-Hodgkin-Katz (GHK) equation for a “leak” K+ current. **, P<0.05 vs. GPR4−/−: pH-insensitive, by 2-way RM-ANOVA with Tukey’s test.
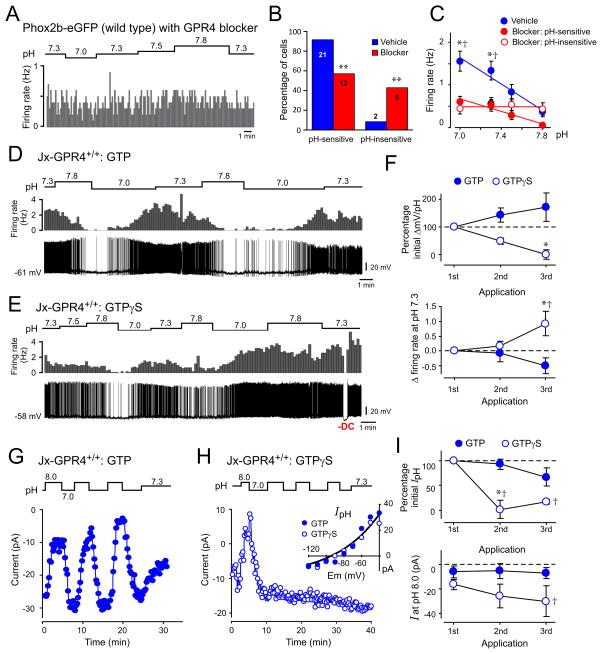
Incubation of brainstem slices from wild-type mice with a small molecule GPR4 receptor antagonist (19) yielded results similar to GPR4 deletion. A prominent group of blocker-treated RTN neurons was insensitive to changes in pH (~40%) whereas a second group retained their pH-sensitivity (~60%; Fig. 3, A to C); basal firing rate was also reduced in cells treated with the GPR4 receptor blocker (Fig. 3C). To test if G protein activation modulates the activity of wild-type RTN neurons, we performed whole cell recordings with pipettes containing either GTP or GTP-γ-S (a poorly-hydrolysable GTP analog that can mimic and irreversibly sustain G protein signaling). In the presence of GTP, repeated bouts of alkalization and acidification yielded reversible and reproducible changes in membrane potential and firing rate of RTN neurons (Fig. 3, D and F). However, RTN cells dialyzed with GTP-γ-S showed diminished membrane potential responses with repeated pH changes, accompanied by progressive membrane depolarization and increased baseline firing (Fig. 3, E and F). Likewise, the acid-induced inward shift in holding current showed little recovery or repeatability in neurons recorded with GTP-γ-S (Fig. 3, G to I). Strong membrane depolarization (or inward current) often occurred immediately upon whole cell access in neurons recorded with GTP-γ-S (n=8); after returning membrane potential and firing rate to normal levels with hyperpolarizing current injection (n=3), those GTP-γ-S-containing cells were insensitive to changes in pH. In addition, bath acidification and alkalization failed to evoke membrane depolarization and hyperpolarization in a subset of RTN neurons recorded with GDP-β-S (n=4/7), a GDP analog that interferes with receptor-stimulated GDP:GTP exchange.
Figure 3. GPR4 and G protein activation contribute to effects of pH on firing and background K+ currents in RTN neurons from wild-type mice.
(A) Firing activity in a wild-type RTN neuron incubated with GPR4 antagonist (Dalton M46, 20 μM); the cell was insensitive to changes in bath pH.
(B) The percentage of pH-sensitive and pH-insensitive wild-type RTN neurons was significantly different after treatment with the GPR4 blocker (**, P<0.0001 by χ2; n provided for each group).
(C) Averaged firing rate at different bath pH from vehicle-treated RTN neurons (n=21) or GPR4 blocker-treated cells that were identified as pH-sensitive (n=12) or pH-insensitive (n=9). *, P<0.05 for vehicle vs blocker:pH-sensitive; and †, P<0.05 for vehicle vs blocker:pH-insensitive, by 2-way RM-ANOVA with Dunnett’s test.
(D, E) Effects of repeated changes in bath pH on firing rate (upper) and membrane potential (lower) recorded from wild-type RTN neurons in the whole cell current clamp configuration with pipettes containing either GTP (D) or GTP-γ-S (E).
(F) Upper: Membrane hyperpolarization (% initial) during multiple bouts of bath alkalization in RTN neurons recorded with GTP and GTP-γ-S. Lower: Change in baseline firing (at pH 7.3) following repeated bath alkalization in RTN neurons recorded with GTP and GTP-γ-S. (2-way RM-ANOVA; *, P<0.05 for GTP vs. GTP-γ-S by Bonferroni test; and †, P<0.05 vs. 1st application by Dunnett’s test; n=8 & 4).
(G, H) Effects of repeated changes of bath pH on holding current in wild-type RTN neurons during whole cell voltage clamp recording with pipettes that contained GTP (G) or GTP-γ-S (H). Inset shows the pH-sensitive I-V obtained during the first sojourn from pH 8 to pH 7 for both cells, overlaid with a fit using the GHK equation.
(I) Normalized pH-sensitive current (pH 8 minus pH 7 at −60 mV, upper) and holding current at pH 8 (lower) during repeated bouts of bath acidification and alkalization. (2-way RM-ANOVA; *, P<0.05 for GTP vs. GTP-γ-S by Bonferroni test; and †, P<0.05 vs. 1st application by Dunnett’s test; n=7 & 3).
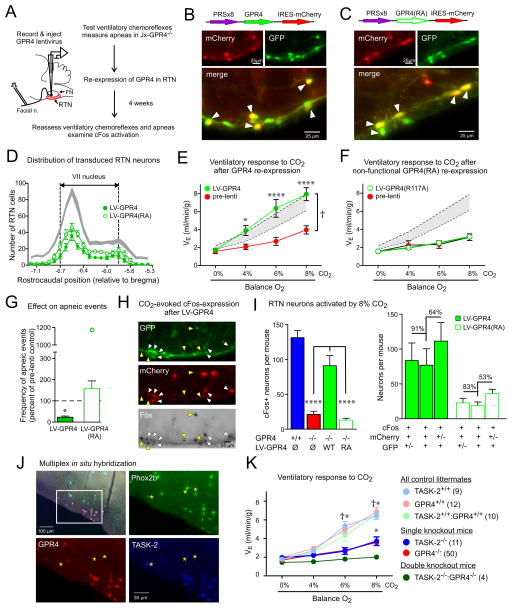
We tested whether targeted re-expression of GPR4 in Phox2b-expressing RTN neurons would rescue the defective respiratory phenotype of mice lacking GPR4. A lentiviral vector was prepared that employed a Phox2b-responsive promoter (PRSx8) to drive GPR4 expression in RTN neurons (5, 20); a control virus expressed GPR4(R117A), a mutated receptor that cannot engage downstream G-protein signaling (fig. S8) (21). Four weeks after virus injection into the RTN region of adult Jx-GPR4−/− mice (Fig. 4A), many RTN neurons and some nearby C1 neurons were transduced (Fig. 4, B to D, fig. S9). Nearly all virally-transduced cells expressed Phox2b (i.e., ~85% of mCherry+ cells were also GFP+). This RTN neuron-selective re-expression of GPR4 yielded a full rescue of the ventilatory response to CO2 (Fig. 4E). By contrast, the signaling-deficient mutant of GPR4 failed to rescue the breathing deficits (Fig 4F). Neither virus modified the hypoxic ventilatory response (fig. S9). An identical rescue was observed using a lentivirus in which GPR4 expression was controlled by a non-selective EF1α promoter (fig. S10). Re-expression of GPR4, but not GPR4(R117A), in RTN neurons reduced the number of apneic events in Jx-GPR4−/− mice (Fig. 4G) and restored CO2-induced cFos expression in RTN neurons (Fig 4, H and I). Virtually all the activated and transduced cells were Phox2b-expressing (~91%), and the majority of the activated RTN neurons were transduced (~64%) (Fig 4I); in a mouse that received a unilateral virus injection, cFos-labeled neurons were found exclusively on the injected side (fig. S11). Despite transduction of some C1 neurons, GPR4 re-expression did not induce CO2-driven cFos expression in those cells (fig. S9). Thus, RTN-selective re-expression of GPR4 in Jx-GPR4−/− mice reinstated CO2-dependent neuronal activation and increased ventilation, and reduced the incidence of apneas.
Figure 4. Re-expression of GPR4 selectively in Phox2b-expressing neurons of the RTN restores CO2-dependent neuronal activation and breathing in Jx-GPR4−/− mice.
(A) Schematic of experimental design.
(B, C) White arrows denote virally-transduced (mCherry+) and Phox2b-expressing (GFP+) RTN neurons after injection of PRSx8-lentivirus driving expression of GPR4-mCherry (B) or GPR4(R117A)-mCherry (C).
(D) Similar number and distribution of RTN neurons transduced with GPR4-mCherry (n=8) and GPR4(R117A)-mCherry (n=7) lentivirus. Shaded area represents 95% confidence interval for distribution of RTN (GFP+/TH-) neurons across all virus-injected animals (n=15).
(E, F) Effect of CO2 on minute ventilation in Jx-GPR4−/− mice before (pre-lenti) and 4 weeks after lentiviral injection into the RTN (E: GPR4-mCherry, n=8; or F: GPR4(R117A)-mCherry, n=7). Shaded areas are 95% confidence intervals for data from Jx-GPR4+/+ mice (from Fig. 1A). (†, P<0.0001 by 2-way RM-ANOVA, comparing mice before and following lentiviral delivery; *, P<0.05, ****, P<0.0001, pairwise comparisons at each CO2 level by Bonferroni test).
(G) The frequency of spontaneous apneic events was reduced following injection of GPR4-mCherry lentiviral vector into RTN (n=8; *, P<0.05 by t-test, ~22% of pre-injection control) but not GPR4(R117A)-mCherry (n=10, P>0.18 by t-test). Note the outlying data point from one mouse that received GPR4(R117A)-mCherry.
(H) Many virally-transduced (mCherry+) RTN neurons (GFP+) were activated (cFos+) by 8% CO2 exposure (white arrows) after injection of PRSx8-lentivirus expressing GPR4 into RTN of Jx-GPR4−/− mice; CO2-activated GFP+ neurons that were not detectably transduced were also observed (yellow arrows).
(I) Left: CO2-activated cFos expression in RTN neurons was rescued following injection of PRSx8-GPR4 but not PRSx8-GPR4(R117A) lentivirus (n=4 & 7, P<0.0001 by 1-way ANOVA, ****, P<0.0001 by pairwise comparison); data from Jx-GPR4+/+ and Jx-GPR4−/− mice are re-plotted from Fig. 1G. Right: CO2-activated, transduced RTN neurons (cFos+/mCherry+/GFP+) in comparison to all CO2-activated, transduced neurons (cFos+/mCherry+) and all CO2-activated RTN neurons (cFos+/GFP+).
(J) Co-expression of Phox2b, GPR4 and TASK-2 in RTN neurons by in situ hybridization; boxed region enlarged in merged image. A few TASK-2-expressing Phox2b+ neurons without GPR4 are indicated (asterisks). Among Phox2b+ neurons that expressed GPR4 and/or TASK-2, 72.9 ± 0.8% expressed both genes, 18.1 ± 1.0% were labeled only for TASK-2 and 9.0 ± 0.2 only for GPR4 (n=3).
(K) Minute ventilation during incremental CO2 challenge for the indicated genotypes. †, all controls greater than all single or double knockouts, P<0.0001; *, both single knockouts greater than double knockouts (n values provided), by 2-way RM-ANOVA with Bonferroni pairwise comparisons.
The preferential brainstem localization of GPR4 to RTN neurons is similar to that of the pH-sensitive TASK-2 channel (8, 9). Indeed, we found extensive co-expression of both GPR4 and TASK-2 in Phox2b-expressing RTN neurons (Fig. 4J). We therefore examined effects of simultaneous genetic ablation of both GPR4 and TASK-2. Of >350 pups obtained by breeding mice heterozygous at both loci, 4 males survived past weaning (from 13 total double knockouts). For those surviving animals, the effect of CO2 on ventilation was nearly completely suppressed (by ~85%, Fig. 4K), suggesting independent contributions of GPR4 and TASK-2.
Our data demonstrate that GPR4 is required for the normal ventilatory response to CO2, and that this reflects its selective expression in Phox2b-expressing RTN chemosensory neurons where it contributes to neuronal excitability and pH sensitivity. GPR4 thus provides proton-sensing capability in chemoreceptor neurons that acutely maintain physiological acid-base balance via regulation of breathing (1, 2). The signaling pathway and channel downstream of GPR4 remain to be determined, but it appears that GPR4 and TASK-2 provide independent molecular pH sensors in overlapping populations of RTN chemosensitive neurons (8), and thus redundancy for this vital function. Astrocytic purinergic signaling appears to represent yet another distinct mechanism for modulating CO2-activated RTN activity (22, 23), since effects of gliotransmitters such as ATP or glutamate do not likely involve GPR4 (fig. S8).
Mice lacking GPR4 recapitulate two cardinal features of CCHS patients – depressed respiratory chemosensitivity and increased apneas (15) – and both defects were rescued after selective GPR4 re-expression in a subset of Phox2b-expressing RTN neurons. Thus, reduced excitability and chemo-responsiveness in RTN neurons, effects more subtle than the outright genetic ablation of those cells observed in CCHS models (6, 7, 9) might predispose individuals to respiratory problems associated with reduced chemosensitivity (e.g., central sleep apnea, apnea of prematurity, sudden infant death syndrome) (24, 25), and activation of GPR4 in RTN neurons might provide a therapeutic option for these common respiratory ailments.
Supplementary Material
Acknowledgments
The authors are grateful to members of the Bayliss and Guyenet laboratories, and to Dr. Q. Al-Awqati for useful suggestions on the studies. They thank Shaofang Shu for technical assistance, Dr. R.L. Stornetta for advice on in situ hybridization, Dr. M.P. Beenhakker for the MATLAB routine used for Poincaré analysis and Drs. K.S. Ravichandran and P.Q. Barrett for comments on the manuscript. SBGA was supported by a National Health and Medical Research Council of Australia Early Career Fellowship (GNT1052674). The use of ZIRP Core Facility for Rodent Phenotyping is acknowledged. The work was supported by the Swiss National Science Foundation (31003A_138143, CAW) and the National Institutes of Health (HL074011, PGG; HL108609, DAB).
References and Notes
- 1.Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulkey DK, et al. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7 doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA. Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2. J Neurosci. 2013;33:7756–7761. doi: 10.1523/JNEUROSCI.5550-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott SBG, et al. Photostimulation of retrotrapezoid nucleus Phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubreuil V, et al. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramanantsoa N, et al. Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci. 2011;31:12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, et al. TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci. 2013;33:16033–16044. doi: 10.1523/JNEUROSCI.2451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gestreau C, et al. TASK2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci U S A. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones WD, Cayirlioglu P, Grunwald Kadow I, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig MG, et al. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 12.Wyder L, et al. Reduced pathological angiogenesis and tumor growth in mice lacking GPR4, a proton sensing receptor. Angiogenesis. 2011;14:533–544. doi: 10.1007/s10456-011-9238-9. [DOI] [PubMed] [Google Scholar]
- 13.Sun X, et al. Deletion of the pH sensor GPR4 decreases renal acid excretion. J Am Soc Nephrol. 2010;21:1745–1755. doi: 10.1681/ASN.2009050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang LV, et al. Vascular abnormalities in mice deficient for the G protein-coupled receptor GPR4 that functions as a pH sensor. Mol Cell Biol. 2007;27:1334–1347. doi: 10.1128/MCB.01909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weese-Mayer DE, et al. An official ATS clinical policy statement: Congenital central hypoventilation syndrome. Am J Respir Crit Care Med. 2010;181:626–644. doi: 10.1164/rccm.200807-1069ST. [DOI] [PubMed] [Google Scholar]
- 16.Teppema LJ, et al. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Fortuna MG, Stornetta RL, West GH, Guyenet PG. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J Physiol. 2009;587:5121–5138. doi: 10.1113/jphysiol.2009.176875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazarenko RM, et al. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong L, et al. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS ONE. 2013;8:e61991. doi: 10.1371/journal.pone.0061991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang DY, Carlezon WA, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum Gene Ther. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- 21.Chen A, et al. Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/Epac pathway. PLoS ONE. 2011;6:e27586. doi: 10.1371/journal.pone.0027586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gourine AV, et al. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenker IC, Kréneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1–Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia AJ, III, Koschnitzky JE, Ramirez J-M. The physiological determinants of Sudden Infant Death Syndrome. Respir Physiol Neurobiol. 2013;189:288–300. doi: 10.1016/j.resp.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antic NA, et al. PHOX2B mutation–confirmed Congenital Central Hypoventilation Syndrome. Am J Respir Crit Care Med. 2006;174:923–927. doi: 10.1164/rccm.200605-607CR. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.