Abstract
Purpose
In intracytoplasmic sperm injection (ICSI) of oocytes with a fragile oolemma (fragile oocytes), breakage can occur at injection. In this study, we produced embryos from oocytes with a fragile and normal oolemma (normal oocytes) by ICSI and compared their ability to be fertilized and develop in vitro. We also investigated whether fragile oocyte-derived embryos could implant after blastocyst transfer to determine whether fragile oocytes should be used for assisted reproductive technology treatment.
Methods
Oocytes were divided into three groups—normal oocytes from cycles containing no fragile oocytes (group A), normal oocytes from cycles containing at least one fragile oocyte (group B), and fragile oocytes (group C), and their fertilization abilities after ICSI and the developmental abilities of resultant embryos were compared.
Results
The fertilization rate in group C (65.3 %) was significantly (P < 0.01) lower than those in groups A (84.6 %) and B (86.9 %), and the degeneration rate in group C (24.2 %) was significantly (P < 0.01) higher than those in groups A (0.71 %) and B (0.28 %). However, there were no significant differences in the blastocyst formation rates (59.7–67.5 %) of embryos among the different groups. In addition, the pregnancy rate after transfer of blastocysts in group C (50.0 %) was not significantly different from those in groups A (35.6 %) and B (45.8 %).
Conclusions
The fertilization ability after ICSI of fragile oocytes is lower than that of normal oocytes but the resultant embryos have the same developmental ability as those of normal oocyte-derived embryos.
Keywords: Embryo, Intracytoplasmic sperm injection, In vitro fertilization, Oocyte plasma membrane, Oolemma, Time-lapse incubator
Introduction
Intracytoplasmic sperm injection (ICSI) is a method of in vitro fertilization (IVF) in which a single sperm is introduced directly into the cytoplasm of a matured oocyte by penetration of the plasma membrane known as the oolemma. The ICSI method is considered an essential technique in modern assisted reproductive technology (ART) [1–4]. However, degeneration of oocytes occurs after ICSI even when maximum care is exercised. Rosen et al. [5] suggested that oocyte degeneration is a common phenomenon experienced by all technicians who perform ICSI. Van Steirteghem et al. [6] reported that an average of 10 % of oocytes may be lost because of degeneration as a result of the procedure. Degeneration of oocytes is particularly evident when the oolemma is very fragile, resulting in sudden breakage of the membrane during ICSI [7, 8]. The observation that oocytes with a fragile oolemma (fragile oocytes) degenerate easily can be explained by the absence of the protective effect of the funnel responsible for sealing the breach. The discontinuity of the oolemma might affect the cortical component of the cytosol and consequently the oocyte cytoskeleton, leading to disturbances in the microtubules responsible for chromatid segregation and expulsion (in the form of polar body) at the second meiotic division [7].
There are patients in which most of the retrieved oocytes have the fragile oolemma, resulting in a high degeneration rate after ICSI [7, 9]. In such cases, we sometimes have no choice but to transfer embryos derived from fragile oocytes. In general, they are cultured for 5 to 6 days and then, embryos that have reached the blastocyst stage are cryopreserved and later warmed to be transferred after the next cycle because the pregnancy rate after the transfer of cryopreserved blastocysts is higher than that of fresh early embryos [10, 11]. For these reasons, we need to examine whether blastocysts can be obtained from fragile oocytes and the blastocysts can be implanted after cryopreservation.
In this study, therefore, we produced embryos from oocytes with fragile or normal oolemma by ICSI and compared their ability to be fertilized and developed in vitro. In addition, we investigated whether fragile oocyte-derived embryos could be implanted after the blastocyst transfer process to determine whether fragile oocytes should be used for ART treatment.
Materials and methods
Patients
Between March 2014 and April 2015, we examined the outcome of 133 retrieval cycles. The mean age ± standard deviation (range) of patients at the retrieval cycles was 36.2 ± 4.1 years (27–47 years). Retrieved oocytes were fertilized by ICSI (76 retrieval cycles) or split cycle combined ICSI and conventional IVF (57 retrieval cycles) using standard techniques [2, 3]. For split cycle, oocytes were randomized to conventional IVF or ICSI.
Ethical considerations
All study participants provided informed consent, and the study design was approved by the appropriate ethics review boards. Written informed consent for their treatment and for their outcomes to be described was obtained from all patients.
Ovarian stimulation and oocyte retrieval
Ovarian stimulation was performed using the gonadotropin-releasing hormone (GnRH) analog buserelin acetate (Fuji Pharmaceutical Co., Ltd., Tokyo, Japan) and the short or long protocol together with follicle-stimulating hormone (FSH) and human menopausal gonadotropin (hMG) (ASUKA Pharmaceutical Co., Ltd., Tokyo, Japan) stimulation. Human chorionic gonadotropin (hCG; Fuji Pharmaceutical Co., Ltd.) or leuprolide were administered when the maximum diameter of two or more follicles reached 18 mm. Cumulus/oocyte complexes were retrieved by ultrasound-guided transvaginal follicle aspiration at approximately 36 h after hCG injection. The concentration of the anti-Müllerian hormone (AMH) was measured during a cycle before oocyte retrieval. Values for FSH and hMG represented the amount of these medications used during the oocyte retrieval cycle. The concentration of estradiol was measured 2 days before oocyte retrieval and used as an index for determining the retrieval date. The retrieved oocytes were pre-cultured for 3 h in a 10 % serum-added human tubal fluid (HTF) (NAKA Medical, Tokyo, Japan). After pre-culture, oocytes were denuded by pipetting in 80 U/ml hyaluronidase solution (NAKA Medical).
ICSI of oocytes
A sperm was immobilized in 7 % polyvinylpyrrolidone (PVP) solution (NAKA Medical), and then ICSI was performed in a drop of 20 % serum-added HEPES-HTF (NAKA Medical). We used an injector, IM-11-2 (Narishige, Tokyo, Japan), and injection pipettes, K-MPIP-3130 (Cook Medical, Bloomington, IN, USA).
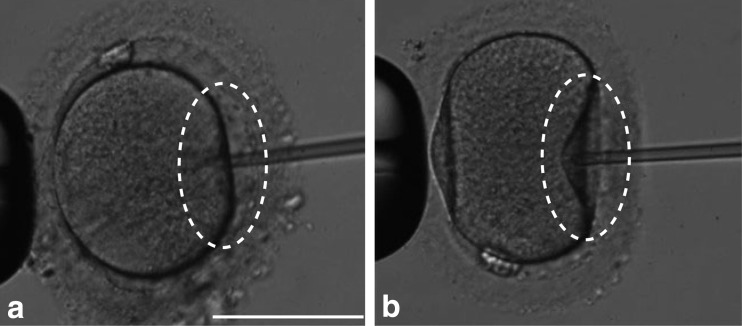
Oocytes whose oolemma was broken at the moment when the injection pipette was inserted into the ooplasm without aspiration during ICSI were defined as fragile oocytes (Fig. 1a). Oocytes whose oolemma was broken by aspiration after the insertion of an injection pipette into the ooplasm were defined as normal oocytes (Fig. 1b). Of 133 cycles examined in this study, 83 cycles (62.4 %) had only normal oocytes and 50 cycles (37.6 %) had at least one fragile oocyte. Normal oocytes from the former and latter were defined as groups A and B, respectively. Fragile oocytes from the latter were defined as group C.
Fig. 1.
Fragile (a) and normal (b) oocytes. As shown in the circles, oolemma was broken by the insertion of an injection pipette in fragile oocytes. Scale bar 100 μm
Culture of embryos
All embryos were transferred to 30 μl of One Step Medium (NAKA Medical) under sterile mineral oil and cultured under 5 % CO2, 5 % O2, and 90 % N2 at 37 °C to the blastocyst stage using EmbryoScope™ (VitroLife, Göteborg, Sweden).
The rates of fertilization, degeneration, blastocyst formation, and good-quality blastocyst formation among the groups A, B, and C were compared. Fertilization was confirmed by the presence of two pronuclei and two polar bodies after ICSI. Oocytes in which ooplasm shrank and darkened within a few hours of ICSI were determined as degenerated oocytes. Good-quality blastocysts were defined as those scoring B or higher for both inner cell mass and trophectoderm grades [12]. In addition, we observed the developmental morphology of the early embryos and compared the second polar body extrusion time, pronuclei appearance, and disappearance times, and the first and second division times among the three groups because these factors can be used to select good-quality embryos [13–15].
Transfer of blastocysts
Fragile oocyte-derived blastocysts were cryopreserved and then transferred to 13 patients for 16 cycles after the next hormone replacement therapy cycle. Their pregnancy rate was compared to that of 78 patients for 132 cycles in which normal oocyte-derived blastocysts were cryopreserved and then transferred at the same time as the first group. Cryopreservation of the embryos using a Cryotop® (Kitazato, Shizuoka, Japan) was performed using established methods [16, 17]. After thawing, a single blastocyst transfer was performed and clinical pregnancy rates were determined based on the presence of a gestational sac as visualized by ultrasound at 3 weeks after embryo transfer.
Statistical analysis
The statistical significance of the differences in the mean values between the two populations was assessed with Student’s t test (Table 1). Statistical analysis was performed using the Bonferroni corrected chi-squared test with continuity correction (Tables 2 and 3) or Kruskal–Wallis analysis of variance (Table 4). P < 0.05 was considered statistically significant.
Table 1.
Patient background
| Group A (83 cycles) | Groups B and C (50 cycles) | |
|---|---|---|
| Age (years) | 36.7 ± 4.0 | 35.3 ± 4.1 |
| No. of oocytes retrieved | 9.8 ± 7.5* | 12.6 ± 5.5** |
| No. of matured oocytes | 8.4 ± 6.7* | 11.0 ± 4.9** |
| FSH (IU) | 1198 ± 225 | 1188 ± 309 |
| hMG (IU)a | 1030 ± 724 | 843 ± 350 |
| E2 (pg/ml)b | 5406 ± 4525* | 7549 ± 6310** |
| AMH (ng/ml)c | 4.1 ± 3.5 | 4.8 ± 4.0 |
Data are presented as the mean ± standard deviation
*, **Values with different superscripts are significantly different (P < 0.05)
FSH follicle-stimulating hormone, hMG human menopausal gonadotropin, E 2 estradiol, AMH anti-Müllerian hormone
aBecause hMG was not measured in 2 patients, data derived from 81 patients were shown in group A
bBecause E2 was not measured in 5 patients, data derived from 78 patients were shown in group A
cBecause AMH was not measured in 16 and 7 patients, data derived from 67 and 43 patients were shown in group A and groups B and C, respectively
Table 2.
Effects of oolemma properties on fertilization after ICSI and subsequent embryonic development
| Group A | Group B | Group C | |
|---|---|---|---|
| No. of oocytes injected | 422 | 351 | 95 |
| No. (%)a of oocytes fertilized | 357 (84.6)* | 305 (86.9)* | 62 (65.3)** |
| No. (%)a of oocytes degenerated | 3 (0.71)* | 1 (0.28)* | 23 (24.2)** |
| No. of embryos cultured | 348d | 305 | 62 |
| No. (%)b of blastocysts | 231 (66.4) | 206 (67.5) | 37 (59.7) |
| No. (%)c of good-quality blastocysts | 134 (58.0) | 123 (59.7) | 24 (64.9) |
*, **Values with different superscripts are significantly different (P < 0.01)
ICSI intracytoplasmic sperm injection
aPercentage per oocytes injected
bPercentage per embryos cultured
cPercentage per blastocysts
dNine fertilized oocytes were cryopreserved or transferred on day 1, 2, or 3
Table 3.
Clinical pregnancy after the transfer of blastocysts derived from normal or fragile oocytes
| Group | No. of embryos transferred | No. (%)a of pregnancies | No. (%)b of miscarriages |
|---|---|---|---|
| A | 73 | 26 (35.6) | 4 (15.4) |
| B | 59 | 27 (45.8) | 7 (25.9) |
| C | 16 | 8 (50.0) | 2 (25.0) |
aPercentage per embryos transferred
bPercentage per pregnancies
Table 4.
Effects of oolemma properties on the developmental process of early embryos
| Group A | Group B | Group C | |
|---|---|---|---|
| No. of embryos observed | 348 | 305 | 62 |
| No. of embryos assessed | 348 | 294a | 57a |
| Second PB extruded ± SD (h) | 3.18 ± 0.95* | 3.32 ± 0.99* | 2.87 ± 0.84** |
| Both PN abutted ± SD (h) | 7.70 ± 1.42 | 7.94 ± 1.59 | 7.81 ± 2.05 |
| Syngamy ± SD (h) | 23.52 ± 3.22 | 23.81 ± 3.33 | 23.93 ± 4.35 |
| First cleavage commenced ± SD (h) | 27.12 ± 3.77 | 27.21 ± 3.88 | 27.60 ± 4.92 |
| Second cleavage commenced ± SD (h) | 39.88 ± 4.56 | 38.99 ± 4.85 | 39.11 ± 5.12 |
*, **Values with different superscripts are significantly different (P < 0.05)
PB polar body, PN pronucleus, SD standard deviation
aEleven and 5 embryos in which at least one of the events was not clearly observed were excluded in groups B and C, respectively
Results
Patients
As shown in Table 1, the number of oocytes retrieved (12.6 ± 5.5) and matured (11.0 ± 4.9) in the patients who provided at least one fragile oocyte (groups B and C) was significantly (P < 0.05) higher than that (9.8 ± 7.5 and 8.4 ± 6.7) in those who provided only normal oocytes (group A), respectively. The estradiol level in groups B and C (7549 ± 6310 pg/ml) was significantly (P < 0.05) higher than that (5406 ± 4525 pg/ml) in group A. There were no significant differences between the two patient groups in terms of the other factors.
Fertilization, degeneration, and blastocyst formation rates
As shown in Table 2, groups A and B showed significantly (P < 0.01) higher fertilization rates (84.6–86.9 %) than group C (65.3 %). The degeneration rates in groups A and B (0.28–0.71 %) were significantly (P < 0.01) lower than those in group C (24.2 %). However, there were no significant differences in the rates of blastocyst formation (59.7–67.5 %) and good-quality blastocyst formation (58.0–64.9 %) among the groups.
Developmental process of early embryos
The second polar body extrusion time in group C (2.87 ± 0.84 h) was significantly (P < 0.05) shorter than those in groups A and B (3.18 ± 0.95–3.32 ± 0.99 h) (Table 4). There were no significant differences among the groups in terms of the other factors.
Pregnancy rates
As shown in Table 3, there were no significant differences in the pregnancy (35.6–50.0 %) and miscarriage (15.4–25.9 %) rates among the groups.
Discussion
The results of this study showed that the numbers of oocytes retrieved and matured and the estradiol level on the 2 days before oocyte retrieval in patients with fragile oocytes are significantly higher than those in patients without fragile oocytes. In ART, we usually perform controlled ovarian stimulation to retrieve multiple oocytes. Joo et al. [18] reported that serum estradiol levels on the day of hCG administration (2 days before oocyte retrieval) have a concentration-dependent effect on the number of oocytes retrieved, and the number of oocytes increases with increasing estradiol levels. This is consistent with our result. On the other hand, Otsuki et al. [19] reported that high serum estradiol levels on the day of hCG administration significantly increased the incidence of smooth endoplasmic reticulum clusters (sERCs) in oocytes and the presence of sERCs significantly decreased the pregnancy rates after the transfer of ICSI oocytes, suggesting that high estradiol levels in patients are associated with the abnormal morphology of human ooplasm. Therefore, the high estradiol level in patients might have increased the incidence of fragile oocytes in this study. Further investigation will be required to clarify this point.
In this study, the fertilization rate after ICSI of fragile oocytes was lower than that of normal oocytes because of the increased rate of degenerated oocytes. This result is consistent with previous reports in which oocyte degeneration was found to be closely related to the characteristics of the oolemma [7, 8]. However, once fragile oocytes were fertilized without degeneration, the developmental ability into the blastocysts of resultant embryos was compared favorably with that of normal oocyte-derived embryos. Therefore, the avoidance of oocyte degeneration after ICSI is the most important to produce blastocysts from fragile oocytes efficiently. A typical oolemma reaction associated with high degeneration rates is sudden breakage when pushing the injection pipette into the oocyte initially [7, 20]. In order to overcome abnormal breakage of the fragile oolemma, Palermo et al. [7] proposed a tangential penetration of the zona pellucida in which the injection pipette is pressed against the zona to penetrate it at 6 or 12 o’clock and then the oocyte is rotated to position the pipette tip at 3 o’clock and the pipette tip is pressed against the oolemma. Moreover, creating a microhole on the zona pellucida of the fragile oocyte by laser beam prior to ICSI provided a less traumatic penetration of the injection pipette into the ooplasm and resulted in lower degeneration and higher embryo development rates [21]. On the other hand, various efforts have also been made to avoid degeneration of normal oocytes after ICSI. To reduce oocyte vulnerability by preventing deformation during ICSI, a laser-assisted opening [20] or partial thinning [22] of the zona pellucida before penetration has been applied. A piezo-micromanipulator is useful for decreasing the degeneration rate of ICSI oocytes [23, 24]. Another report showed that it is possible to improve ICSI outcomes by using an ultra-thin micropipette [25]. These techniques would improve the fertilization rate after ICSI of fragile oocytes, which brings about the efficient production of blastocysts from them.
From the time-lapse observation, it was shown that the second polar body extrusion time after ICSI of fragile oocytes is significantly shorter than that of normal oocytes. In embryonic development, there is an appropriate time for extrusion of the polar body, formation and disappearance of the pronucleus, and cleavage [15, 26, 27]. The results of this study suggest that fragile oocytes cannot extrude the second polar body within an appropriate timeframe, which is probably associated with their abnormal oolemma. Palermo et al. [7, 28] also reported that fragile membranes have the reduced ability to extrude the second polar body. It is unclear whether this phenomenon is related to the increased degeneration rate of fragile oocytes. However, the phenomenon would not affect the subsequent fertilization and developmental process of fragile oocytes because the mean times for abutment of male and female pronuclei, syngamy, first cleavage, and second cleavage of fragile oocytes were not different from those in normal oocytes and each time was similar to that previously reported [15, 26, 27]. The results of this study indicated that the pregnancy and miscarriage rates after the transfer of fragile oocyte-derived blastocysts are at the same level as those of normal oocyte-derived blastocysts, although the number of fragile oocyte-derived blastocysts transferred was limited to 16. These results suggest that fragile oocyte-derived blastocysts have the same ability to be implanted and develop into babies as that of normal oocyte-derived blastocysts. In addition, there were no significant differences in the fertilization rates of oocytes, blastocyst formation rates of embryos, and pregnancy rates between groups A and B. Therefore, the presence of fragile oocytes would not influence the quality of normal oocytes in the same cycle.
In conclusion, this study demonstrated that fragile oocytes are eligible for ART because the fertilization ability after ICSI of fragile oocytes is lower than that of normal oocytes but resultant embryos have the same developmental ability as that of normal oocyte-derived embryos. In the future, the establishment of a method that increases the fertilization rate of fragile oocytes will be essential for their use in ART treatment efficiently.
Acknowledgments
We would like to thank the staff of the Aiiku Ladies Clinic (Kagoshima, Japan).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
This study was approved by the Institutional Review Board of AIIKUKAI Medical Corporation.
Informed consent
Written informed consent for their treatment and for their outcomes to be described was obtained from all patients.
Footnotes
Capsule In intracytoplasmic sperm injection (ICSI) of oocytes with fragile oolemma, breakage can occur at injection. We produced embryos by ICSI of oocytes with fragile and normal oolemma, compared fertilization and development in vitro, and implanted fragile oocyte-derived embryos after blastocyst transfer to determine usability of fragile oocytes in assisted reproduction.
References
- 1.Dumoulin JM, Coonen E, Bras M, Bergers-Janssen JM, Ignoul-Vanvuchelen RC, van Wissen LC, et al. Embryo development and chromosomal anomalies after ICSI: effect of the injection procedure. Hum Reprod. 2001;16:306–12. doi: 10.1093/humrep/16.2.306. [DOI] [PubMed] [Google Scholar]
- 2.Orief Y, Dafopoulos K, Al-Hassani S. Should ICSI be used in non-male factor infertility? Reprod BioMed Online. 2004;9:348–56. doi: 10.1016/S1472-6483(10)62152-9. [DOI] [PubMed] [Google Scholar]
- 3.Palermo G, Joris H, Devroey P, Van Steirteghen AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 4.Vanderzwalmen P, Bertin G, Lejeune B, Nijs M, Vandamme B, Schoysman R. Two essential steps for a successful intracytoplasmic sperm injection: injection of immobilized spermatozoa after rupture of the oolemma. Hum Reprod. 1996;11:540–7. doi: 10.1093/HUMREP/11.3.540. [DOI] [PubMed] [Google Scholar]
- 5.Rosen MP, Shen S, Dobson AT, Fujimoto VY, McCulloch CE, Cedars MI. Oocyte degeneration after intracytoplasmic sperm injection: a multivariate analysis to assess its importance as a laboratory or clinical marker. Fertil Steril. 2006;85:1736–43. doi: 10.1016/j.fertnstert.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Van Steirteghem A, Nagy Z, Joris H, Liu J, Staessen C, Smitz J, et al. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod. 1993;8:1061–6. doi: 10.1093/oxfordjournals.humrep.a138192. [DOI] [PubMed] [Google Scholar]
- 7.Palermo GD, Alikani M, Bertoli M, Colombero LT, Moy F, Cohen J, et al. Oolemma characteristics in relation to survival and fertilization patterns of oocytes treated by introplasmic sperm injection. Hum Reprod. 1996;11:172–6. doi: 10.1093/oxfordjournals.humrep.a019012. [DOI] [PubMed] [Google Scholar]
- 8.Yanagida K, Katayose H, Suzuki K, Suganuma A, Sat A. Flexibility of oolemma is an important factor for oocyte survival after ICSI. J Mamm Ova Res. 2001;18:93–8. doi: 10.1274/jmor.18.93. [DOI] [Google Scholar]
- 9.Nagy ZP, Liu J, Joris H, Bocken G, Desmet B, Van Ranst H, et al. The influence of the site of sperm deposition and mode of oolemma breakage at intracytoplasmic sperm injection of fertilization and embryo developmental rates. Hum Reprod. 1995;10:3171–7. doi: 10.1093/oxfordjournals.humrep.a135881. [DOI] [PubMed] [Google Scholar]
- 10.Cruz M, Gadea B, Garrido N, Pedersen KS, Martínez M, Pérez-Cano I, et al. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time-lapse imaging. J Assist Reprod Genet. 2001;28:569–73. doi: 10.1007/s10815-011-9549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuh D, Zhanq J, Cao S, Zhang J, Heng BC, Huang M, et al. Vitrified-warmed blastocyst cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles—time for a new embryo transfer strategy? Fertil Steril. 2011;95:1691–5. doi: 10.1016/j.fertnstert.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 13.Pribenszky C, Matyas S, Kovacs P, Losonczi E, Zadori J, Vajta G. Pregnancy achieved by transfer of a single blastocyst selected by time-lapse monitoring. Reprod BioMed Online. 2010;21:533–6. doi: 10.1016/j.rbmo.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–71. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 15.Mizobe Y, Akiyoshi T, Minami S, Matsuo K, Fukushima R, Yamaguchi A, et al. Effect of a time-lapse incubator (EmbryoScope®) on in vitro culture of human embryos. J Mamm Ova Res. 2014;31:40–4. doi: 10.1274/jmor.31.40. [DOI] [Google Scholar]
- 16.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod BioMed Online. 2005;11:300–8. doi: 10.1016/S1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 17.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67:73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril. 2010;93:442–6. doi: 10.1016/j.fertnstert.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 19.Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–7. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 20.Nagy ZP, Oliveira SA, Abdelmassih V, Abdelmassih R. Novel use of laser to assist ICSI for patients with fragile oocytes: a case report. Reprod BioMed Online. 2002;4:27–31. doi: 10.1016/S1472-6483(10)61911-6. [DOI] [PubMed] [Google Scholar]
- 21.Abdelmassih S, Cardoso J, Adbelmassih V, Dias JA, Abdelmassih R, Nagy ZP. Laser-assisted ICSI: a novel approach to obtain higher oocyte survival and embryo quality rates. Hum Reprod. 2002;17:2694–9. doi: 10.1093/humrep/17.10.2694. [DOI] [PubMed] [Google Scholar]
- 22.Takahahi I, Hatori A, Nakano H. The usefulness of partial thinning of zona pellucida using a laser-assisted hatching system in ICSI. J Mamm Ova Res. 2013;30:49–52. doi: 10.1274/jmor.30.49. [DOI] [Google Scholar]
- 23.Katayose H, Yanagida K, Shinoki T, Kawahara T, Horiguchi T, Sato A. Efficient injection of bull spermatozoa into oocytes using a piezo-driven pipette. Theriogenology. 1999;52:1215–24. doi: 10.1016/S0093-691X(99)00213-7. [DOI] [PubMed] [Google Scholar]
- 24.Yanagida K, Katayose H, Yazawa H, Kimura Y, Konnai K, Sato A. The usefulness of a piezo-micromanipulator in intracytoplasmic sperm injection in humans. Hum Reprod. 1999;14:448–53. doi: 10.1093/humrep/14.2.448. [DOI] [PubMed] [Google Scholar]
- 25.Hiraoka K, Hiraoka K, Tamaki T, Nada Y, Kiriake C, Yoshie M, et al. Clinical efficiency of an improved piezo-ICSI method using an ultra-thin micropipette. J Mamm Ova Res. 2013;30:53–8. doi: 10.1274/jmor.30.53. [DOI] [Google Scholar]
- 26.Mio Y, Maeda K. Time-lapse cinematography of dynamic changes occurring during in vitro development of human embryos. Am J Obstet Gynecol. 2008;199:660.e1–5. doi: 10.1016/j.ajog.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Mio Y, Iwata K, Yumoto K, Kai Y, Sargant HC, Mizoguchi C, et al. Possible mechanism of polyspermy block in human oocytes observed by time-lapse cinematography. J Assis Reprod Genet. 2012;29:951–6. doi: 10.1007/s10815-012-9815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palermo G, Munne S, Cohen J. The human zygote inherits its mitotic potential from the male gamete. Hum Reprod. 1994;9:1220–5. doi: 10.1093/oxfordjournals.humrep.a138682. [DOI] [PubMed] [Google Scholar]