Abstract
Background
Low-income, unemployed women with low levels of education are more likely to smoke during pregnancy compared to their higher income, employed, and well-educated counterparts. The reserve capacity model (RCM) offers a theoretical framework to explain how psychosocial factors may serve as pathways connecting socioeconomic status (SES) to health behaviors. Research supports the link between prenatal smoking and several psychosocial variables such as chronic stressors, depressive symptoms, and social support. How these variables interrelate to explain the predominance of prenatal smoking in lower socioeconomic groups of pregnant women has not been fully elucidated.
Objective
To test the RCM to evaluate the roles of levels of chronic stress, quality of the primary intimate relationship, and depressive symptoms in early pregnancy in explaining the relationship between SES and persistent prenatal smoking.
Methods
A secondary analysis of data from 370 pregnant non-smokers, spontaneous quitters, and persistent prenatal smokers was conducted. Based on the RCM, chronic stressors, depressive symptoms, and the quality of the primary intimate relationship were evaluated as potential mediating variables linking SES with persistent prenatal smoking using path analysis.
Results
Path analyses indicated that a simple model with all three psychosocial variables as mediators of the relationship between SES and persistent prenatal smoking provided the best fit.
Discussion
Findings indicated that chronic stressors, depressive symptoms, and the quality of the primary intimate relationship play important roles in the pathway from SES to prenatal smoking status. This knowledge can assist in the development of prevention and intervention strategies to target these variables and ultimately reduce prenatal smoking.
Keywords: pregnant women, psychosocial aspects, smoking, socioeconomic status
Decades of research highlight the harmful effects of smoking on pregnancy outcomes which include preterm birth, intrauterine growth restriction, low birthweight, fetal demise, and Sudden Infant Death Syndrome (Tong et al., 2013). Prenatal smoking remains a significant, national health problem with an overall national rate of 12.3% (Tong et al., 2013), which far exceeds the desired goal of 1.4% as cited in Healthy People 2020 (U.S. Department of Health and Human Services, 2014). Furthermore, evidence indicates that current prenatal smoking cessation interventions show limited effectiveness—particularly among women of low socioeconomic status (SES) who are most at risk for this behavior (Lumley et al., 2009). The lack of effective smoking cessation interventions is reflected in the wide degree of geographic variation in prenatal smoking prevalence ranging from 4.5% in Utah to 30.5% in West Virginia (Tong et al., 2013).
Research has consistently linked three indicators of low SES with increased prenatal smoking behavior: income, education, and employment. Low-income women are more likely to engage in prenatal smoking compared to women with higher income levels (Tong, Jones, Dietz, D’Angelo, & Bombard, 2009). Lower levels of education (less than high school) are strongly associated with prenatal smoking status (Tong et al., 2009), and pregnant women are more likely to be smokers if they have unskilled jobs or are unemployed (Penn & Owen, 2002). Reviews of epidemiologic and empirical studies confirm the association of these SES indicators with prenatal smoking (Lu, Tong, & Oldenburg, 2001; Schneider & Schütz, 2008), in addition to other potential socioeconomic indicators, such as social status, home ownership, and urban versus nonurban residence (Schneider & Schütz, 2008). Although several different psychosocial variables have been associated with prenatal smoking, the influence of these variables on the relationship between SES and prenatal smoking has not been fully elucidated.
Background
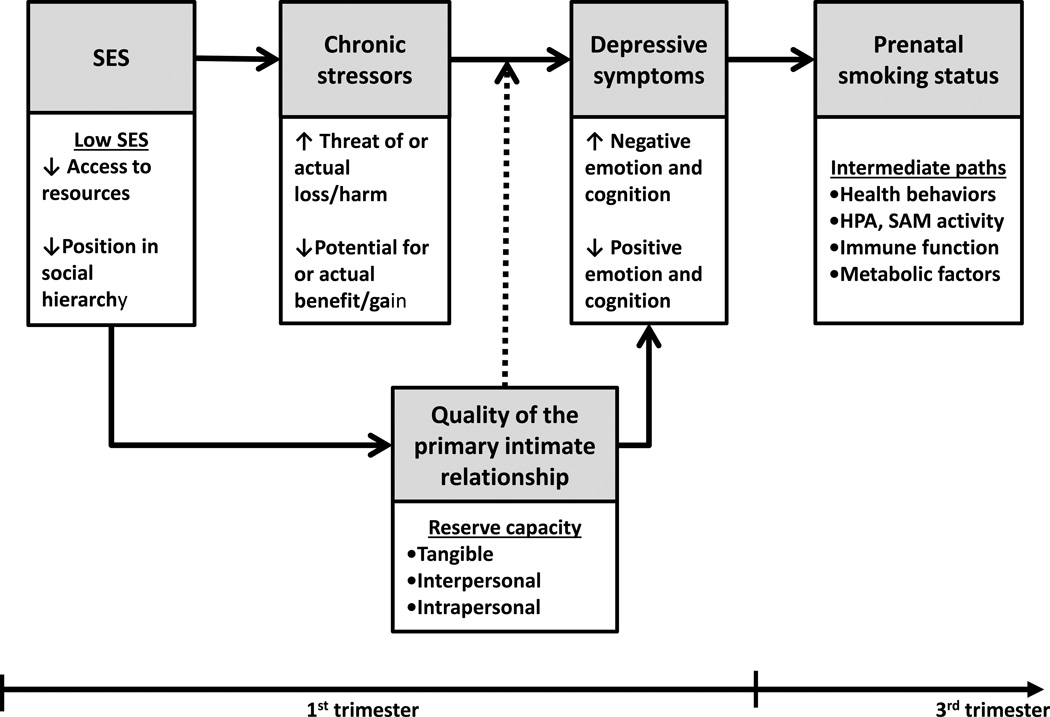
The reserve capacity model (RCM) is a framework proposed to explain how psychosocial factors, over time, serve as pathways connecting SES to health outcomes (Gallo & Mathews, 2003). The model hypothesizes a pathway where exposure to stressors and their related emotional responses function as multiple mediators in a causal chain linking SES to health behaviors like prenatal smoking. The framework further suggests that individuals maintain a bank of resources—both tangible and interpersonal—from which to draw on to deal with stressful events. This bank of resources or “reserve capacity” serves as both a mediator between SES and emotional response, as well as a moderator of the relationship between stress exposure and emotional response (see Figure 1). In the RCM, low SES environments are associated with increased exposure to stressful situations or decreased exposure to beneficial situations. These factors have direct negative effects on emotion and cognition. Individuals with a low SES also have a smaller set of resources—or reserve capacity—to cope with stressful events. This reserve capacity moderates the effect of exposure to stressful or beneficial situations on emotion and cognition which, in turn, has an indirect effect on health outcomes via health behaviors and/or physiologic markers of chronic stress.
FIGURE 1.
Proposed relationships among SES, chronic stressors, quality of the primary intimate relationship, depressive symptoms and prenatal smoking status based on the reserve capacity model (RCM; Matthews et al., 2010).
Since its inception, aspects of the RCM have been supported in studies of various health outcomes. Matthews, Räikkönen, Gallo, and Kuller (2008) found support for the mediation pathway from SES through reserve capacity (operationalized as optimism, self-esteem, and social support) and negative emotion (operationalized as depressive symptoms, anger, and tension) to metabolic syndrome. Similar support was found in a study of the association between poverty and health status appraisals in patients postmyocardial infarction (Bennett, Buchanan, Jones, & Spertus, 2015) and a study of RCM mediational pathways between SES and health-related quality of life (Howarter & Bennett, 2013). Support for the moderating effect of reserve capacity factors on the relationship between stress exposure and negative emotion has also been demonstrated. Education moderated the relationship of perceived racism on negative affect (Brondolo et al., 2008), and numerous studies have shown that interpersonal resources can buffer the pathogenic impact of stress (Cohen & Wills, 1985). These studies provide evidence for the usefulness of the RCM in understanding psychosocial pathways that connect SES with health behaviors like prenatal smoking.
The RCM incorporates several psychosocial variables implicated in prenatal smoking behavior. The three psychosocial variables commonly found in prenatal smoking research are stress, social support, and depressive symptoms. Sources of stress reported to affect prenatal smoking status include: financial stress (Bullock, Mears, Woodcock, & Record, 2001), parenting challenges, living in disruptive home environments, and lack of social support (Pletsch, Morgan, & Pieper, 2003). Although a lack of social support is commonly defined as being unmarried/single, a more discriminating indicator of social support may be the quality of a woman’s social relationships. The effect of the quality of the social relationships on prenatal smoking status is not well understood. Additionally, the link between depressive symptoms and prenatal smoking is strong. Prenatal smokers have a higher rate of depressive symptoms than nonsmokers (Linares Scott, Heil, Higgins, Badger, & Bernstein, 2009). Depressive symptoms also predict prenatal smoking status (Zhu & Valbø, 2002). Thus, the three psychosocial variables of stress, social support, and depressive symptoms have been independently linked to prenatal smoking.
Interrelationships among stress, depressive symptoms, and social support and their relationship to low SES have been alluded to in the literature. For example, research on stress and prenatal smoking indicates that sources of stress are related to low SES and low social support (Bullock et al., 2001). Women identified feelings of social isolation and lack of partner support as contributing to feelings of stress (Pletsch et al., 2003). Depressive symptoms also appear to interact with social resources to affect prenatal smoking behavior. Nichter et al. (2007) found that persistent prenatal smokers have poor support for quitting, a lack of control over their environment, a dearth of social and financial resources, and higher rates of depression. Exactly how these variables interrelate with one another and SES and lead to prenatal smoking behavior is unclear. A more in-depth understanding of the psychosocial pathways that link SES to prenatal smoking behavior is needed.
Study Aims
The purpose of this study was to test whether aspects of the RCM could be used to elucidate socioeconomic discrepancies in prenatal smoking behavior. Specifically, we tested the model’s proposed pathways linking SES to health behaviors. Our hypotheses were derived from a portion of the RCM. We predicted that early pregnancy levels of chronic stressors, depressive symptoms, and quality of the primary intimate relationship would mediate the effects of SES on third trimester prenatal smoking status; and that quality of the primary intimate relationship (the conceptualization of reserve capacity) would moderate the effect of chronic stressors on depressive symptoms (Figure 1).
Methods
Data Source
We conducted secondary analysis of a nonexperimental multicenter study of 370 pregnant women. Data for these women were collected between June 2008 and May 2013 at three prenatal time points (5–13 weeks; 14–26 weeks; 27–36 weeks gestation). (The purposes of the original study were to explore the hypothesis that preterm birth and low birthweight are associated with higher levels of prenatal inflammatory markers in saliva, serum and cervico-vaginal fluid, and determine if psychosocial and biobehavioral variables in combination with these inflammatory markers pose a significant risk for adverse birth outcomes; Ashford et al., 2016).
The current study is a cross-sectional prevalence study combined with a longitudinal panel study of psychosocial predictors of prenatal smoking status at the third trimester of pregnancy. Women with a singleton pregnancy, no history of diabetes, heart disease, sexually transmitted disease, multifetal pregnancy, or second trimester bacterial vaginosis, and at least 18 years of age, were included. Women with a current history of illegal or prescription drug abuse were excluded. Participants were recruited from two different prenatal clinics. Institutional Review Board approval was obtained from the University of Louisville.
Variables and Measurement
All SES and psychosocial variable measures were collected at the same time point in early pregnancy. Smoking status was determined from third trimester data to evaluate the ability of SES and psychosocial pathways to predict persistent prenatal smoking behavior. Summed scores for the socioeconomic status composite variable and each psychosocial mediator variable were used for the path analysis.
Smoking status
The women were divided into three groups based on their smoking status in the third trimester: nondmoker (NS), spontaneous quitter (SQ), and persistent prenatal smoker (PPS). Smoking status was operationalized as an ordered-categorical variable based on self-report questions indicating the length of time smoked. The three ordered levels consisted of: nonsmokers (pregnancies with no tobacco exposure); spontaneous quitters (pregnancies with early tobacco exposure); and persistent prenatal smokers (pregnancies with ongoing exposure). Levels of exposure were verified with urine cotinine. Urine cotinine and self-report were not correlated in the first trimester, however, they were weakly correlated in the second and third trimesters (rs = .13, p < .05, rs = .16, p < .05). This lack of/weak association is not surprising given the poor concordance reported in the literature between self-reported smoking and biomarkers of smoking behavior (Britton, Brinthaupt, Stehle, & James, 2004).
Socioeconomic status
In the present study, a composite variable was created to capture the multidimensional nature of SES by summing scores of three variables: income, education, and employment status. Annual household income was a categorical variable (0 = ≤ $20,000; 1 = $20,000–$39,999; and 2 = ≥ $40,000); both education (0 = ≤ high school; 1 = > high school) and employment status (0 = Unemployed; 1 = Employed) were dichotomized. Scores on the composite SES variable ranged from 0–4; higher scores reflected a higher level of SES. Correlations between each component variable and the composite score were strong (range: .62−.89, p < .001).
Chronic stressors
The Everyday Stressors Index (ESI) measures low-income mothers’ perceptions of chronic stressors they face on a daily basis (Hall, 1983). The 20-item ESI assesses five problem areas: role overload, financial concerns, parenting worries, employment problems, and interpersonal conflict. Respondents rate how much each problem worries, upsets, or bothers them using a 4-point scale of 0 = not at all bothered to 3 = bothered a great deal. Scores are summed and can range from 0–60 (Hall, 1983). In samples of mothers of young children, the ESI demonstrated reliability, with alphas ranging from .81 to .86 (Hall, Williams, & Greenberg, 1985; Peden, Rayens, Hall, & Grant, 2004). Content and construct validity of the ESI were also supported in a number of studies (Hall, Kotch, Browne, & Rayens, 1996; Pollock, Amankwaa, & Amankwaa, 2005). Cronbach’s alpha in the current sample was .87.
Quality of the Primary Intimate Relationship (QPIR)
The Autonomy and Relatedness Inventory (ARI) is a 32-item instrument that assesses the QPIR in the following eight areas: autonomy, relatedness, acceptance, support, listening, control, detachment/rejection, and hostile control (Hall, 1983; Schaefer & Edgerton, 1982). Women responded to items in reference to the person they identified as most important in their lives. Responses are given on a 5-point Likert scale ranging from 1 = not at all like to 5 = very much like the intimate (Hall & Kiernan, 1992). Negative items are reverse scored and all item responses are summed; 32 is subtracted from the total to form a cumulative score ranging from 0 to 120. The ARI demonstrated good reliability and validity in studies with mothers and married couples. Cronbach’s alphas ranged from .75 to .90; subscale alphas ranged from .53 to .77 (Hall & Kiernan, 1992; Hall et al., 1985). The measure also demonstrated good content, convergent, and factorial validity (Hall & Kiernan, 1992). Cronbach’s alpha in the current sample was .94.
Depressive symptoms
The Edinburgh Postnatal Depression Scale (EPDS; Cox, Holden, & Sagovsky, 1987) is a 10-item, self-rated scale used to screen for postpartum depression. Items are scored on a three-point scale from 0 to 3. Responses are summed to form a cumulative score that ranges from 0 to 30. The suggested threshold for follow up in a routine primary care setting is a score of 9–10; higher scores may indicate depressive illness (Cox, Holden, & Sagovsky, 1987). Cronbach’s alphas in samples of pregnant women ranged from .82–.84; test-retest reliability across all three trimesters ranged from .55–.63 (Bergink et al., 2011; Bunevicius, Kusminskas, Pop, Pedersen, & Bunevicius, 2009). Construct validity was supported by substantial correlations between the EPDS and the anxiety and somatization subscales of the Symptom Checklist-90 (Bergink et al., 2011). Cronbach’s alpha in the current sample was .86.
Secondhand smoke exposure
Participants were asked, “How many hours in a day are you exposed to other people’s tobacco smoke indoors at home?” Based on their response, secondhand smoke (SHS) exposure in the home was dichotomized to reflect any exposure (0 = 0 hours, no exposure; 1 = > 0 hours, exposure to SHS).
Demographic characteristics
Information about age, parity, and marital status was collected via self-report. Race was dichotomized as either Caucasian or non-Caucasian due to the lack of racial variability among the participants (67% of the total sample self-identified as White).
Data Analysis
Descriptive statistics and differences among smoking status groups were compared using SPSS software version 22.0 (IBM Corp., 2013). Missing data were imputed using maximum likelihood estimation in AMOS 22. Rates of missing data for all variables ranged from 0.27% to 3.51%. Parity data was missing for 17.57% of participants. This was due to a failure to collect this data item from a significant number of women. The overall missing rate was 2.76%. The Little’s MCAR test obtained for this study’s data resulted in a χ2 = 3.79 (df = 3, p = .29) indicating that the missing at random null hypothesis could not be rejected.
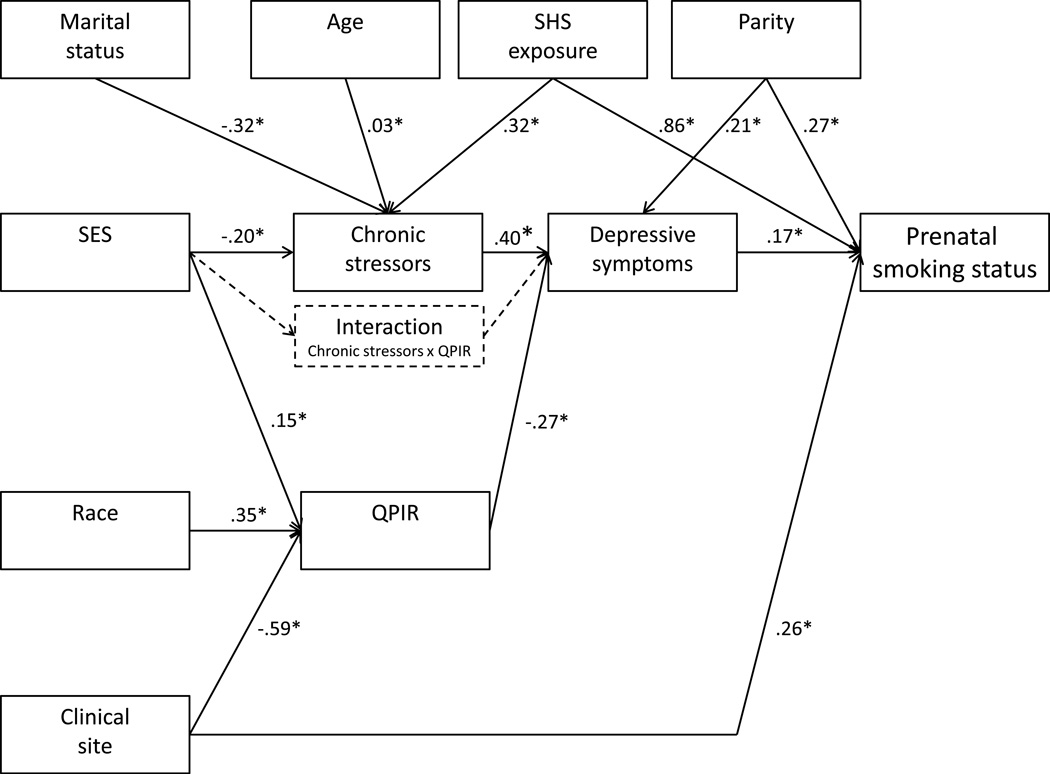
Associations among variables were tested prior to testing the model. Theoretical models with and without the hypothesized interaction term were translated to path models that were estimated and tested. Key theoretical relationships are shown in Figure 2. Covariates (parity, age, race, marital status, SHS exposure, and clinical site) predicted all other variables except SES. A probit model was estimated due to the ordinal nature of the outcome variable (smoking status). Bayesian estimates were obtained using the Markov Chain Monte Carlo approach (MCMC) in AMOS version 22 (Arbuckle, 2013). Significant paths (determined according to a 95% confidence interval) were retained for the estimation of a reduced model.
FIGURE 2.
Path model testing chronic stressors, depressive symptoms, and quality of the primary intimate relationship as multiple mediators in the relationship between SES and prenatal smoking status. This model also tested quality of the primary intimate as a moderator of the relationship between chronic stressors and depressive symptoms.
Two Bayesian estimation fit measures were used to evaluate path model fit. These are the Deviance Information Criterion (DIC) and the posterior predictive p-value (PP p-value). The DIC provides a Bayesian measure of model fit or adequacy similar to model fit criteria like the AIC (Akaike information criterion) or the BIC (Schwarz Bayesian information criterion) used in the classical modeling framework (Spiegelhalter, Best, Carlin, & Van Der Linde, 2002). As with the more traditionally used criteria, a smaller DIC indicates better fit. The PP p-value is a simple method for assessing the goodness of fit of a posited model in Bayesian analysis. A value of about .5 indicates a plausible model and values toward the extremes of 0 or 1 indicate implausibility (Lee & Song, 2003).
Results
Participant Characteristics
The mean age of the 370 participants was 25.9 years, SD = 5.2. Other sociodemographic and personal characteristics of the sample are summarized in Table 1. The majority of the sample was evenly split between the lowest and the highest income levels. Most of the women had some post-high school education, were employed either full or part time, White, married/partnered, and primiparous. The mean SES composite score was 2.3, SD = 1.4. Of the women, 202 (54.4%) were nonsmokers, 84 (22.7%) were spontaneous quitters, and 84 (22.7%) were persistent prenatal smokers. Overall, the women had a low level of depressive symptoms (M = 5.7, SD = 5.0), a moderate level of chronic stressors (M = 30.61, SD = 8.5), and a high QPIR (M = 110.10, SD = 16.0). Most participants identified a husband, boyfriend, or partner as their primary intimate (n = 237; 64.1%). Almost 22% (n = 80) listed their mothers as the intimate; others indicated another family member or friend (n = 44; 12%). Primary intimate data were missing for nine women (2.4%).
TABLE 1.
Association of Sociodemographic and Personal Characteristics with Smoking Status
| All (N = 370) |
NS (n = 202) |
SQ (n = 84) |
PPS (n = 84) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | (%) | n | (%) | n | (%) | n | (%) | p | ES |
| Household income (US$)a | <.001 | .50 | ||||||||
| <20,000 | 143 | (40) | 44 | (22) | 51 | (64) | 48 | (59) | ||
| 20,000–39,999 | 75 | (21) | 34 | (17) | 17 | (21) | 24 | (29) | ||
| ≥40,000 | 142 | (39) | 120 | (61) | 12 | (15) | 10 | (12) | ||
| Education (>HS)b | 239 | (65) | 166 | (83) | 34 | (41) | 39 | (46) | <.001 | .42 |
| Employment (yes)c | 235 | (64) | 147 | (73) | 49 | (58) | 39 | (46) | <.001 | .23 |
| SES (composite)d | <.001 | .38 | ||||||||
| 0 | 49 | (14) | 8 | (4) | 19 | (23) | 22 | (27) | ||
| 1 | 74 | (21) | 20 | (10) | 27 | (32) | 27 | (33) | ||
| 2 | 66 | (18) | 35 | (18) | 19 | (23) | 12 | (15) | ||
| 3 | 56 | (16) | 31 | (16) | 11 | (13) | 14 | (17) | ||
| 4 | 113 | (32) | 98 | (51) | 8 | (10) | 7 | (9) | ||
| Race (Caucasian)e | 245 | (67) | 153 | (77) | 33 | (40) | 59 | (70) | <.001 | .31 |
| Married/partnered (yes)f | 264 | (72) | 164 | (82) | 47 | (56) | 53 | (63) | <.001 | .25 |
| Term deliveries (past, none)g | 208 | (68) | 117 | (73) | 48 | (73) | 43 | (55) | .02 | .16 |
| SHS exposure (yes) | 100 | (27) | 14 | (7) | 33 | (41) | 53 | (63) | <.001 | .53 |
Note. ES = effect size; NS = nonsmoker; PPS = persistent prenatal smoker; SHS = second hand smoke; SQ = spontaneous quitter.
n = 360.
n = 368.
n = 369.
n = 358.
n = 367.
n = 369.
n = 305.
n = 366.
Variables Associated with Smoking Status
All of the categorical sociodemographic and personal characteristics were significantly associated with prenatal smoking status (Table 1). Compared to nonsmokers, persistent smokers were significantly more likely to: (a) have a lower annual household income; (b) have a high school education or less; and (c) be unemployed, single/divorced/separated, and multiparous. This was reflected in differences in SES composite score. Those with the lowest and highest SES levels had significantly different proportions of nonsmokers and persistent smokers. Persistent smokers also were more likely to be exposed to indoor SHS compared to both spontaneous quitters and nonsmokers. Similarly, they were more likely to have smoked over 20 cigarettes per day prior to pregnancy compared to the other two groups.
Mean age differed across the three smoking status groups. Post-hoc comparisons using the Tukey honestly significant difference (HSD) test indicated that the mean age of the persistent smokers was significantly lower than the nonsmokers. Spontaneous quitters were younger than nonsmokers but did not differ in age compared to persistent smokers. Mean baseline scores on the ARI, the ESI, and the EPDS differed significantly by prenatal smoking status. Post-hoc comparisons indicated that persistent smokers and spontaneous quitters had lower mean ARI scores and higher mean ESI and EPDS scores compared to nonsmokers. The means of spontaneous quitters and persistent smokers did not differ on any of the three psychosocial variables (Table 2).
TABLE 2.
Psychosocial Variables by Smoking Status Groups
| Variable | Statistic | NS | SQ | PPS | p |
|---|---|---|---|---|---|
| Age (years)a | M | 27.0 | 24.8 | 24.6 | <.001 |
| SD | (5.15) | (5.11) | (5.08) | ||
| Autonomy/Relatedness Inventoryb | M | 114.0 | 102.7 | 108.1 | <.001 |
| SD | (12.43) | (18.96) | (17.53) | ||
| Everyday Stressors Indexc | M | 27.8 | 33.5 | 34.4 | <.001 |
| SD | (6.43) | (9.47) | (9.50) | ||
| Edinburgh Postnatal Depression Scaled | M | 4.1 | 7.1 | 8.2 | <.001 |
| SD | (4.03) | (5.02) | (5.61) |
Note. NS = nonsmoker; PPS = persistent prenatal smoker; SD = standard deviation; SQ = spontaneous quitter.
n = 368.
n = 357.
n = 369.
n = 369.
Model Testing
Correlations among all study variables are presented in Table 3. Path analysis was used to test the pathways in the hypothesized model. The model easily converged, with a C.S. of 1.0017. The model adequately fit the data with a PP p-value = .50. DIC was 2634.33. Because the coefficients to and from the interaction variable were not significant, a simplified model without the interaction term was re-estimated (Figure 2; see Supplemental Digital Content for detailed results). Control variables were all left in for significant effects on various endogenous variables in the model. The PP p-value remained unchanged at .50. Slight improvement in model fit was indicated by an improved DIC of 1949.63. Path coefficients for the main pathway effects in the simplified model were as follows. SES was negatively related to levels of chronic stressors (b = −0.20, 95% CI [-0.30,-0.11]) and positively related to QPIR (b = 0.15, 95% CI [0.06, 0.25]). Level of chronic stressors and QPIR were both, in turn, associated with level of depressive symptoms (b = 0.40, 95% CI [0.31, 0.48] and b = −0.27, 95% CI [-0.37, −0.18], respectively). Finally, level of depressive symptoms was positively associated with prenatal smoking status (b = 0.17, 95% CI [0.10, 0.24]) on a probit scale; meaning, that for every standard deviation increase in depressive symptoms, the underlying z-score for smoking status increased by 0.17. Category-specific marginal effects were computed to measure the effect of unit change in depressive symptoms on the specific group probability, while holding covariates at their mean values. Findings indicate that for a unit standard deviation increase in depressive symptoms, the probabilities of being in the nonsmoker and spontaneous quitter group decreased by .044 and .023, respectively, and that of being in the persistent smoker group increased by .068. Additionally, with the mediators in the model, there was no direct pathway between SES and prenatal smoking status, suggesting complete mediation.
TABLE 3.
Correlations Among Modeled Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | SES composite | -- | |||||||||||
| 2. | Clinical site | −.394** | -- | ||||||||||
| 3. | Chronic stressors | −.373** | .167** | -- | |||||||||
| 4. | QPIR | .387** | −.366** | −.405** | -- | ||||||||
| 5. | DS | −.384** | .224** | .569** | −.487** | -- | |||||||
| 6. | Interactiona | .057 | −.026 | −.286** | .453** | −.260** | -- | ||||||
| 7. | Prenatal smoking (yes) | −.482** | .211** | .356** | −.217** | .365** | −.135** | -- | |||||
| 8. | Parity (past, ≥1) | −.142* | −.256** | .127* | .038 | .167** | −.029 | .136* | -- | ||||
| 9. | Race (Caucasian) | .408** | −.350** | −.242** | .347** | −.283** | .120* | −.152** | −.055 | -- | |||
| 10. | Married (yes) | .441** | −.212** | −.287** | .225** | −.282** | .119* | −.217** | .002 | .436** | -- | ||
| 11. | Age (years) | .479** | −.219** | −.060 | .150** | −.086 | −.083 | −.191** | .090 | .174** | .314** | -- | |
| 12. | SHS exposure (yes) | −.367** | .082 | .239** | −.119* | .205** | .010 | .531** | .001 | −.064 | −.215** | −.280** | -- |
Note. N = 370; missing data varies by variable. Displayed variables codes are higher values. Pearson correlations are shown except for correlations involving ordinal level data. DS = depressive symptoms; QPIR = Quality of the Primary Intimate Relationship; SES = socioeconomic status composite.
Chronic stressors x QPIR.
p < .05.
p < .01.
These results support our first hypothesis. Levels of chronic stressors, depressive symptoms, and QPIR fully mediated the relationship between SES and prenatal smoking status, thereby lending support to the psychosocial mechanisms proposed by the Reserve Capacity Model. Our second hypothesis was not supported. The quality of a woman’s primary intimate relationship did not moderate the relationship between level of chronic stressors and depressive symptoms.
Discussion
These findings support the use of the RCM as a framework for understanding the relationship between SES and prenatal smoking. The RCM proposes that exposure to stress, negative emotion and cognition, and reserve capacity function as mediation pathways connecting SES to health behaviors (Gallo & Matthews, 2003). This study tested multiple mediator variables, including level of chronic stressors, the quality of the primary intimate relationship as an interpersonal source of reserve capacity, and level of depressive symptoms as a negative emotion. As expected, there were significant negative bivariate associations between SES and prenatal smoking status. These results are consistent with other studies linking low SES with increased prenatal smoking behavior (Higgins et al., 2009; Tong et al., 2009).
Path analyses indicated that a simple model with all three psychosocial variables as mediators provided the best fit to the data. SES indirectly increased the likelihood of prenatal smoking. In our sample, women with low SES levels had higher levels of chronic stressors and depleted levels of reserve capacity measured in terms of quality of the primary intimate relationship. Conversely, higher levels of SES predict lower levels of chronic stressors and higher quality of the primary intimate relationship. Moreover, as stress levels increased and quality of the primary intimate relationship decreased, levels of depressive symptoms increased.
Our findings are consistent with prior research that reported similar associations among low SES, stress, depressive symptoms, and social support. Individuals with low SES are more likely to encounter or live in stress-inducing environments and have a higher rate of depression (Gallo & Matthews, 2003). In previous prenatal research, low SES women had higher stress levels and negative affect (Crittenden, Manfredi, Cho, & Dolecek, 2007) and lower levels of social support (Bullock et al., 2009) compared to those with high SES. High levels of chronic stressors were associated with high levels of depressive symptoms in low-income mothers (Hall, Gurley, Sachs, & Kryscio, 1991; Peden et al., 2004), and there is a direct association between a woman’s depressive symptoms and the emotional support she receives from her partner (Manuel, Martinson, Bledsoe-Mansori, & Bellamy, 2012).
Our results suggest that the three psychosocial variables of chronic stressors, depressive symptoms, and the quality of the primary intimate relationship play a key role in the association of SES to prenatal smoking status. By recognizing these mediational pathways, prevention and intervention strategies can be designed to target these variables and ultimately improve prenatal smoking outcomes. For example, in the RCM, stress is the first variable in the pathway that leads to adverse health behaviors. Stress management for low SES women, however, needs to be tailored to relevant sources of stress. Two key stressors identified by low SES women were parenting challenges and personal health concerns (Pletsch et al., 2003). Examples of interventions targeting these sources of stress may include offering parenting support groups or taking advantage of the prenatal window for health interventions that address other personal health issues. The impact of these interventions could then be tested using the RCM framework for their effectiveness in mitigating the pathways leading to persistent prenatal smoking.
The effect of QPIR as a moderator of the relationship between chronic stressors and depressive symptoms was not evident in our analysis; however, the main effects of chronic stressors and QPIR on depressive symptoms were significant. Chronic stressors were positively related to depressive symptoms, whereas QPIR was negatively related to depressive symptoms. Our findings differ from some literature that supports the hypothesis that social support buffers the effect of stress on adverse psychological outcomes (Cohen & Wills, 1985). Other studies, including the current one, however, did not find that the quality of social support from a partner acted as a moderator (Manuel et al., 2012). The significant main effect of the quality of the primary intimate relationship on depressive symptoms was demonstrated in previous research (Hall et al., 1985), as was overall emotional support from a woman’s partner (Manuel et al., 2012).
This study offers preliminary evidence for the usefulness of the RCM in understanding prenatal smoking behavior, particularly as a behavior marked by socioeconomic differences between women who continue to smoke during pregnancy and women who do not. The framework offers several targets for intervention. In addition to the individualized psychosocial targets of intervention mentioned above, public policies that have a positive impact on SES variables (income, education, and employment) and smoke-free and cigarette taxation policies that reduce the level of tobacco exposure and availability often seen in low-SES populations are likely to reduce the prevalence of persistent prenatal smoking.
To the best of our knowledge, this is the first study to show that quality of a woman’s primary intimate plays an important role in understanding prenatal smoking behavior. Our findings contribute to the clarification of how SES interacts with these psychosocial mechanisms to increase the likelihood of prenatal smoking. Future research should explore ways to improve this model with the addition of other potential mediators, such as nicotine dependence, biological measures of stress, external environmental factors (neighborhood disadvantage, perceived safety or social standing, levels of racial discrimination), access to prenatal care, and behavior/motivation to change factors.
Limitations
The greatest limitation is that the findings were based on secondary analysis of existing data. Therefore, the method of smoking status assignment in this study may not have allowed for precise discrimination of the women’s smoking status. The NS group, for example, included women who were never smokers and women whose survey answers indicated that they had quit smoking over one year ago. The grouping method also may not have captured occasional smokers or those who reduced the number of cigarettes smoked per day. Secondly, the exclusion criteria imposed for reducing confounding variables in the parent study limits the generalizability of the findings. Finally, the purpose of this study was to investigate the pathways linking psychosocial variables in early pregnancy to late trimester smoking; though, future studies are needed to examine longitudinal data for mediator variables in order to assess temporal interactions between each mediator variable.
Conclusion
Our findings support the reserve capacity model as a framework for understanding the relationship between SES and prenatal smoking. Evidence for the moderating role of the quality of the primary intimate relationship was absent; however, all three psychosocial variables were instrumental as mediators of the relationship between SES and prenatal smoking status. Chronic stressors, depressive symptoms, and the quality of the primary intimate relationship all contributed to the explanation of the relationship between SES and prenatal smoking behavior. Healthcare providers and policymakers cannot ignore the influence of psychosocial factors on prenatal smoking. Specifically, interventions for socioeconomically disadvantaged pregnant women may be more effective if they target variables that mediate the SES prenatal smoking relationship, i.e., chronic stressors unique to low SES women, counseling for depressive symptoms, and addressing issues with a woman’s primary intimate. These interventions may be more effective than standard cessation interventions by targeting stressors and circumstances uniquely pertinent to those most vulnerable to this behavior.
Supplementary Material
Acknowledgments
The authors acknowledge that this project was supported by grant #1P30 GM110788-01 from the National Institute of General Medical Sciences, grant #T32 NR01275 from the National Institute of Nursing Research, and a Dissertation Completion Award from the University of Louisville School of Interdisciplinary and Graduate Studies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, the National Institute of Nursing Research, or the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to report.
Supplemental Digital Content 1. Simplified model summary is provided in combined file.pdf
Contributor Information
Irene Yang, Emory University, School of Nursing, Atlanta, GA.
Lynne Hall, University of Louisville, School of Nursing, Louisville, KY.
Kristin Ashford, University of Kentucky, College of Nursing, Lexington, KY.
Sudeshna Paul, Emory University, School of Nursing, Atlanta, GA.
Barbara Polivka, University of Louisville, School of Nursing, Louisville, KY.
S. Lee Ridner, University of Louisville, School of Nursing, Louisville, KY.
References
- Arbuckle JL. IBM® SPSS® Amos™ 22 user’s guide. Crawfordville, FL: Amos Development Corporation; 2013. [Google Scholar]
- Ashford KB, Chavan N, Wiggins A, McCubbin AK, Barnett J, O’Brien JM. Patterns of systemic and cervicovaginal fluid (CVF) inflammatory analytes throughout pregnancy: Relation to preterm birth. American Journal of Obstetrics and Gynecology. 2016;214(Suppl. 1):S213–S214. [Google Scholar]
- Bennett KK, Buchanan DM, Jones PG, Spertus JA. Socioeconomic status, cognitive-emotional factors, and health status following myocardial infarction: Testing the Reserve Capacity Model. Journal of Behavioral Medicine. 2015;38:110–121. doi: 10.1007/s10865-014-9583-4. [DOI] [PubMed] [Google Scholar]
- Bergink V, Kooistra L, Lambregtse-van den Berg MP, Wijnen H, Bunevicius R, van Baar A, Pop V. Validation of the Edinburgh Depression Scale during pregnancy. Journal of Psychosomatic Research. 2011;70:385–389. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Britton GRA, Brinthaupt J, Stehle JM, James GD. Comparison of self-reported smoking and urinary cotinine levels in a rural pregnant population. Journal of Obstetric, Gynecologic & Neonatal Nursing. 2004;33:306–311. doi: 10.1177/0884217504264866. [DOI] [PubMed] [Google Scholar]
- Brondolo E, Brady N, Thompson S, Tobin JN, Cassells A, Sweeney M, Contrada RJ. Perceived racism and negative affect: Analyses of trait and state measures of affect in a community sample. Journal of Social and Clinical Psychology. 2008;27:150–173. doi: 10.1521/jscp.2008.27.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock L, Everett KD, Mullen PD, Geden E, Longo DR, Madsen R. Baby BEEP: A randomized controlled trial of nurses’ individualized social support for poor rural pregnant smokers. Maternal and Child Health Journal. 2009;13:395–406. doi: 10.1007/s10995-008-0363-z. [DOI] [PubMed] [Google Scholar]
- Bullock LFC, Mears JLC, Woodcock C, Record R. Retrospective study of the association of stress and smoking during pregnancy in rural women. Addictive Behaviors. 2001;26:405–413. doi: 10.1016/s0306-4603(00)00118-0. [DOI] [PubMed] [Google Scholar]
- Bunevicius A, Kusminskas L, Pop VJ, Pedersen CA, Bunevicius R. Screening for antenatal depression with the Edinburgh Depression Scale. Journal of Psychosomatic Obstetrics & Gynecology. 2009;30:238–243. doi: 10.3109/01674820903230708. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychology Bulletin. 1985;98:310. [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Crittenden KS, Manfredi C, Cho YI, Dolecek TA. Smoking cessation processes in low-SES women: The impact of time-varying pregnancy status, health care messages, stress, and health concerns. Addictive Behaviors. 2007;32:1347–1366. doi: 10.1016/j.addbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychology Bulletin. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- Hall LA. Supports, stressors, and depressive symptoms in low-income mothers of young children. Chapel Hill, NC: University of North Carolina at Chapel Hill; 1983. Unpublished doctoral dissertatation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LA, Gurley DN, Sachs B, Kryscio RJ. Psychosocial predictors of maternal depressive symptoms, parenting attitudes, and child behavior in single-parent families. Nursing Research. 1991;40:214–220. [PubMed] [Google Scholar]
- Hall LA, Kiernan BS. Psychometric assessment of a measure of the quality of primary intimate relationships. Health Values. 1992;16(4):30–39. [Google Scholar]
- Hall LA, Kotch JB, Browne D, Rayens MK. Self-esteem as a mediator of the effects of stressors and social resources on depressive symptoms in postpartum mothers. Nursing Research. 1996;45:231–238. doi: 10.1097/00006199-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Hall LA, Williams CA, Greenberg RS. Supports, stressors, and depressive symptoms in low-income mothers of young children. American Journal of Public Health. 1985;75:518–522. doi: 10.2105/ajph.75.5.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Badger GJ, Skelly JM, Solomon LJ, Bernstein IM. Educational disadvantage and cigarette smoking during pregnancy. Drug and Alcohol Dependence. 2009;104:S100–S105. doi: 10.1016/j.drugalcdep.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarter AD, Bennett KK. Perceived discrimination and health-related quality of life: Testing the Reserve Capacity Model in Hispanic Americans. Journal of Social Psychology. 2013;153:62–79. doi: 10.1080/00224545.2012.703973. [DOI] [PubMed] [Google Scholar]
- IBM Corp. SPSS Statistics for Windows (Version 22.0) Armonk, NY: IBM Corp; 2013. [Google Scholar]
- Lee S-Y, Song X-Y. Bayesian analysis of structural equation models with dichotomous variables. Statistics in Medicine. 2003;22:3073–3088. doi: 10.1002/sim.1544. [DOI] [PubMed] [Google Scholar]
- Linares Scott TJ, Heil SH, Higgins ST, Badger GJ, Bernstein IM. Depressive symptoms predict smoking status among pregnant women. Addictive Behaviors. 2009;34:705–708. doi: 10.1016/j.addbeh.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Tong S, Oldenburg B. Determinants of smoking and cessation during and after pregnancy. Health Promotion International. 2001;16:355–365. doi: 10.1093/heapro/16.4.355. [DOI] [PubMed] [Google Scholar]
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy (Review) Cochrane Library. 2009 doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel JI, Martinson ML, Bledsoe-Mansori SE, Bellamy JL. The influence of stress and social support on depressive symptoms in mothers with young children. Social Science & Medicine. 2012;75:2013–2020. doi: 10.1016/j.socscimed.2012.07.034. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Räikkönen K, Gallo L, Kuller LH. Association between socioeconomic status and metabolic syndrome in women: Testing the reserve capacity model. Health Psychology. 2008;27:576–583. doi: 10.1037/0278-6133.27.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichter M, Nichter M, Muramoto M, Adrian S, Goldade K, Tesler L, Thompson J. Smoking among low-income pregnant women: An ethnographic analysis. Health Education & Behavior. 2007;34:748–764. doi: 10.1177/1090198106290397. [DOI] [PubMed] [Google Scholar]
- Peden AR, Rayens MK, Hall LA, Grant E. Negative thinking and the mental health of low-income single mothers. Journal of Nursing Scholarship. 2004;36:337–344. doi: 10.1111/j.1547-5069.2004.04061.x. [DOI] [PubMed] [Google Scholar]
- Penn G, Owen L. Factors associated with continued smoking during pregnancy: Analysis of socio-demographic, pregnancy and smoking-related factors. Drug and Alcohol Review. 2002;21:17–25. doi: 10.1080/09595230220119291. [DOI] [PubMed] [Google Scholar]
- Pletsch PK, Morgan S, Pieper AF. Context and beliefs about smoking and smoking cessation. MCN: American Journal of Maternal/Child Nursing. 2003;28:320–325. doi: 10.1097/00005721-200309000-00012. [DOI] [PubMed] [Google Scholar]
- Pollock MA, Amankwaa LC, Amankwaa AA. First-time fathers and stressors in the postpartum period. Journal of Perinatal Education. 2005;14:19–25. doi: 10.1624/105812405X44682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ES, Edgerton ME. Autonomy and relatedness inventory. Chapel Hill, NC: University of North Carolina; 1982. Unpublished manuscript. [Google Scholar]
- Schneider S, Schütz J. Who smokes during pregnancy? A systematic literature review of population-based surveys conducted in developed countries between 1997 and 2006. European Journal of Contraception & Reproductive Health Care. 2008;13:138–147. doi: 10.1080/13625180802027993. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64:583–639. [Google Scholar]
- Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, England LJ. Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. Morbidity and Mortality Weekly Report, Surveillance Summaries. 2013;62:1–19. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6206a1.htm?utm_source=rss&utm_me. [PubMed] [Google Scholar]
- Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy: Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. Morbidity and Mortality Weekly Report, Surveillance Summaries. 2009;58:1–29. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, ODPHP. Healthy People 2020. 2014 Retrieved from http://www.healthypeople.gov/2020/topics-objectives/topic/maternal-infant-and-child-health/objectives.
- Zhu S-H, Valbø A. Depression and smoking during pregnancy. Addictive Behaviors. 2002;27:649–658. doi: 10.1016/s0306-4603(01)00199-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.