Abstract
Introduction
The World Health Organization (WHO) and other health agencies have concluded that yellow fever booster vaccination is unnecessary since a single dose of vaccine confers lifelong immunity.
Areas Covered
We reviewed the clinical studies cited by health authorities in their investigation of both the safety profile and duration of immunity for the YFV-17D vaccine and examined the position that booster vaccination is no longer needed. We found that antiviral immunity may be lost in 1-in-3 to 1-in-5 individuals within 5 to 10 years after a single vaccination and that children may be at greater risk for primary vaccine failure. The safety profile of YFV-17D was compared to other licensed vaccines including oral polio vaccine (OPV) and the rotavirus vaccine, RotaShield, which have subsequently been withdrawn from the US and world market, respectively.
Expert Commentary
Based on these results and recent epidemiological data on vaccine failures (particularly evident at >10 years after vaccination), we believe that current recommendations to no longer administer YFV-17D booster vaccination be carefully re-evaluated, and that further development of safer vaccine approaches should be considered.
Keywords: Yellow fever virus, neurotropic, viscerotropic, vaccination, flaviviruses, antibody duration, immunity
1. Introduction
Yellow fever virus (YFV) represents an important mosquito-borne human pathogen that is endemic in approximately 40 countries in sub-Saharan Africa and South America [1]. Although disease may be mild in some instances, clinical presentation often involves severe acute onset with fever, nausea, vomiting, hepatitis, hemorrhage and renal failure. The case fatality rate (CFR) for severe cases of yellow fever ranges from 20–60% [2,3]. There are no licensed antiviral drugs to treat yellow fever and prevention or reduction of disease burden is mainly accomplished through vaccination as well as through vector control measures.
The live, attenuated YFV-17D vaccine was developed in 1936 and although there are two main substrains that are used in commercial manufacturing (17D and 17DD), there appear to be no major differences in safety or immunogenicity between YFV-17D vaccines [2,4–6]. Yellow fever vaccination is recommended for people living in endemic areas and for travelers who may visit endemic areas with the notable exception of infants <9 months of age and people with HIV, thymus disease, or other immunodeficiencies, since these represent vulnerable populations in which yellow fever vaccination is contraindicated [7]. For many years, YFV-17D has been considered “one of the safest vaccines in the world” [8] and has often been referred to as a “gold standard” among vaccines due to its remarkable ability to induce lifelong immunity. Based on this premise, several heath advisory groups convened with the task of determining whether the recommendation to administer booster vaccinations at 10-year intervals is needed. In April 2013, the Strategic Advisory Group of Experts (SAGE) on Immunization, the primary advisory counsel to the World Health Organization (WHO), concluded that a single dose of YFV-17D vaccine provided lifelong protection and that no booster dose was needed [9,10]. In May 2014, the World Health Assembly adopted this change in guidance and the requirement for a booster dose to be administered at 10-year intervals is expected to be removed from the International Health Regulations in 2016. Evidence pertaining to the potential risks and benefits of yellow fever vaccination were evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework [11] and in February 2015, the Advisory Committee on Immunization Practices (ACIP) reviewed these documents and voted to approve the use of only a single dose of vaccine for most travelers [12].
The change in recommendations for YFV-17D vaccines has been controversial and several groups have questioned the decision by these health agencies to no longer recommend booster vaccination [13–15]. Indeed, some endemic countries such as Brazil have indicated that they will continue to recommend at least one booster vaccination among the endemic population as well as continue to recommend booster vaccination for travellers who plan to visit other yellow fever-endemic countries [16,17]. The risks of rare but serious side effects following YFV-17D vaccination (including the risk of vaccine-related hospitalization and death that mainly occur during primary vaccination) must be weighed in proportion to the potential risks to individuals in yellow fever endemic countries who have primary vaccine failures (i.e., lack of seroconversion after vaccination) or whose antiviral immunity wanes to below the protective threshold. Here, we have reviewed the literature on YFV-17D vaccine safety and immune memory that was used to justify the removal of booster vaccination from the YFV-17D schedule. Our review of these documents indicates that although live attenuated YFV-17D vaccines are not completely safe, they are needed for control of yellow fever disease burden in endemic areas and the recommendation for booster vaccination should be reconsidered in order to protect those with primary vaccine failures as well as those whose immunity has declined to non-protective levels. Based on the rapid loss of immunity in a subpopulation of vaccine recipients, it should be considered whether the first booster dose of vaccine be administered within 5 years after primary vaccination instead of 10 years after vaccination as previously proposed.
2. Can memory T cells provide protective immunity against reinfection?
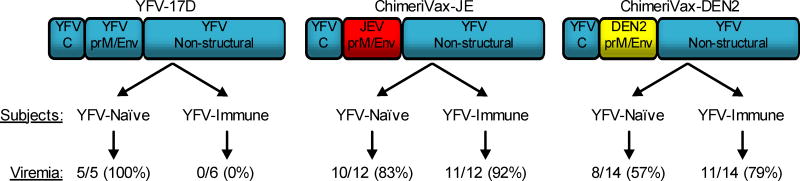
YFV-17D is a highly immunogenic vaccine, resulting in strong innate immunity [18–21], antiviral T cell immunity [22–26] and neutralizing antibody responses [27–29]. Before one can estimate the duration of immunity after YFV-17D vaccination, it is important to determine whether memory T cells or pre-existing antibodies play the predominant role in protection against infection. When the CDC concluded that YFV-17D vaccination induced lifelong immunity, this was based, at least in part, on the potential role of T cells to provide protection in the absence of neutralizing antibody responses. In the Conclusions section of GRADE Table 3 [11], the authors noted, “Further evidence suggests that even with no detectable neutralizing antibodies, immunity might be given due to cell-mediated protective effects”. Niedrig et al. [27] and Reinhardt et al. [30] were cited as evidence for T cells demonstrating a protective role in antiviral immunity. However, the Niedrig study performed no T cell analysis, and concluded their paper by stating, “The relative contributions of cellular and humoral immunity mediating protection against YF infection are not known. So far our data indicate that NT [neutralizing titer] results are the most appropriate parameters for monitoring 17D vaccine efficacy” [27]. The Reinhardt study did not directly measure YFV-17D-specific T cells but only measured the frequency of total circulating CD8+ T cells in the blood after primary or secondary vaccination [30]. In their discussion, they mention that the early expansion of T cells during primary YFV-17D infection, “strongly suggested that CD8+T cells were involved in the primary immune reaction to YF vaccination”. However, this observation cannot distinguish between a coincidental relationship or a causal relationship in CD8+ T cell responses and clearance of YFV-17D viremia. YFV-17D induces T cell responses to all viral proteins with strong T cell responses directed towards non-structural proteins [23,24]. However, the presence of T cell memory does not necessarily equate to the presence of protective T cell-mediated immunity. Fortunately, this question has been resolved by comparing YFV-17D to chimeric flaviviruses based on the YFV-17D backbone [31–34]. The relative role of vaccine-mediated T cell memory has been elegantly determined in two clinical studies involving YFV-17D and chimeric versions of YFV-17D in which the neutralizing epitopes of the envelope and PrM proteins of YFV-17D were replaced by the envelope and PrM proteins from dengue virus serotype 2 [31] or Japanese encephalitis virus (JEV) [32] (Figure 1). When YFV-naïve subjects were inoculated with YFV-17D, ChimeriVax-JE (recombinant YFV-17D expressing JEV-specific neutralizing epitopes) or ChimeriVax-DEN2 (recombinant YFV-17D expressing dengue serotype 2-specific neutralizing epitopes), viremia was observed in 100%, 83%, and 57% of subjects, respectively. This shows that each of these viruses is capable of inducing transient, systemic viral infection in a naïve human host. In contrast, when YFV-17D-immune subjects were inoculated with one of these three different viruses (Figure 1), there was complete protection against YFV-17D replication (0% of subjects with detectable viremia) whereas ChimeriVax-JE and ChimeriVax-DEN2 viruses replicated as efficiently in YFV-immune subjects as that observed in YFV-17D-naïve subjects (92% and 79% viremia, respectively). If memory T cells are able to control YFV infection, then YFV-immune subjects should have controlled infection by the ChimeriVax-JE and ChimeriVax-DEN2 viruses since 8/10 of the proteins (including all of the nonstructural proteins) are from YFV-17D and therefore have identical T cell epitopes. Instead, these studies indicate that in the absence of YFV-17D-specific neutralizing antibodies, the pre-existing YFV-17D-specific memory T cell response is unable to provide protection against repeat infection. Indeed, area-under-the-curve (AUC) analysis showed that there was no reduction in the peak or duration of viremia and there was no evidence that YFV-17D-specific T cells reduced disease symptoms after infection (reviewed in [34]). In contrast, passive transfer of YFV-specific immune serum provides protective immunity against lethal YFV infection in mice [28,35,36], hamsters [37], and rhesus macaques [35]. Together, these studies indicate that neutralizing antibodies are both necessary and sufficient for protection against yellow fever [33]. This also indicates that the assumption that memory T cells might play a role in protection against yellow fever in seronegative vaccinees [11] is unsubstantiated and one should use caution before using this as evidence to support changing the YFV-17D vaccine schedule.
Figure 1. YFV-17D-specific T cells fail to protect against infection in the absence of neutralizing antibodies.
Clinical studies were conducted to determine if pre-existing YFV-specific immunity would impact virus replication upon challenge with YFV-17D or chimeric versions of YFV-17D in which the prM (pre-membrane) and envelope (Env) proteins of YFV-17D were replaced with either JEV [32] or DENV2 [31]. These recombinant viruses, ChimeriVax-JE and ChimeriVax-DEN2, were composed of the YFV-17D capsid (C) structural protein and 7 YFV-17D non-structural proteins, but could no longer be neutralized by YFV-17D-specific antibodies. This provided the opportunity to determine the impact of T cell-mediated protection in the absence of a neutralizing antibody response. YFV-17D infection elicits T cells to both structural and nonstructural proteins [23,24] but pre-existing antiviral T cell memory had no measurable impact on the peak or duration of viremia as determined by area-under-the-curve measurements [31,32,34].
3. Yellow fever correlate of immunity and duration of antibody responses
Rhesus macaques are highly susceptible to yellow fever and the correlate of protective immunity was first identified by vaccinating rhesus macaques with graded doses of live YFV-17D vaccine and measuring neutralizing antibody responses prior to lethal challenge with virulent YFV-Asibi [38]. At 20 weeks after vaccination, all but one of the vaccinated animals with a pre-existing log neutralizing index (LNI) of ≥0.7 were protected against YFV-Asibi challenge whereas animals with titers of <0.7 LNI were susceptible to YFV-Asibi infection. The LNI procedure requires two serum samples (pre-challenge and post-challenge) and is measured using a constant-serum, diluted-virus approach in which the neutralizing activity of the immune serum is calculated after subtracting the neutralizing activity of pre-challenge serum. This approach is feasible in the setting of prospective clinical trials but is difficult to perform when studying long-term immunity since pre-vaccination serum samples are often not available, especially when examining immune responses more than 10 years after vaccination. For this reason, none of the studies cited by the CDC [11], WHO [10], or ACIP [12] used LNI to determine the durability of vaccine-mediated immunity after YFV-17D vaccination. Instead, researchers used other approaches such as mouse protection studies or an in vitro constant-virus, diluted-serum approach in which the neutralizing titer is the reciprocal of the dilution of serum that reduces infectious virus by a pre-specified percentage (e.g., 50% plaque reduction neutralizing titer is designated as PRNT50 and focus reduction neutralizing titer is designated FRNT50). Studies involving adoptive transfer of YFV-immune serum into naïve hamsters have provided further insight into the level of neutralizing antibodies that are required for protection against lethal YFV infection [37]. Animals with pre-existing PRNT50 titers of ≥40 were fully protected against viremia and death after YFV challenge whereas animals with PRNT50 titers of 10 to 20 showed breakthrough of viremia although 83% to 88% of animals were still protected from YFV-associated mortality, respectively. We have tested an experimental hydrogen peroxide-inactivated YFV vaccine in rhesus macaques and our preliminary data also indicates that a pre-existing PRNT50 titer of >20 is protective against lethal yellow fever (Authors, unpublished data). Although more studies are needed, a PRNT50 >20 may represent an immunological correlate of immunity in cases wherein measuring LNI is unfeasible.
The WHO [10], ACIP [12] and CDC [11] reports utilized similar references in their deliberations but the CDC report provided the most comprehensive list of studies that measured long-term immunity after YFV-17D vaccination. They found that an average of 88% of subjects remain seropositive at ≥10 years after YFV-17D vaccination and this represents one of the main pieces of evidence for the decision to no longer require booster vaccination. Of the 13 studies included in Table 3 of the CDC publication (references [13,27,28,30,39–46] and an unpublished analysis of CDC Arbovirus laboratory testing), there were 4 that used mouse protection assays to determine the serostatus of the vaccinated individuals. It remains unclear how mouse protection experiments relate to protective immunity in humans since an early study demonstrated that mice could be protected by transfer of immune human serum that was non-protective in the more clinically relevant rhesus macaque model [35]. Of the remaining 9 studies that measured neutralizing titers at ≥10 years after YFV-17D vaccination, 4 were performed in endemic countries [13,40,41,45] and 5 were performed in non-endemic countries (references [27,28,30,46] and an unpublished analysis of CDC Arbovirus laboratory testing). When stratified into these two groups, 97.6% (359/368) of subjects from YFV-endemic areas maintained detectable neutralizing antibodies whereas only 83.7% (264/337) of subjects who lived in non-endemic countries remained seropositive. In addition to technical differences in measuring neutralizing antibody levels (e.g., PRNT50, PRNT75, PRNT80, PRNT90), there could be additional confounders such as age, race, and gender differences, etc. However, the disparity in protective levels observed between these two groups indicates that it may not be prudent to include the data from endemic countries for estimating the inherent duration of vaccine-mediated immunity after YFV-17D immunization due to the potential for periodic boosting of immunity via exposure to yellow fever or other potentially cross-reactive flaviviruses. Most importantly, these results indicate that on average, nearly 1 in 5 subjects from non-endemic areas may lose measurable antibody responses within 10 or more years after vaccination.
Two studies [27,28] cited by the CDC [11] as evidence of lifelong immunity after YFV-17D vaccination are particularly informative. One of the studies in Table 3 of the CDC report [11] was published by Poland et al. in a non-endemic country [28] and 91/116 subjects (78%) were considered seropositive by the CDC based on having a neutralizing titer (PRNT90) of ≥2. However, serum samples with a neutralizing titer of 2 or even 4 were inconsistent in mouse protection studies (providing only 33–50% protection), making it difficult to know if these neutralizing titers should be considered above a protective threshold. Neutralizing titers of ≥16 consistently protected mice [28] and based on more recent PRNT50 studies in hamsters [37] these levels are more likely to be above a protective threshold. If this more conservative cutoff value is used, then the long-term serostatus among vaccinated subjects drops from 78% seropositive [11] to 62.9% seropositive [28].
Another study from Table 3 of the CDC report [11] by Niedrig et al. [27] provides a rich source of information regarding the kinetics and durability of neutralizing antibody responses from a few weeks to up to 35 years after YFV-17D vaccination (Figure 2). Their data shows that sera with a neutralizing titer of >1:10 decreased from 94% in the first year after vaccination to 74.5% after 10 years. Upon closer examination of the dataset (Figure 2), it appears that immunity declines rapidly during the first 1–4 years after vaccination and if the samples from 5–35 years post-vaccination are combined, then only 69% (51/74) of subjects retain neutralizing antibodies of >1:10. This rapid decline in YFV-specific antibodies shortly after vaccination is supported by another study based on the long-term analysis of neutralizing antibody responses after YFV-17D booster vaccination of laboratory workers at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) [47]. Laboratory researchers with potential risk of exposure were screened for neutralizing antibodies and revaccinated if their titers declined to below 1:40 or (as of 1996) 1:20. The decay rate kinetics of YFV-specific neutralizing antibody responses were determined in 1,029 laboratory personnel. The antibody decay curves indicated that neutralizing antibody titers dropped below 40 within 3–4 years for approximately half of the subjects after booster vaccination and the authors noted that vaccine recipients with a low pre-booster titer declined to below 40 within 3 years. These independent studies [27,47] together indicate that a sizeable proportion of vaccinated individuals may lose antiviral immunity in less than 5 to 10 years after a single YFV-17D vaccination.
Figure 2. Neutralization titers after yellow fever vaccination with 17D (n = 209).
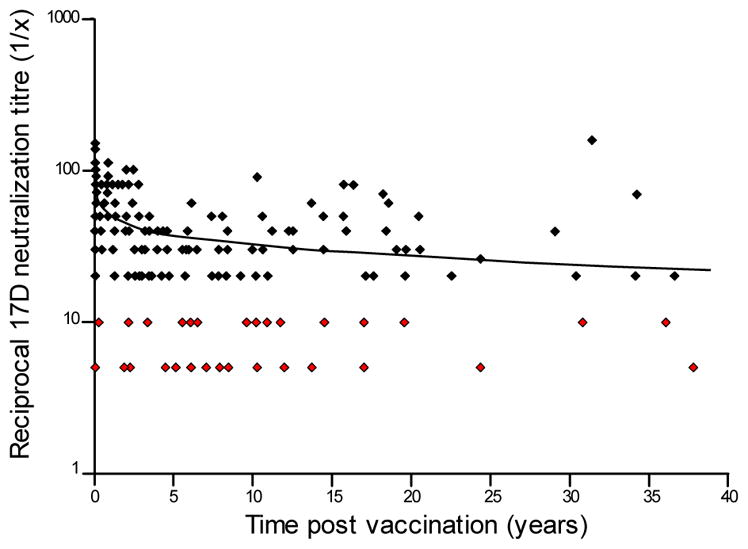
Serum plaque reduction neutralizing titers (PRNT90) were determined at the indicated time points following vaccination with YFV-17D. Neutralizing titers of >1:10 indicated a protective level of immunity. Neutralizing titers of 1:10 were considered borderline and titers of <1:10 were considered negative and both are highlighted in red. Adapted with permission from [27].
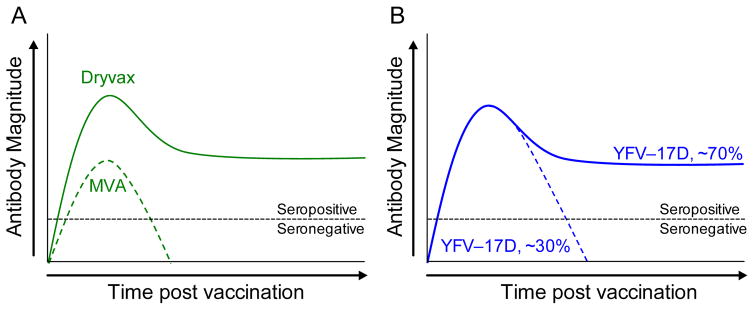
Why is it that lifelong immunity is retained for decades after viral infection in some cases, whereas in others, immunity is rapidly lost? We believe that this may be due to differences in host:pathogen interactions in which viruses that are cleared too quickly may not replicate sufficiently to prime the host immune system to generate long-term immunity (Figure 3). For example, the immune responses elicited by two live smallpox vaccine strains, Dryvax and Modified Vaccinia-Ankara (MVA), have very divergent outcomes. Unmodified strains of vaccinia virus such as Dryvax are able to replicate at the site of inoculation for several days to several weeks, with the viral infection typically cleared at the time that the vaccination scab detaches from the skin. Similar to yellow fever, virus-specific T cell memory is unlikely to protect against orthopoxvirus infection [48] but smallpox vaccination elicits antiviral T cell responses that can be detected for decades [49,50] and antibody responses that are maintained with a 72 year half-life [51]. In contrast, MVA is a further-attenuated, replication-deficient strain of vaccinia that is unable to establish an acute infection in humans and is therefore rapidly cleared. Antiviral immune responses to this over-attenuated strain of vaccinia are lower than that achieved with wild-type vaccine strains of replication-competent vaccinia (Figure 3A) and antiviral immunity declines sharply even after 2 or 3 doses of vaccine have been administered [52,53]. For example, within 6 months after a second vaccination as few as 35–54% of subjects maintain a measurable neutralizing antibody titer of PRNT50 ≥10 [52]. Despite reaching peak geometric mean neutralization titers of up to 77.6 shortly after booster vaccination, by the 6 month time point the antiviral antibody titers of the remaining seropositive subjects ranged from 11.7 to 10.2 which is near the limit of detection (PRNT50 = 10). Similar results were found in an earlier study in which 71% of subjects seroconverted after two MVA immunizations but only 28.8% maintained neutralizing antibody responses by 2 months after booster vaccination [53]. In contrast to these viruses, in which the difference in host-pathogen interactions are clearly due to differences in vaccine strain attenuation, the vaccine strains of YFV-17D are genetically stable viruses [54] and show similar safety and immunogenicity profiles [2,4–6]. It is therefore unlikely that the long-lived immunity or short-lived immunity observed among different vaccinated individuals (Figure 2) is due to differences in vaccine virus strains. Indeed, even under circumstances in which lethal viscerotropic disease after YFV-17D vaccination has occurred, sequencing of the virus from blood samples has shown that it maintains its genetic identity and does not typically revert to a virulent phenotype [8,54,55]. In this scenario, we postulate that the differences in immunological memory following yellow fever vaccination (Figure 3B) are more likely to be linked to rates of viral clearance that are associated with differences in host susceptibility. The initial viral load after YFV-17D vaccination varies between human subjects [56] and below a certain threshold the level of systemic viral replication determines the peak in effector CD8+ T cell responses. It is plausible that the extent and duration of viral replication may also impact the peak and duration of antiviral antibody responses as well (similar to smallpox vaccines, Figure 3A). Interestingly, YFV-17D may persist in other organs for a longer period of time than one might predict based on measuring serum viremia. One study found YFV-17D genomic RNA excreted in the urine of vaccinated individuals at time points in which paired serum samples taken at the same time were negative [57]. Moreover, viral RNA could be detected for up to 1–3 weeks after vaccination in a subpopulation of vaccinated individuals and positive urine samples appeared to be more common among those with YFV-17D-associated adverse events. Taken together with the clinical data on yellow fever showing clear examples of both short-term and long-term immunity after vaccination (Figure 2) [27,28,47] and what is known in terms of the durability of antiviral antibody responses with other viruses that are either rapidly cleared (e.g., MVA) vs. those that mount an acute viral infection that is cleared within a few weeks (e.g., Dryvax) (Figure 3A) [58], we propose the following model (Figure 3B): Following YFV-17D vaccination, >95% of adults mount neutralizing antibody responses above the protective threshold of ≥0.7 LNI [59]. Among ~70% of YFV-17D-vaccinated individuals, the magnitude and duration of virus replication is sufficient to induce long-lived plasma cells and antibody responses that are maintained essentially for life. In contrast, approximately 30% of vaccinated individuals may clear the viral infection too quickly, resulting in reduced antigenic load and instead of inducing lifelong immunity, antiviral antibodies wane to below the protective threshold within 5–10 years. Although this is only a hypothesis at this point, we believe that this represents an area worthy of further investigation.
Figure 3. Models of host-pathogen interactions and potential impact on long-term immunity.
Live virus vaccines may elicit different patterns of serum antibody maintenance based on pathogen-specific or host-specific differences in replication and overall antigen load. (A) The smallpox vaccines, Dryvax and MVA (Modified Vaccinia Ankara), are closely related strains of vaccinia virus that differ in their attenuation and ability to replicate in the human host. Dryvax is able to replicate locally in the skin for several days or weeks after inoculation and this induces a long-lived immune response in the majority of vaccine recipients [49,50]. MVA is replication-deficient and the virus is rapidly cleared, resulting in lower antibody responses that decay more rapidly than that observed with Dryvax [52,53]. (B) YFV-17D vaccine strains of virus elicit a viremic infection of variable magnitude and duration [56]. Approximately 70% of vaccine recipients achieve long-term, potentially lifelong immunity whereas ~30% of individuals lose antiviral antibody responses within 5 to 10 years after vaccination [27]. Since YFV-17D is genetically stable, one hypothesis is that differences in host susceptibility to YFV-17D infection represent an underlying factor that influences the magnitude and duration of the acute viral infection, and in turn, the differences in viral replication and systemic antigen load result in either short-term or long-term antiviral immunity.
4. YFV immunology, epidemiology and evidence of waning protective immunity
In determining that one dose of yellow fever vaccine induces lifelong immunity, the WHO provided evidence that there have been only 12 reported vaccine failures out of >540 million doses of YFV-17D vaccine administered [9,10]. This remarkable statistic was revised slightly upwards by the CDC to 23 reported failures, but further modified to only 18 out of >540 million doses because 5 cases of yellow fever occurred <10 days after vaccination, and this may have been too early for vaccine-mediated protection to have formed [11]. These statistics were subsequently referenced by the ACIP publication on YFV-17D vaccine booster recommendations [12]. This finding is surprising, but also somewhat difficult to support since no vaccine is 100% effective and a small proportion of vaccine failures will leave a proportion of individuals at risk for subsequent infection. Clinical trials involving YFV-17D vaccines produced by two different manufacturers found that between 98.6% and 99.3% of adult subjects elicit neutralizing antibody responses above the protective threshold of ≥0.7 LNI within 31 days after vaccination [59]. If we consider that primary YFV-17D vaccination is 99% effective, then one might have predicted 5 million primary vaccine failures (based on failure to seroconvert) among 500 million doses of administered vaccine.
If one uses the meta-analysis performed by the CDC in reference to long-term immunity after YFV-17D vaccination, then 88% of vaccinated individuals will remain seropositive [11]. If we round this up to 90%, then one would have anticipated 50 million secondary vaccine failures (defined as seropositive subjects who become seronegative) among more than 500 million doses of administered YFV-17D vaccine. As noted earlier (Figure 2 and Figure 3), based on immunological memory studies performed in non-endemic areas ([27,28,30,46] and an unpublished analysis of CDC Arbovirus laboratory testing) it is likely that immunity is lost in 30–40% of individuals within 5–10 years after YFV-17D vaccination and this would further increase the susceptible population. HIV infection is known to interfere with the development of vaccine-mediated immunity and one study showed that only 83% of HIV+ individuals were seropositive less than one year after YFV-17D vaccination compared to 97% seropositivity in HIV− individuals [60]. Further, among the 102 HIV+ patients studied, there were 11 who initially seroconverted but became seronegative after a median of only 1.8 years after vaccination (range; 0.9–5.1 years), indicating that HIV+ status may profoundly impact the durability of antiviral immunity after YFV-17D vaccination. Importantly, seroconversion rates are also lower among children after YFV-17D vaccination (88% within 1–2 months after primary YFV-17D vaccination; [11]). A large clinical trial involving 1,966 children comparing 17D-213/77 and 17DD vaccines found seroconversion rates of only 84.8% and 85.8%, respectively [4]. However, among children aged 12 months or older, the seroconversion rate was only 69% when concomitant vaccination with measles, mumps, and rubella (MMR) was performed [4]. These results are supported by prior studies in which only 552/792 children (70%) seroconvert after YFV vaccination when administered with MMR vaccination [6]. Based on these two large independent studies that clearly demonstrate poor seroconversion rates among children after primary vaccination, the recommendation to only administer a single dose of YFV-17D vaccine should be reconsidered.
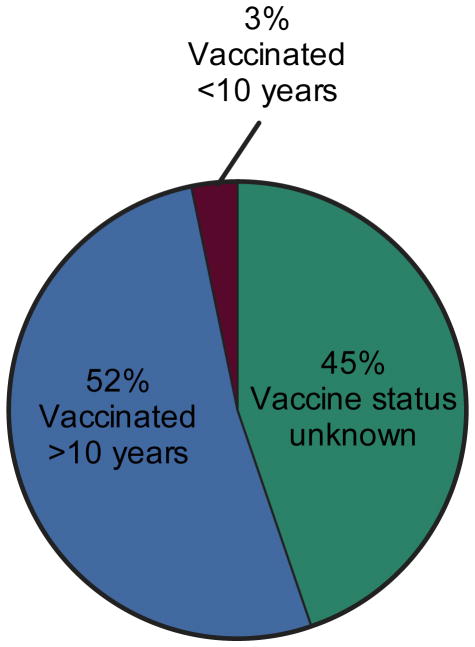
After examining the frequency of primary vaccine failures and subsequent waning immunity (Figure 2), one might question the reliability of the evidence indicating that only a maximum of 23 vaccine failures have occurred among >540 million vaccinated individuals. There are two likely explanations for the remarkably low rate of vaccine failures noted by the WHO study. First, it is unlikely that all 540 million individuals were directly exposed to YFV-infected mosquitoes. If a person is not exposed to yellow fever, a potential vaccine failure will not be identified. Secondly, yellow fever is endemic in many remote regions of South America and Africa and in many developing countries with limited epidemiological infrastructure, there are substantial problems with under-reporting. Together, this makes it difficult to determine the “true” number of vaccine failures or to determine if protective immunity does indeed wane over time. Fortunately, an extensive study examining sylvatic cases of yellow fever in Brazil has provided much needed insight into this important question [3]. In this study, patients were not only queried about their vaccination status, but they were also asked whether they had been vaccinated within 10 years prior to contracting yellow fever (Figure 4). There were 831 cases of yellow fever identified between 1973–2008 with a case fatality rate of 51%. Vaccination status was unavailable for 372/831 (45%) of patients but the remaining 459 cases had received prior YFV vaccination and yet still contracted yellow fever. Since all of the cases of yellow fever were virologically confirmed, we believe that this is likely to be a more accurate representation of vaccine failures than the previous estimate of only 23 worldwide vaccine failures occurring between 1944–2013 [11,12]. Of the individuals who contracted yellow fever, 3% had been vaccinated <10 years before disease onset whereas 52% had been vaccinated >10 years previously. Although the cumulative level of direct exposure to yellow fever-infected mosquitoes in each group is unknown, this appears to indicate that there is a distinct difference in the likelihood of contracting yellow fever based on the number of years since last vaccination. This data is consistent with a low frequency of primary vaccine failures in addition to waning immunity after YFV-17D vaccination (Figure 2) and supports the opinion that YFV-17D booster vaccination be performed within 5–10 years after primary vaccination to prevent a lapse in protective immunity.
Figure 4. Proportion of yellow fever cases in relation to YFV-17D vaccination history.
The vaccine status of 831 cases of sylvatic yellow fever from 1973 – 2008 were reported in Brazil [3]. In 45% of the cases (372/831), vaccine status of the patient was unknown. Of the remaining cases, 52% of patients (432/831) had been vaccinated >10 years earlier and 3% (27/831) of patients had been vaccinated <10 years prior to disease onset. All cases of yellow fever were virologically confirmed and the case fatality rate was 51%.
5. Rates of YFV-17D serious adverse events (SAEs)
By some estimates, YFV-17D is considered one of the least safe vaccines in use [61]. Serious adverse events (SAEs) are commonly defined as causing in-patient hospitalization, long-term disability, or death [62]. Although co-administration of multiple vaccines can complicate the interpretation of some adverse events, known examples of YFV-17D-associated SAEs include anaphylaxis, neurologic disease, viscerotropic disease, or vaccine-related fatalities (Table 1). These are rare events and estimating the true rate of any rare adverse event can be challenging, particularly under conditions of passive surveillance or in locations with poor health-monitoring infrastructure. When determining that only a single dose of YFV-17D vaccine is required for lifelong protection against yellow fever, the CDC identified a total of 1,255 SAEs worldwide among 333,455,887 vaccine doses distributed from 1990–2013 [11]. This low level of YFV-17D-associated SAEs was based on eight published studies and an additional unpublished analysis of the US VAERS system from 2007–2013 [11]. However, two studies in particular [63,64] dominated the dataset, and together accounted for 314 million vaccinations, or 94% of the total YFV-17D doses analyzed by the CDC. The accuracy of such analysis is questionable, since these two studies relied heavily on reporting from endemic countries with limited healthcare infrastructure that are prone to under-reporting [63,64]. In one study, adverse events were followed in eight African countries after mass YFV-17D vaccination campaigns that totaled 38 million doses [64]. Only 164 SAEs were reported (4.3 SAEs per million doses), with the authors acknowledging that this rate was substantially lower than prior observations in several non-endemic studies. They considered that this could be due to differences in the populations under surveillance, but also conceded that underreporting was a significant concern. Indeed, the authors estimated that based on crude mortality rates they should have detected around 38,000 deaths during their surveillance period but only 33 deaths were reported [64]. This has substantial implications with regard to the limited sensitivity in their surveillance system. Similar issues were highlighted in another study which reported SAEs following distribution of 276 million doses of YFV-17D worldwide from 1993–2010 [63]. As noted by the authors, while doses distributed in non-endemic countries accounted for only 5.9% of the total distributed doses (16.2 million doses), they accounted for 95% of all reported adverse events. In their analysis of YFV-17D vaccine-associated SAEs, the overall rate was reported at 3 SAEs per million doses, but this number reached 51 SAEs per million doses in the non-endemic (i.e. traveler) population [63]. These studies are important because they highlight the limited sensitivity in the vaccine surveillance networks in developing countries and show how this may dramatically skew vaccine safety results.
Table 1.
Safety profile of YFV-17D compared to withdrawn live vaccines, OPV and RotaShield
| Adverse Event Classification | YFV-17D | OPV | RotaShield |
|---|---|---|---|
| Serious Adverse Events | 13–51 per million doses administered1 | 9.0 per million doses administered2 | 90–214 per million doses administered3 |
| Anaphylaxis | 2.0–18 per million doses administered4 | 1.9 per million doses administered5 | Not applicable |
| Neurological Disease | 1.3–38.7 per million doses administered6 | 0.4–0.5 per million doses administered7 | Not applicable |
| Viscerotropic Disease | 0.7–4 per million doses adminstered8 | Not applicable | Not applicable |
| Death | 0.1–2.6 per million doses administered9 | 0.012–0.015 per million doses administered10 | <1 per million doses administered11 |
Passive surveillance in non-endemic locations, including reported rates of 47 per million [62]; 13 per million, based on 42 total SAEs from 3.16 million administered doses (2.23 million civilian and 0.93 million military) [68]; 25 per million [69]; and a rate of 51 per million reported among non-endemic travelers [63]. Note that references [62] and [68] have overlapping datasets, but this does not impact reported rates of SAEs.
Passive surveillance of non-fatal serious events from the US VAERS system [112].
Passive surveillance in non-endemic locations including 7.6 per million, based on 40 cases from 5.24 million doses [113]; 18 per million [62]; and a rate of 2.0 per million from the non-endemic subpopulation [63].
All cases occurred in patients that received concurrent administration of injectable vaccines including DTP-Hib, hepatitis B, and MMR [114].
Passive surveillance in non-endemic locations, including rates of 8 per million [62]; 3.2 per million based on 10 reports from 3.16 million administered doses (2.23 million civilian and 0.93 million military) [68]; 1.3 per million [77]; and 1.5 per million among non-endemic populations [63], or endemic locations following enhanced surveillance to include neurological disorders such as aseptic meningitis (38.7 cases per million) [66]. The rate of aseptic meningitis is supported by similar results in a smaller non-endemic study, which reported rates as high as 99 per million [78].
Derived from studies performed in non-endemic locations, including 4 per million [62]; 2.5 per million based on 8 reports from 3.16 million administered doses (2.23 million civilian and 0.93 million military) [68]; 1.3 per million [77]; and 0.7 per million among non-endemic populations [63]. A smaller study of 47,742 people in Peru reported 116 cases per million doses [84].
Passive surveillance in non-endemic locations, including 1.3 per million, based on 4 deaths associated with 1.53 million vaccinations [62]; 1.6 per million based on 5 vaccine-associated deaths from 3.16 million administered doses (2.23 million civilian and 0.93 million military) [68]; 0.33 per million based on 1 death reported among 3 million doses [77]; and a reported rate of 0.1 per million among non-endemic populations [63].
Derived by applying an estimated 3% mortality rate [92] to the reported rate of VAPP following OPV vaccination [90,91].
Based on 1 death potentially associated with an estimated 1.5 million RotaShield vaccinations [97].
The differences between endemic and non-endemic communities within a single study [63] appeared to be similar to the differences observed between studies cited by CDC when segregated by geographic location (see Table 4 in [11]). To better measure these differences, the average SAE rates from studies in only endemic regions [64–67] were compared to those from only non-endemic regions ([62,68,69] and unpublished US VAERS analysis in [11]). For endemic areas, a total of 234 SAEs were identified among approximately 43 million doses of YFV-17D, equaling 5.4 SAEs per million. By comparison, for the non-endemic locations a total of 216 SAEs were reported from approximately 14.4 million doses, resulting in 15 SAEs per million. However, even this analysis of the non-endemic SAE rate appears to be an underestimate due to the differences in one study between the number of YFV-17D doses purchased versus the number of doses that were administered [68]. The CDC [11] referenced a study of US civilian and military vaccinees that included 9.6 million total doses (2.2 million civilian doses and 7.4 million military doses) [68]. However, in that study there was a large difference between doses purchased and doses administered for the military population and the authors clarified these differences, basing their subsequent analysis on 2.2 million civilian doses and only 0.93 million military doses [68]. Using the studies cited by the CDC [11] in non-endemic countries (corrected for the total number of administered doses in [68]), and the non-endemic subset analysis from [63], it is estimated that the SAE rate ranges from 13–51 SAEs per million doses of YFV-17D (Table 1).
6. Yellow fever vaccine-associated neurotropic disease (YEL-AND)
Neurological symptoms represent an SAE that was first widely recognized after YFV-17D immunization of infants <6 months of age [70]. From 1952–1953, 5 cases of meningoencephalitis were reported among 1,800 children (≤12 months old) vaccinated at the Pasteur Institute in Paris, yielding a rate of 2,800 cases per million vaccinations in this age group [70]. In the report, 9 additional cases of encephalitis in YFV-17D vaccinated infants (all <6 months old) were also identified [70], underpinning the current contraindication against vaccination of infants <9 months old [71]. The clinical definition of yellow fever vaccine-associated neurotropic disease (YEL-AND) now includes a wider range of neurological complications including meningoencephalitis, Guillain-Barré syndrome, acute disseminated encephalomyelitis, and bulbar palsy [72]. Although not typically lethal, at least one reported case of virologically confirmed YEL-AND resulted in the death of a 3-year-old child [73,74]. The virus recovered from this patient had mutated to become more neurovirulent after inoculation into mice or non-human primates [74].
Despite the contraindication of YF-17D in infants, this SAE continues to be observed in the broader population following vaccination. Based on 8 studies from both endemic and non-endemic regions [11], the CDC identified 208 vaccine-associated neurologic cases among 462 million doses (0.45 YEL-AND cases per million doses). However, this number may be heavily skewed since 432 million doses (94%) were distributed in endemic countries. When the analysis is specifically limited to endemic countries ([64,65,75,76] and the endemic subpopulation in [63]) there are 0.38 cases of YEL-AND per million doses (165 cases from approximately 432 million vaccinations). After correcting for the number of administered military doses in [68], analysis from this study and the three other references cited by the CDC [11] in non-endemic regions ([62,68,77], and the non-endemic subpopulation in [63]), together totaled 43 cases of YEL-AND among approximately 22.5 million vaccinations (1.9 YEL-AND per million doses).
As with SAEs in general, reporting rates for YEL-AND in endemic countries appear problematic due to under-reporting. Of the 24 cases of YEL-AND identified by Cottin et al., only 1 occurred outside of their traveler vaccine subpopulation, which equates to 1 case per 259.8 million vaccinations (0.004 YEL-AND per million doses) [63]. In 2014, 63 cases of YEL-AND were reported in Brazil among 31.4 million immunizations (2.0 YEL-AND per million vaccinations) [75]. However, when limiting their analysis to only the state of Rio Grande do Sul in 2009, where training of healthcare staff and surveillance were specifically intensified due to a vaccination campaign, the YEL-AND rate jumped to 10.8 cases per million. This emphasizes the importance of strong surveillance systems when studying rare adverse events. Indeed, additional analysis has continued to identify subpopulations that may be at even greater risk of YEL-AND due to age. Children aged 5–9 had YEL-AND rates as high as 26 per million vaccinations [75]. Those of advanced age (≥ 60 years old) also appear to be at increased risk, with the rate of YEL-AND estimated at 14 cases per million vaccinations [68]. This appears to be a vaccine-specific problem since no such age-related increase in adverse events was noted with other travelers vaccines (Hepatitis A or Typhoid) within the same dataset [68]. When serious adverse events in the general population were expanded to include a wider range of neurological symptoms such as aseptic meningitis (in addition to encephalitis), one study in Brazil recorded rates as high as 38.7 per million after a 2001 vaccine campaign involving 344,195 doses of vaccine [66]. While this number may seem quite high, an even higher rate of 99 cases per million doses (meningitis or encephalitis) was observed in a smaller retrospective study performed in France that was based on 40,404 doses [78]. When considering the spectrum of neurologic symptoms associated with YFV-17D, the results from large studies of >100,000 vaccinations yield a range of 1.3–38.7 cases per million (Table 1). In total, modern experience with the YFV-17D vaccine indicates that there is a much higher rate of neurologic symptoms in the general population than was originally appreciated at the time of the vaccine’s initial development.
7. Yellow fever vaccine-associated viscerotropic disease (YEL-AVD)
In 2001, a previously uncharacterized SAE with a high mortality rate, termed yellow fever vaccine-associated viscerotropic disease (YEL-AVD), was first formally described [72,79]. YEL-AVD results from a widespread dissemination of the vaccine virus, closely mimicking wild-type fulminant yellow fever [29]. The case-fatality rate has been estimated at ~65% [29] and although sex and advanced age appear to be significant risk factors [68,80,81], a substantial number of cases have also been observed in healthy young adults, as well as children [81]. Following the initial description of YEL-AVD, increased monitoring has led to a growing number of case reports across multiple continents [72,81], with retrospective analysis dating cases to as early as the 1970s [82].
While precise measurements of the incidence of YEL-AVD in large populations are limited by the lack of adequate prospective data, several retrospective reviews have addressed the rate of this life-threatening condition [62,65,68,80,81,83]. The CDC identified a total of 72 cases from 437 million doses administered from 1990–2010, for an estimated rate of 0.16 cases of YEL-AVD per million doses administered [11]. As with the preceding discussion of total SAEs and YEL-AND, when limiting the analysis to the studies performed in endemic countries (endemic subpopulation in [63] and [64,65,75,76]), there were only 0.10 cases of YEL-AVD per million doses of vaccine administered (43 cases among an estimated 424 million vaccinations). By contrast, 30 cases of viscerotropic disease were found following a CDC-estimated 29.7 million vaccinations in non-endemic locations ([62,68,77], and the non-endemic population in [63]). After correcting for the total number of administered doses in the military population [68], there were on average 1.3 cases of YEL-AVD per million doses of YFV-17D, with a range of 0.7–4 cases per million, and an overall mortality rate of 0.10–2.6 deaths per million (Table 1). This statistic represents more than a 10-fold increase in the occurrence of YEL-AVD in non-endemic countries compared to endemic countries.
The discrepancy in SAE reporting rates is illustrated in Cottin et al., in which endemic and non-endemic reports were compared within the same study [63]. Of the 12 cases of YEL-AVD reported, 11 were from the non-endemic cohort, and the location of 1 was listed as unknown. This means that there was <1 case per 259.8 million doses reported in endemic regions (i.e., <0.004 YEL-AVD per million doses) compared to a rate of 0.7 per million doses (11 cases per 16.2 million doses) in non-endemic countries. The distinction in YEL-AVD rates between endemic and non-endemic populations could suggest differences in demographics or in the surveillance and reporting capabilities of the various locations. The latter explanation appears more likely since estimates from other studies in endemic countries have yielded results comparable to non-endemic countries if enhanced surveillance is undertaken. For instance, a study of Brazilian adverse events identified only 20 cases of YEL-AVD following 107.6 million vaccinations from 1999–2009 [76] for a rate of 0.19 cases per million vaccinations. However, when the Brazilian analysis was limited to specific vaccination campaigns in Rio Grande do Sul or São Paulo, the YEL-AVD rates increased substantially, ranging from 1.1 to 3.1 cases of YEL-AVD per million doses, respectively. This is strikingly similar to the range reported from non-endemic countries (Table 1). Likewise, during an Argentinian vaccination campaign involving 1.9 million doses of YFV-17D administered from 2008–2009, with specific surveillance systems established prior to initiating the campaign, there were 12 cases of YEL-AVD identified, yielding a rate of 6 cases per million doses [65]. Since advanced age is a known risk factor for YEL-AVD [68,80], the Brazilian National Immunization Program recommends that unvaccinated individuals older than 60 years of age consult with their physician to evaluate the risk:benefit ratio before considering yellow fever vaccination [16,17].
The most notable documented cluster of YEL-AVD occurred in the Ica Region of Peru [84] and provides a cautionary note against implementing YFV-17D vaccination campaigns in the absence of a clear threat of exposure. A single lot of YFV-17D (substrain 17DD) was used to vaccinate 42,742 people as part of a post-disaster health response to a destructive earthquake [84]. YFV was not considered endemic to this area of Peru and the vaccine campaign resulted in 5 cases of YEL-AVD, and 4 deaths. In this instance, the YEL-AVD rate of 116 per million vaccinations represents an incidence that is nearly 30 times greater than the highest rate previously observed in the general population (Table 1). Genetic analysis of the vaccine lot revealed no obvious changes in the virus genome sequence, limiting the likelihood that this was a batch-specific issue [84]. Of the male patients, one was advanced in age (79 years old), which is a risk factor [68,80]. The other male was 49 years old with a history of appendectomy, though whether or not this represents a YEL-AVD risk factor is unknown. Of the female patients, one was 49 years old and found to have underlying immune disorders (systemic lupus erythematosus and rheumatoid arthritis) that may have pre-disposed her to YEL-AVD. The two remaining female patients were younger (23 and 24 years old), apparently healthy and had no obvious risk factors. As the investigators noted, “The occurrence of YEL-AVD in these and other young women is concerning and unexplained.” [84]. This lack of understanding of host susceptibility represents a continuing and uncertain risk during YFV-17D mass vaccination campaigns.
8. YFV-17D vaccine risks in comparison to other withdrawn live virus vaccines
The YFV-17D vaccine has been employed in vaccination programs since the late 1930’s and had been broadly considered one of the most successful, and safest, vaccines ever developed [85]. However, certain risks were recognized early in the history of YFV-17D vaccine use. Following initial field trials it was recognized that controlling passage level was imperative to reduce the risk of both over-attenuation and under-attenuation [29]. For example, following a Brazilian vaccine campaign in 1941 using 7 lots of vaccine derived from the same substrain (17D-NY 104), 199 cases of adverse events with ‘evidence of central nervous system involvement’ were reported among approximately 55,000 vaccinees, with the authors linking these adverse events directly to the YFV-17D vaccine [29,86]. It was concluded that passage history played a role in this instance [86] and subsequent clinical studies indicated that this high rate of encephalitis was a characteristic of this particular substrain [29]. Further, it was concluded that this trait developed following a small number of subcultures from the parent strain, and recognition of this point contributed to further changes in the seed-lot system by 1942 [70], including the establishment of better characterized primary and secondary seed lots to reduce the potential for pathogenic changes, as well as restricting vaccine lots to a single passage past the secondary lot [29]. This seed-lot system culminated with the current 17D substrains used in vaccine production today, which include 17D-204, 17D-213 and 17DD [87]. Encephalitis among YFV-17D-vaccinated infants led to early contraindications in this age group [70], and additional contraindications now include immunosuppressed patients, as well as those with hypersensitivity to egg products [71]. However, even in the seed-lot era and with contraindications for vulnerable populations in place, serious adverse events (SAEs) have persisted at relatively high rates (Table 1).
YFV-17D-associated SAEs may include anaphylaxis, neurotropic disease, viscerotropic disease, and death (Table 1). Although often described as one of the safest vaccines ever developed, YFV-17D vaccines have safety issues at rates similar to other licensed live viral vaccines that have been subsequently withdrawn from the US vaccine market due to safety concerns (Table 1). For example, the oral polio vaccine (OPV) was first introduced in 1961 as an alternative to the existing inactivated-polio vaccine (IPV), and soon became the dominant form used in the US [88]. While the vaccine was effective in reducing wild-type polio transmission, it was clear that it also posed a risk of virulence reversion [89], leading to a condition termed vaccine-associated paralytic poliomyelitis (VAPP). Once wild type polio transmission was eliminated in the US, OPV became the sole source responsible for endemic cases of paralytic poliomyelitis [88], at a rate of 0.4–0.5 cases per million doses administered [90,91], with ~3% of those cases resulting in death (0.012–0.015 per million doses administered) [92]. Based on this evidence, the Advisory Council on Immunization Practices (ACIP) began recommending the reintroduction of IPV starting in 1997 [88], with an IPV-only schedule adopted by 2000 [93]. Similar to OPV, the first rotavirus vaccine, RotaShield®, represents another example of an attenuated live virus vaccine that was eventually deemed unsafe for routine clinical use [94]. RotaShield was licensed for US markets in 1998, but following an unexpectedly high rate of intussusception reports, ACIP recommendations were rescinded by November of 1999 and the product was removed from the market. More highly attenuated rotavirus vaccines (Rotarix® and RotaTeq®) only became available 7–10 years later [94]. Retrospective analysis indicates that the increased risk of intussusception from RotaShield was approximately 90–214 excess cases per million vaccinated children [95,96], and the vaccine may have been associated with one death out of an estimated 1.5 million doses administered (<1 per million doses) [97]. For OPV and RotaShield, these rates of serious adverse events were considered too high for routine use and the vaccines were withdrawn from the US and world market, respectively. It is somewhat surprising to note that serious vaccine-related events are similar between YFV-17D and either OPV or RotaShield, and the YFV-17D-associated mortality rate is also much higher than that observed among these two withdrawn vaccines (Table 1). Increased awareness of the risks associated with YFV-17D vaccination has prompted a reassessment of the current live attenuated vaccine and led to calls for safer alternatives [7,98].
9. Yellow fever risk:benefit ratio for international travelers
The overall risk of contracting yellow fever among unvaccinated US travelers has been estimated at approximately 0.5–5 cases per million travelers during a 2-week stay in yellow fever endemic areas [62]. The theoretical incidence of YFV-associated deaths in travelers has been estimated to be as high as 300 per year [99], but the actual observed incidence comes in substantially lower, at approximately 0.7 cases per million travelers, based on 2 cases of yellow fever among 3 million unvaccinated travelers per year [99]. In comparison, the rate of YEL-AVD ranges from 0.7–4 cases per million doses administered, with a death rate of 0.1–2.6 per million doses (Table 2). Although relative risk calculations can change depending on individual travel plans, this comparison suggests that the current live YFV-17D vaccine might actually pose as much risk to most US travelers as the disease itself (Table 2) [99]. The challenge in making the decision to vaccinate the travelers population has been underscored by recent expert analysis [7], in which it was highlighted that among travelers from 1990–2010, 6 deaths were associated with wild-type yellow fever, while twice as many deaths (12 total) were associated with YEL-AVD following YFV-17D vaccination. As noted in the review [7], this is a problematic comparison since denominators for at-risk travelers (either vaccinated or unvaccinated) are difficult to estimate, and one cannot know for sure how many cases of wild-type infection were averted in the vaccinated population. Still, the comparison acknowledges the point that the risk:benefit ratio for international travelers is often unclear. While travel to Africa during epidemic activity warrants vaccination, the risk:benefit ratio during non-epidemic periods is less certain [7]. The situation in South America is even less clear, where risk of YFV exposure has been estimated at approximately 10-fold less than Africa [99]. However, considering that YFV can circulate in intense periods of local transmission [7], it is important for each individual to consult with their physician based on their specific health status and travel plans.
Table 2.
Estimated risks of contracting viscerotropic yellow fever1
| Illness | Death | |
|---|---|---|
| Travel to West Africa | 500 per million travelers | 100 per million travelers |
| Travel to South America | 50 per million travelers | 10 per million travelers |
| Overall risk to all US travelers | 0.5–5 per million travelers | 0.7 per million travelers |
| YFV-17D vaccination risk | 13–51 per million doses | 0.1–2.6 per million doses |
The travel risks of contracting yellow fever or dying from the disease have been estimated for a two week journey during inter-epidemic periods of yellow fever in West Africa or South America [99]. The overall risk to all US travelers has been estimated at lower rates, based on data analysis for US travelers from 1996–2004 [62]. The risk of yellow fever-associated death among US travelers is based on two deaths among an estimated 3 million unvaccinated US travelers [99] and the risks of illness or death due to YFV-17D vaccination are described in Table 1.
10. Yellow fever vaccine cost:benefit analysis and broader considerations
Vaccines play a critical role in modern healthcare, and are considered the most effective medical means to reducing mortality and supporting population growth [100]. However, understanding the ultimate purpose of vaccines in our society can be subtle and challenging. As articulated previously [101], one must consider if vaccines are simply a tool for reducing healthcare costs, or do they serve a deeper purpose for reducing human suffering? Although these positions are not mutually exclusive, there are instances wherein strict cost:benefit calculations intersect with scientific and moral obligations. Polio vaccination provides an important illustration of this point. The seriousness and impact of polio disease is unquestionable, and the effectiveness of OPV in preventing paralysis and death is indisputable [102]. However as described earlier, OPV is associated with rare but serious side-effects (Table 1) that can mimic wild type polio in vaccinees and their susceptible contacts [90,91]. Once wild-type poliovirus was eliminated in the US, it became clear that all endogenous cases of paralytic polio were due to the vaccine, with approximately 10 children stricken with paralysis each year [88]. Despite the existence of IPV, a safe and effective vaccine alternative, economic analysis indicated that the switch to IPV would not be cost-beneficial compared to simply compensating children injured through vaccination [103]. Nonetheless, governing bodies ultimately decided that eliminating the risk of directly harming vaccinees outweighed strict economic considerations [88], and subsequent economic analysis has demonstrated that IPV continues to be a cost-effective vaccination strategy [102]. Yellow fever vaccines pose a similar dilemma. Wild type yellow fever is severe, and vaccination with live YFV-17D is effective in preventing disease. However, the vaccine comes with small but verified risks, including fulminant disease and in rare instances, death (Table 1). Unlike poliovirus, licensed inactivated YFV vaccines are currently unavailable and live YFV-17D vaccination remains the only option for travelers and those living in endemic countries. Nevertheless, recent advances in vaccine technologies bring new hope to the field. Several groups are developing potentially safer non-replicating vaccines [104–106] and at least one candidate has progressed to early stage clinical trials [107]. Still, until new vaccine alternatives become available, healthcare providers and individual patients will be left with challenging questions on how to balance the safety of the vaccine versus the likelihood of contracting yellow fever.
11. Expert Commentary
Yellow fever represents a serious mosquito-borne disease with hospitalized cases associated with up to 20–60% mortality. Introduced in 1936, YFV-17D vaccines have been in use for 80 years and represent an important tool for the prevention and control of yellow fever outbreaks. However, these vaccines are not entirely safe nor do they induce lifelong immunity among all vaccine recipients. Bearing this in mind, changes to the recommended vaccination schedule must be carefully weighed in relation to the safety and long-term efficacy of the current live attenuated YFV-17D vaccines. In order to make informed decisions on YFV-17D vaccine recommendations, the available epidemiological literature must be carefully reviewed with the understanding that there are inherent inefficiencies in the passive reporting of vaccine-related adverse events, especially among developing nations that may not have the necessary infrastructure to identify and report vaccine-associated SAEs. We believe that a conservative approach be used in which the higher rates of adverse events found among vaccine recipients in non-endemic countries be taken as the more accurate indication of safety due to the likelihood of more efficient reporting and follow-up of patient outcomes. In terms of the duration of antibody responses after YFV-17D vaccination, it is true that these vaccines can elicit lifelong immunity. However, this does not mean that every vaccinated individual will be endowed with lifelong immunity. This point may explain why the ACIP recommends no booster vaccination for most travelers, but still recommends booster vaccination at least every 10 years for travelers who might enter a higher-risk setting based on season, location, activities, and duration of their travel [12]. Likewise, they recommend that laboratory workers who routinely handle wild-type yellow fever virus to either have their serum tested for neutralizing titers or receive booster vaccination at least every 10 years. In other words, the ACIP still recommends booster vaccination for individuals with a high risk of yellow fever exposure [12]. Upon examination of the literature used as the basis for recommending only a single dose of YFV-17D vaccine, we found evidence indicating that antiviral immunity wanes after primary vaccination, resulting in up to 1-in-3 to 1-in-5 individuals losing immunity due to a combination of primary vaccine failure (especially among children) and waning immunity during the first 5 to 10 years after vaccination. This immunological data is supported by a large epidemiological study in Brazil spanning 1973–2008, in which more than half (55%) of the yellow fever patients had received YFV-17D vaccination prior to contracting virologically confirmed yellow fever. Of the 831 yellow fever patients described in the study, there were 27 cases found among those vaccinated <10 years previously and 432 cases among those vaccinated >10 years previously. Notably, the yellow fever case fatality rate was 51% - an important reminder that this is a devastating and highly virulent disease among those without vaccine-mediated protection. Combined with the data on primary vaccine efficacy and waning immunity, we believe that this epidemiological study supports the use of YFV-17D booster vaccination within 5–10 years after primary immunization. However, based on the safety concerns associated with live attenuated YFV-17D vaccines, the target population should be limited to only those individuals with a credible risk of exposure.
12. Five-year view
As noted previously [7], YFV-17D vaccination will remain an important component of immunization programs within endemic countries and continue to be required by the International Health Regulations for international travel to prevent epidemics that could otherwise spread across borders. The updated regulatory guidance to no longer recommend booster vaccination will likely provide initial cost savings but the potential impact on long-term immunity is uncertain and more studies on the durability of antiviral antibody responses are needed among both adult and pediatric cohorts. Immunological studies use a variety of methods to measure neutralizing antibodies (LNI, PRNT50, PRNT75, PRNT80, PRNT90, mouse protection, etc.) and this makes comparisons between groups difficult to interpret. One consideration would be to agree on using a harmonized approach in which future studies provide neutralizing data utilizing the same or similar methodology. The LNI approach has historical significance and an LNI of ≥0.7 is the only correlate of protective immunity after YFV-17D vaccination [38]. However, this approach requires relatively large volumes of serum in addition to pre- and post-vaccination samples, making it difficult (and in many cases unfeasible) to be used for long-term immunological studies. We would propose the use of PRNT50 since this has been used to determine a putative correlate of protection in hamsters [37] and eventually could be correlated with LNI results. The decision to recommend YFV-17D vaccination is further complicated by the risk:benefit ratio for different populations. Although YFV-17D vaccination is necessary within many endemic regions, the risk of vaccine-associated serious adverse events among elderly or immunocompromised individuals can in some cases outweigh the risk of contracting natural yellow fever, especially among international travelers. The need for safer vaccines against yellow fever has been raised on more than one occasion [7,98] and there are several non-replicating vaccines in various stages of development [7,54]. There are many gaps remaining in our knowledge of YFV-17D vaccine safety and durability (Table 12 of [10]) and further study into safer vaccine alternatives should be added to this list. Two of the most common arguments against the development of an inactivated or subunit vaccine against yellow fever include a) the feasibility to administer multiple doses of a given vaccine in developing countries and b) the cost of vaccine production. According to the WHO, 3-dose coverage of tetanus/diphtheria/pertussis (DTP) vaccination of children in Africa has increased dramatically from 5% in 1980 to 77% in 2014 [108] – indicating that it is becoming more feasible to administer multiple doses of vaccine in developing countries. Vaccine production costs on the other hand are typically product-specific and harder to predict, but this was also considered one of the largest obstacles when the decision was made to replace live oral polio vaccine (OPV) with inactivated polio vaccine (IPV). The successful transition from OPV to IPV (and the associated improvements in safety) lends credence to the belief that eventually live attenuated YFV-17D vaccines will be replaced by safer alternatives and with various improvements in modern vaccine manufacturing technologies, it is likely that this clinical need will be addressed. There has been a long-held misconception that live attenuated vaccines induce lifelong immunity whereas inactivated/non-replicating vaccines induce only short-lived immunity and therefore require more booster vaccinations. In reality, nearly all vaccines require at least one booster vaccination [109] and highly immunogenic multivalent vaccines such as those developed against human papilloma virus [110] and hepatitis A virus [111] elicit remarkably long-lived immunity. Indeed, two doses of the inactivated whole-virus hepatitis A vaccine has been estimated to maintain protective immunity in >95% of vaccine recipients for more than 30 years [109,111]. If a similar vaccine were to be developed for yellow fever, then not only would the risk:benefit ratio likely be improved, but long-term antiviral immunity may be attained with durability potentially reaching the levels observed with live attenuated YFV-17D vaccines. Continued epidemiological studies on yellow fever and rare YFV-17D vaccine-associated adverse events will be needed to gain a clearer picture of the risk:benefit ratio for vaccinating different populations and there is optimism that new, safer vaccine technologies are on the horizon.
13. Key issues.
YFV-17D vaccines are highly immunogenic but have a questionable safety profile.
Analysis of YFV-17D SAEs indicate substantial under-reporting from endemic areas and this may explain why it was originally considered to have an excellent safety profile.
The rate of SAEs associated with YFV-17D vaccination is similar to OPV and RotaShield, two vaccines withdrawn from the US and world market, respectively, due to safety concerns.
Primary vaccine failures among YFV-17D-vaccinated children are more common than among adults, with seroconversion rates of only 69%–85.8% in some instances.
Immunity after YFV-17D vaccination wanes over time with seropositive rates declining from >90% among adults shortly after vaccination to as low as 70% within 5 to 10 years.
Among 831 cases of yellow fever, 3% (n = 27) had been vaccinated within the previous 10 years whereas 52% (n = 432) had been vaccinated >10 years prior to contracting the disease.
Footnotes
Declaration of Interests
This project was funded in part with federal funds from the National Institute of Allergy and Infectious Diseases R44 AI079898 (to MK Slifka and IJ Amanna), R01 AI098723 (to MK Slifka) and Oregon National Primate Research Center grant, 8P51 OD011092-53 (to MK Slifka). OHSU, MK Slifka, and IJ Amanna have a financial interest in Najít Technologies, Inc., a company that is developing a new yellow fever vaccine based on a hydrogen peroxide-based inactivation approach. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Russell MN, Cetron MS, Eidex RB. The US-Certified Yellow Fever Vaccination Center Registry: a tool for travelers, state health departments, and vaccine providers. J Travel Med. 2006;13(1):48–49. doi: 10.1111/j.1708-8305.2006.00011.x. [DOI] [PubMed] [Google Scholar]
- 2.Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 3**.Camara FP, de Carvalho LM, Gomes AL. Demographic profile of sylvatic yellow fever in Brazil from 1973 to 2008. Trans R Soc Trop Med Hyg. 2013;107(5):324–327. doi: 10.1093/trstmh/trt014. Describes 831 cases of yellow fever in Brazil with 459 (55%) of the patients described as having a history of YFV-17D vaccination. This indicates that YFV-17D vaccine failures are more common than previously realized. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Group for Studies on Yellow Fever Vaccines. A randomised double-blind clinical trial of two yellow fever vaccines prepared with substrains 17DD and 17D-213/77 in children nine-23 months old. Mem Inst Oswaldo Cruz. 2015;110(6):771–780. doi: 10.1590/0074-02760150176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camacho LA, da Freire MS, da Leal ML, et al. Immunogenicity of WHO-17D and Brazilian 17DD yellow fever vaccines: a randomized trial. Rev Saude Publica. 2004;38(5):671–678. doi: 10.1590/s0034-89102004000500009. [DOI] [PubMed] [Google Scholar]
- 6.Nascimento Silva JR, Camacho LA, Siqueira MM, et al. Mutual interference on the immune response to yellow fever vaccine and a combined vaccine against measles, mumps and rubella. Vaccine. 2011;29(37):6327–6334. doi: 10.1016/j.vaccine.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Monath TP. Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev Vaccines. 2012;11(4):427–448. doi: 10.1586/erv.12.6. [DOI] [PubMed] [Google Scholar]
- 8.Engel AR, Vasconcelos PF, McArthur MA, Barrett AD. Characterization of a viscerotropic yellow fever vaccine variant from a patient in Brazil. Vaccine. 2006;24(15):2803–2809. doi: 10.1016/j.vaccine.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Vaccines and vaccination against yellow fever. WHO position paper -- June 2013. Wkly Epidemiol Record. 2013;88(27):269–283. [PubMed] [Google Scholar]
- 10.World Health Organization. Vaccine Position Papers. SAGE Working Group; Geneva, Switzerland: 2013. Background Paper on Yellow Fever Vaccine. [Google Scholar]
- 11.Centers for Disease Control and Prevention. GRADE Evidence Tables - Recommendations in MMWR. Atlanta, GA: 2015. Grading of recommendations, assessment, development, and evaluation (GRADE) for yellow fever vaccine booster doses. [Google Scholar]
- 12*.Staples JE, Bocchini JA, Jr, Rubin L, Fischer M. Yellow Fever Vaccine Booster Doses: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(23):647–650. An updated ACIP recommendation for YF-17D vaccination that includes the discontinuation of booster doses for most travelers, although individuals at high exposure risk should continue to get vaccinated at least every 10 years. [PMC free article] [PubMed] [Google Scholar]
- 13.Collaborative Group for Studies on Yellow Fever Vaccines. Duration of post-vaccination immunity against yellow fever in adults. Vaccine. 2014;32(39):4977–4984. doi: 10.1016/j.vaccine.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Patel D, Simons H. Yellow fever vaccination: is one dose always enough? Travel Med Infect Dis. 2013;11(5):266–273. doi: 10.1016/j.tmaid.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Grobusch MP, Goorhuis A, Wieten RW, et al. Yellow fever revaccination guidelines change - a decision too feverish? Clin Microbiol Infect. 2013;19(10):885–886. doi: 10.1111/1469-0691.12332. [DOI] [PubMed] [Google Scholar]
- 16.Domingues CMAS. Recommendations of yellow fever vaccination for travelers, following a statement of the World Health Organization. Ministério da Saúde, Departamento de Vigilância das Doenças Transmissíveis; Brasília: 2015. Parecer No. 05/CGPNI/DEVIT/SVS/MS. Recomendações de vacinação contra febre amarela para viajantes, após a declaração da Organização Mundial da Saúde. [Google Scholar]
- 17.Maranhão AGK. Recommendations of vaccination against yellow fever, following a statement of the World Health Organization. Ministério da Saúde, Departamento de Vigilância das Doenças Transmissíveis; Brasília: 2014. Nota informativa No. 143/CGPNI/DEVIT/SVS/MS. Recomendações da vacinação contra febre amarela, após a declaração da Organização Mundial da Saúde. [Google Scholar]
- 18.Querec T, Bennouna S, Alkan S, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203(2):413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Querec TD, Akondy RS, Lee EK, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9(10):741–747. doi: 10.1038/nri2629. [DOI] [PubMed] [Google Scholar]
- 21.Kohler S, Bethke N, Bothe M, et al. The early cellular signatures of protective immunity induced by live viral vaccination. Eur J Immunol. 2012;42(9):2363–2373. doi: 10.1002/eji.201142306. [DOI] [PubMed] [Google Scholar]
- 22.Miller JD, van der Most RG, Akondy RS, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Akondy RS, Monson ND, Miller JD, et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol. 2009;183(12):7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James EA, LaFond RE, Gates TJ, Mai DT, Malhotra U, Kwok WW. Yellow fever vaccination elicits broad functional CD4+ T cell responses that recognize structural and nonstructural proteins. J Virol. 2013;87(23):12794–12804. doi: 10.1128/JVI.01160-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blom K, Braun M, Ivarsson MA, et al. Temporal dynamics of the primary human T cell response to yellow fever virus 17D as it matures from an effector- to a memory-type response. J Immunol. 2013;190(5):2150–2158. doi: 10.4049/jimmunol.1202234. [DOI] [PubMed] [Google Scholar]
- 26.Schulz AR, Malzer JN, Domingo C, et al. Low Thymic Activity and Dendritic Cell Numbers Are Associated with the Immune Response to Primary Viral Infection in Elderly Humans. J Immunol. 2015;195:4699–4711. doi: 10.4049/jimmunol.1500598. [DOI] [PubMed] [Google Scholar]
- 27*.Niedrig M, Lademann M, Emmerich P, Lafrenz M. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health. 1999;4(12):867–871. doi: 10.1046/j.1365-3156.1999.00496.x. This study shows the durability of antibody responses in YFV-17D vaccinated individuals for up to 35 years after vaccination. [DOI] [PubMed] [Google Scholar]
- 28.Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull World Health Organ. 1981;59(6):895–900. [PMC free article] [PubMed] [Google Scholar]
- 29*.Monath TP, Gershman M, Staples JE, Barrett AD. Vaccines. Plotkin, SA, Orenstein, WA, Offit, PA: Saunders/Elsevier; 2013. Yellow fever vaccine; pp. 870–968. This chapter provides a comprehensive description of yellow fever disease in addition to in-depth analysis of the safety and efficacy of yellow fever vaccination. [Google Scholar]
- 30.Reinhardt B, Jaspert R, Niedrig M, Kostner C, L’Age-Stehr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol. 1998;56(2):159–167. doi: 10.1002/(sici)1096-9071(199810)56:2<159::aid-jmv10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Guirakhoo F, Kitchener S, Morrison D, et al. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: Phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum Vaccin. 2006;2(2):60–67. doi: 10.4161/hv.2.2.2555. [DOI] [PubMed] [Google Scholar]
- 32.Monath TP, McCarthy K, Bedford P, et al. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20(7–8):1004–1018. doi: 10.1016/s0264-410x(01)00457-1. [DOI] [PubMed] [Google Scholar]
- 33.Amanna IJ, Slifka MK. Wanted, dead or alive: new viral vaccines. Antiviral Res. 2009;84(2):119–130. doi: 10.1016/j.antiviral.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slifka MK. Vaccine-mediated immunity against dengue and the potential for long-term protection against disease. Front Immunol. 2014;5(195):1–7. doi: 10.3389/fimmu.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Sawyer WA. Persistence of yellow fever immunity. J Prev Med. 1931;5:413–428. The first demonstration that neutralizing serum antibodies could protect against lethal yellow fever. [Google Scholar]
- 36.Thibodeaux BA, Garbino NC, Liss NM, et al. A humanized IgG but not IgM antibody is effective in prophylaxis and therapy of yellow fever infection in an AG129/17D-204 peripheral challenge mouse model. Antiviral Res. 2012;94(1):1–8. doi: 10.1016/j.antiviral.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Julander JG, Trent DW, Monath TP. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine. 2011;29(35):6008–6016. doi: 10.1016/j.vaccine.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl Microbiol. 1973;25(4):538–544. doi: 10.1128/am.25.4.539-544.1973. This study determined that 0.7 LNI is the correlate of protective immunity in rhesus macaques after YFV-17D vaccination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dick GW, Gee FL. Immunity to yellow fever nine years after vaccination with 17D vaccine. Trans R Soc Trop Med Hyg. 1952;46(4):449–458. doi: 10.1016/0035-9203(52)90062-x. [DOI] [PubMed] [Google Scholar]
- 40.de Melo AB, da da Silva MP, Magalhaes MC, et al. Description of a prospective 17DD yellow fever vaccine cohort in Recife, Brazil. Am J Trop Med Hyg. 2011;85:739–747. doi: 10.4269/ajtmh.2011.10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machado VW, Vasconcelos PF, Silva EV, Santos JB. Serologic assessment of yellow fever immunity in the rural population of a yellow fever-endemic area in Central Brazil. Rev Soc Bras Med Trop. 2013;46(2):166–171. doi: 10.1590/0037-8682-0007-2012. [DOI] [PubMed] [Google Scholar]
- 42.Rosenzweig EC, Babione RW, Wisseman CL., Jr Immunological studies with group B arthropod-borne viruses. IV. Persistence of yellow fever antibodies following vaccination with 17D strain yellow fever vaccine. Am J Trop Med Hyg. 1963;12:230–235. [PubMed] [Google Scholar]
- 43.Courtois G. Duration of immunity after yellow fever vaccination. Ann Soc Belg Med Trop. 1954;34(1):9–12. [PubMed] [Google Scholar]
- 44.Groot H, Riberiro RB. Neutralizing and haemagglutination-inhibiting antibodies to yellow fever 17 years after vaccination with 17D vaccine. Bull World Health Organ. 1962;27:699–707. [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez SY, Ocazionez RE. Yellow fever virus 17D neutralising antibodies in vaccinated Colombian people and unvaccinated ones having immunity against dengue. Rev Salud Publica (Bogota) 2008;10(5):796–807. doi: 10.1590/s0124-00642008000500012. [DOI] [PubMed] [Google Scholar]
- 46.Coulange Bodilis H, Benabdelmoumen G, Gergely A, et al. Long term persistence of yellow fever neutralising antibodies in elderly persons. Bull Soc Pathol Exot. 2011;104(4):260–265. doi: 10.1007/s13149-011-0135-7. [DOI] [PubMed] [Google Scholar]
- 47*.Hepburn MJ, Kortepeter MG, Pittman PR, et al. Neutralizing antibody response to booster vaccination with the 17d yellow fever vaccine. Vaccine. 2006;24:2843–2849. doi: 10.1016/j.vaccine.2005.12.055. This study of 1,029 laboratory researchers describes a rapid decline in neutralizing antibodies, particularly in the first 1–3 years after booster vaccination. [DOI] [PubMed] [Google Scholar]
- 48.Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 49.Hammarlund E, Lewis MW, Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nature Medicine. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 50.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 51.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 52.Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naive subjects. Vaccine. 2015;33(39):5225–5234. doi: 10.1016/j.vaccine.2015.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Krempelhuber A, Vollmar J, Pokorny R, et al. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine. 2010;28(5):1209–1216. doi: 10.1016/j.vaccine.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beck AS, Barrett AD. Current status and future prospects of yellow fever vaccines. Expert Rev Vaccines. 2015;14(11):1479–1492. doi: 10.1586/14760584.2015.1083430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doblas A, Domingo C, Bae HG, et al. Yellow fever vaccine-associated viscerotropic disease and death in Spain. J Clin Virol. 2006;36:156–158. doi: 10.1016/j.jcv.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Akondy RS, Johnson PL, Nakaya HI, et al. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc Natl Acad Sci U S A. 2015;112(10):3050–3055. doi: 10.1073/pnas.1500475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Domingo C, Yactayo S, Agbenu E, et al. Detection of yellow fever 17D genome in urine. J Clin Microbiol. 2011;49(2):760–762. doi: 10.1128/JCM.01775-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammarlund E, Lewis MW, Hanifin JM, Simpson EL, Carlson NE, Slifka MK. Traditional smallpox vaccination with reduced risk of inadvertent contact spread by administration of povidone iodine ointment. Vaccine. 2008;26(3):430–439. doi: 10.1016/j.vaccine.2007.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monath TP, Nichols R, Archambault WT, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg. 2002;66(5):533–541. doi: 10.4269/ajtmh.2002.66.533. [DOI] [PubMed] [Google Scholar]
- 60.Veit O, Niedrig M, Chapuis-Taillard C, et al. Immunogenicity and safety of yellow fever vaccination for 102 HIV-infected patients. Clin Infect Dis. 2009;48:659–666. doi: 10.1086/597006. [DOI] [PubMed] [Google Scholar]
- 61.Monath TP. Dengue and yellow fever--challenges for the development and use of vaccines. N Engl J Med. 2007;357:2222–2225. doi: 10.1056/NEJMp0707161. [DOI] [PubMed] [Google Scholar]
- 62.Lindsey NP, Schroeder BA, Miller ER, et al. Adverse event reports following yellow fever vaccination. Vaccine. 2008;26:6077–6082. doi: 10.1016/j.vaccine.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Cottin P, Niedrig M, Domingo C. Safety profile of the yellow fever vaccine Stamaril®: a 17-year review. Expert Rev Vaccines. 2013;12(11):1351–1368. doi: 10.1586/14760584.2013.836320. [DOI] [PubMed] [Google Scholar]
- 64.Breugelmans JG, Lewis RF, Agbenu E, et al. Adverse events following yellow fever preventive vaccination campaigns in eight African countries from 2007 to 2010. Vaccine. 2013;31(14):1819–1829. doi: 10.1016/j.vaccine.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 65.Biscayart C, Carrega ME, Sagradini S, et al. Yellow fever vaccine-associated adverse events following extensive immunization in Argentina. Vaccine. 2014;32(11):1266–1272. doi: 10.1016/j.vaccine.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 66.Fernandes GC, Camacho LA, Sa Carvalho M, Batista M, de Almeida SM. Neurological adverse events temporally associated to mass vaccination against yellow fever in Juiz de Fora, Brazil, 1999–2005. Vaccine. 2007;25:3124–3128. doi: 10.1016/j.vaccine.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 67.Fitzner J, Coulibaly D, Kouadio DE, et al. Safety of the yellow fever vaccine during the September 2001 mass vaccination campaign in Abidjan, Ivory Coast. Vaccine. 2004;23:156–162. doi: 10.1016/j.vaccine.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 68.Khromava AY, Eidex RB, Weld LH, et al. Yellow fever vaccine: an updated assessment of advanced age as a risk factor for serious adverse events. Vaccine. 2005;23:3256–3263. doi: 10.1016/j.vaccine.2005.01.089. [DOI] [PubMed] [Google Scholar]
- 69.Schumacher Z, Bourquin C, Heininger U. Surveillance for adverse events following immunization (AEFI) in Switzerland--1991–2001. Vaccine. 2010;28:4059–4064. doi: 10.1016/j.vaccine.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Stuart G. Yellow Fever Vaccination. WHO; Geneva, Switzerland: 1956. Reactions following vaccination against yellow fever; pp. 143–189. [Google Scholar]
- 71.YF-VAX® Package Insert. Sanofi Pasteur; Swiftwater, PA Rev: May 02, 2013. [Google Scholar]
- 72.Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- 73.Sencer DJ, Langmuir AD, Kokko UP, et al. Fatal viral encephalitis following 17D yellow fever vaccine inoculation. Report of a case in a 3-year-old child. JAMA. 1966;198(6):671–672. doi: 10.1001/jama.1966.03110190153047. [DOI] [PubMed] [Google Scholar]
- 74.Jennings AD, Gibson CA, Miller BR, et al. Analysis of a yellow fever virus isolated from a fatal case of vaccine-associated human encephalitis. J Infect Dis. 1994;169(3):512–518. doi: 10.1093/infdis/169.3.512. [DOI] [PubMed] [Google Scholar]
- 75.de Martins RM, Pavao AL, de Oliveira PM, et al. Adverse events following yellow fever immunization: Report and analysis of 67 neurological cases in Brazil. Vaccine. 2014;32(49):6676–6682. doi: 10.1016/j.vaccine.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 76.de Martins RM, de Maia ML, dos Santos EM, et al. Yellow fever vaccine post-marketing surveillance in Brazil. Proc Vaccinol. 2010;2:178–183. [Google Scholar]
- 77.Kitchener S. Viscerotropic and neurotropic disease following vaccination with the 17D yellow fever vaccine, ARILVAX. Vaccine. 2004;22(17–18):2103–2105. doi: 10.1016/j.vaccine.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 78.Guimard T, Minjolle S, Polard E, et al. Short report: Incidence of yellow fever vaccine-associated neurotropic disease. Am J Trop Med Hyg. 2009;81:1141–1143. doi: 10.4269/ajtmh.2009.09-0295. [DOI] [PubMed] [Google Scholar]
- 79.Vasconcelos PF, Luna EJ, Galler R, et al. Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Lancet. 2001;358:91–97. doi: 10.1016/s0140-6736(01)05326-0. [DOI] [PubMed] [Google Scholar]
- 80.Rafferty E, Duclos P, Yactayo S, Schuster M. Risk of yellow fever vaccine-associated viscerotropic disease among the elderly: a systematic review. Vaccine. 2013;31(49):5798–5805. doi: 10.1016/j.vaccine.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 81.Seligman SJ. Risk groups for yellow fever vaccine-associated viscerotropic disease (YEL-AVD) Vaccine. 2014;32(44):5769–5775. doi: 10.1016/j.vaccine.2014.08.051. [DOI] [PubMed] [Google Scholar]
- 82.Monath TP. Suspected yellow fever vaccine-associated viscerotropic adverse events (1973 and 1978), United States. Am J Trop Med Hyg. 2010;82:919–921. doi: 10.4269/ajtmh.2010.10-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas RE, Lorenzetti DL, Spragins W, Jackson D, Williamson T. Active and passive surveillance of yellow fever vaccine 17D or 17DD-associated serious adverse events: systematic review. Vaccine. 2011;29:4544–4555. doi: 10.1016/j.vaccine.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 84.Whittembury A, Ramirez G, Hernandez H, et al. Viscerotropic disease following yellow fever vaccination in Peru. Vaccine. 2009;27:5974–5981. doi: 10.1016/j.vaccine.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 85.Cetron MS, Marfin AA, Julian KG, et al. Yellow fever vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2002. MMWR Recomm Rep. 2002;51(RR-17):1–11. [PubMed] [Google Scholar]
- 86.Fox JP, Lennette EH, Manso C, Souza-Aguiar JR. Encephalitis in man following vaccination with 17D yellow fever virus. Amer J Hyg. 1942;36(2):117–142. [Google Scholar]
- 87.WHO Expert Committee on Biological Standardization. WHO Expert Committee on Biological Standardization. WHO; Geneva, Switzerland: 2012. Recommendations to Assure the Quality, Safety and Efficacy of Live Attenuated Yellow Fever Vaccines; pp. 241–314. [Google Scholar]
- 88.Advisory Committee on Immunization Practices. Poliomyelitis prevention in the United States: introduction of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46(RR-3):1–25. [PubMed] [Google Scholar]
- 89.World Health Organization. Evidence on the safety and efficacy of live poliomyelitis vaccines currently in use, with special reference to type 3 poliovirus. Bull World Health Organ. 1969;40(6):925–945. [PMC free article] [PubMed] [Google Scholar]
- 90.Prevots DR, Sutter RW, Strebel PM, Weibel RE, Cochi SL. Completeness of reporting for paralytic poliomyelitis, United States, 1980 through 1991. Implications for estimating the risk of vaccine-associated disease. Arch Pediatr Adolesc Med. 1994;148(5):479–485. doi: 10.1001/archpedi.1994.02170050037007. [DOI] [PubMed] [Google Scholar]
- 91.Hao L, Toyokawa S, Kobayashi Y. Poisson-model analysis of the risk of vaccine-associated paralytic poliomyelitis in Japan between 1971 and 2000. Jpn J Infect Dis. 2008;61(2):100–103. [PubMed] [Google Scholar]
- 92.Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: What does the evidence show? Vaccine. 2015;33(29):3288–3292. doi: 10.1016/j.vaccine.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Advisory Committee on Immunization Practices. Poliomyelitis prevention in the United States. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49(RR-5):1–22. [PubMed] [Google Scholar]
- 94.Walton LR, Orenstein WA, Pickering LK. Lessons learned from making and implementing vaccine recommendations in the U.S. Vaccine. 2015;33(Suppl 4):D78–82. doi: 10.1016/j.vaccine.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 95.Kramarz P, France EK, Destefano F, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J. 2001;20(4):410–416. doi: 10.1097/00006454-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344(8):564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 97.Zanardi LR, Haber P, Mootrey GT, Niu MT, Wharton M. Intussusception among recipients of rotavirus vaccine: reports to the vaccine adverse event reporting system. Pediatrics. 2001;107(6):E97. doi: 10.1542/peds.107.6.e97. [DOI] [PubMed] [Google Scholar]
- 98.Hayes EB. Is it time for a new yellow fever vaccine? Vaccine. 2010;28(51):8073–8076. doi: 10.1016/j.vaccine.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 99.Monath TP, Cetron MS. Prevention of yellow fever in persons traveling to the tropics. Clin Infect Dis. 2002;34:1369–1378. doi: 10.1086/340104. [DOI] [PubMed] [Google Scholar]
- 100.Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Saunders/Elsevier; Philadelphia, PA: 2008. pp. 1–16. [Google Scholar]
- 101.Black S. The role of health economic analyses in vaccine decision making. Vaccine. 2013;31(51):6046–6049. doi: 10.1016/j.vaccine.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 102.Thompson KM, Tebbens RJ. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal. 2006;26:1423–1440. doi: 10.1111/j.1539-6924.2006.00831.x. [DOI] [PubMed] [Google Scholar]
- 103.Miller MA, Sutter RW, Strebel PM, Hadler SC. Cost-effectiveness of incorporating inactivated poliovirus vaccine into the routine childhood immunization schedule. JAMA. 1996;276(12):967–971. [PubMed] [Google Scholar]
- 104.Monath TP, Lee CK, Julander JG, et al. Inactivated yellow fever 17D vaccine: development and nonclinical safety, immunogenicity and protective activity. Vaccine. 2010;28:3827–3840. doi: 10.1016/j.vaccine.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 105.Amanna IJ, Raue HP, Slifka MK. Development of a new hydrogen peroxide-based vaccine platform. Nat Med. 2012;18(6):974–979. doi: 10.1038/nm.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gaspar LP, Mendes YS, Yamamura AM, et al. Pressure-inactivated yellow fever 17DD virus: implications for vaccine development. J Virol Methods. 2008;150:57–62. doi: 10.1016/j.jviromet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 107.Monath TP, Fowler E, Johnson CT, et al. An inactivated cell-culture vaccine against yellow fever. N Engl J Med. 2011;364(14):1326–1333. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]
- 108.World Health Organization. Immunization, Vaccines and Biologicals - African Region. Geneva: 2015. http://www.who.int/immunization/monitoring_surveillance/data/AFR/en/ [Google Scholar]
- 109.Slifka MK, Amanna I. How advances in immunology provide insight into improving vaccine efficacy. Vaccine. 2014;32(25):2948–2957. doi: 10.1016/j.vaccine.2014.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Safaeian M, Porras C, Pan Y, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013;6(11):1242–1250. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bovier PA, Bock J, Ebengo TF, et al. Predicted 30-year protection after vaccination with an aluminum-free virosomal hepatitis A vaccine. J Med Virol. 2010;82(10):1629–1634. doi: 10.1002/jmv.21883. [DOI] [PubMed] [Google Scholar]
- 112.Wattigney WA, Mootrey GT, Braun MM, Chen RT. Surveillance for poliovirus vaccine adverse events, 1991 to 1998: impact of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine. Pediatrics. 2001;107(5):E83. doi: 10.1542/peds.107.5.e83. [DOI] [PubMed] [Google Scholar]
- 113.Kelso JM, Mootrey GT, Tsai TF. Anaphylaxis from yellow fever vaccine. J Allergy Clin Immunol. 1999;103:698–701. doi: 10.1016/s0091-6749(99)70245-9. [DOI] [PubMed] [Google Scholar]
- 114.Bohlke K, Davis RL, Marcy SM, et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003;112(4):815–820. doi: 10.1542/peds.112.4.815. [DOI] [PubMed] [Google Scholar]