Abstract
Purpose
The goal for the present study was to implement a technique for protein extraction and identification in human cumulus cells (CCs).
Methods
Forty samples of CCs were collected after ovum pick-up from patients undergoing intracytoplasmic sperm injection (ICSI). Samples were split into the blastocyst group (n = 10), including patients in which all embryos converted into blastocysts, and the non-blastocyst group (n = 10), including patients in which none of the embryos reached the blastocyst stage or the positive-pregnancy (n = 10) and negative-pregnancy group (n = 10). Proteins were extracted and injected into a liquid chromatography system coupled to a mass spectrometer. The spectra were processed and used to search a database.
Results
There were 87 different proteins in samples from the blastocyst and non-blastocyst groups, in which 30 were exclusively expressed in the blastocyst group and 17 in the non-blastocyst group. Among the 72 proteins detected in the pregnancy groups, 19 were exclusively expressed in the positive, and 16 were exclusively expressed in the negative-pregnancy group.
Conclusions
CC proteomics may be useful for predicting pregnancy success and the identification of patients that should be included in extended embryo culture programs.
Keywords: Cumulus cell, Blastocyst, Mass spectrometry, Pregnancy, Proteomics
Introduction
Since the first successful in vitro fertilization (IVF) [1], assisted reproduction techniques (ART) have advanced considerably. Despite these advances, it has been reported that around 70–80 % of embryos produced in vitro fail to implant, and 66 % of IVF cycles fail to result in pregnancy [2]. The low efficiency contributes to the practice of multiple embryo transfer, which seeks to overcome implantation failure and maximize pregnancy rates. Unfortunately, this procedure frequently leads to the inconvenient outcome of multiple pregnancies [3, 4].
A key step in ART is the assessment of gamete and embryo viability to identify the embryo(s) that are most likely to result in a pregnancy. Nevertheless, our knowledge of the molecular determinants of embryo viability is limited, and therefore, current embryo assessment strategies are based on embryo morphology.
Prolonging the duration of culture to day 5 and transfer of blastocyst stage embryos is associated with increased implantation rates compared with the transfer of cleavage stage embryos [5, 6]. Embryo transfer on day 5 allows chromosomally competent embryos to develop to the blastocyst stage and enables a better selection of embryos that have the potential for continued development, because the laboratory assessment occurs after the expression of the embryonic genome [7]. Moreover, transfer of embryos on day 5 has the advantage of physiological synchronization between endometrium function and embryonic development [8, 9]. Single blastocyst transfers have the additional advantages of increasing pregnancy rates and reducing multiple gestations because of their high implantation rates [10, 11].
While blastocyst transfer is beneficial in good prognosis patients, the same is not true for unselected groups of patients [12]. Therefore, some assisted reproduction centers may avoid adopting extended embryo cultures due to an unpredictable rate of blastocyst development.
Therefore, the development of reliable and non-invasive methods is of pivotal importance when selecting patients who could benefit from extended embryo culture programs. In the post-genomic era, many “omics” efforts are focusing on increasing our understanding of the relationships between the genome, DNA transcripts, proteins, metabolites, and phenotypes in cells and organisms [13]. Non-invasive approaches for embryonic development potential have the advantages of increasing the knowledge of embryo physiology, therefore allowing the development of methods to predict developmental competence and viability [14]. These approaches include genomic and proteomic profiling and analytical examination of the embryonic metabolome [13, 15–20].
Bidirectional communication between the oocyte and surrounding cumulus cells (CCs) is essential for the acquisition of oocyte competence [21]. It has been previously reported that because of their close connection with the oocyte, CCs may retain a footprint of the follicular conditions experienced by the oocyte [22].
The knowledge that CCs have a central role in the support of oocyte development and maturation has led various groups to focus their research on the analysis of CC gene expression (for review, see [23]). Although many studies have focused on the analysis of CC gene expression in the search for novel markers of oocyte competence and pregnancy outcome [14, 24–28], this approach does not actually reflect the cell phenotype. In fact, protein synthesis is the major outcome of gene expression and is directly associated with the observed phenotype, which is not always the case with RNA. Therefore, it is clear that protein analysis should be the preferred end-point of all physiological analyses.
Earlier embryonic proteomic studies utilized two-dimensional (2D) gel electrophoresis in combination with analysis of gel images [29, 30]. For known proteins [31] or to correlate protein phosphorylation with embryonic development [32], Western blot analysis has been used. More recently, mass spectrometry (MS) fingerprinting has been demonstrated to provide a reliable approach for the identification of groups of proteins within limited amounts of samples [33, 34].
The goal for the present study was to utilize the analytical power of MS with minimal sample preparation and minute analysis to identify patients that would benefit from extended embryo culture programs and to predict the pregnancy outcome by differential protein expression in CCs.
Materials and methods
Samples
CC samples were collected and stored at −20 °C, immediately after ovum pick-up from 40 patients undergoing intracytoplasmic sperm injection (ICSI) with embryo transfer performed on day 5. Samples were split according to blastocyst formation rate and pregnancy outcome.
There were two experimental groups based on blastocyst formation rate. The blastocyst group (n = 10) included patients in which all embryos had formed blastocysts. The non-blastocyst group (n = 10) included patients in which none of the embryos had reached the blastocyst stage. There were also two experimental groups considering the pregnancy outcome, including the positive-pregnancy group (n = 10) and negative-pregnancy group (n = 10).
The inclusion criteria were as follows: (i) ICSI cycle using ejaculated sperm with embryo transfer performed on day 5; (ii) patient’s age (≤35 years old); (iii) number of retrieved mature oocytes (MII) ≥ 4; (iv) patients without pelvic and/or uterine abnormalities of clinical significance, absence of endometriosis grade III and IV, absence of polycystic ovary syndrome (PCOS); and (v) the presence of both ovaries. All of the cases with severe spermatogenic alterations were excluded from the study.
To meet the inclusion criteria and reach the number of samples in each group, 182 CC samples were collected.
Written informed consent in which patients agreed to share the outcomes of their cycles for research purposes was obtained. The study was conducted in accordance with the principles set out in the Declaration of Helsinki and was approved by the local institutional review board.
Controlled ovarian stimulation and oocyte retrieval
Controlled ovarian stimulation was achieved by using recombinant FSH (Gonal-F; Serono, Geneva, Switzerland), as a daily dose, beginning on day 3 of the cycle. Pituitary blockage was performed by using a GnRH antagonist (Cetrotide, Serono, Geneva, Switzerland), when at least one follicle of at least 14 mm was visualized.
Follicular growth was monitored using transvaginal ultrasound examination starting on day 4 of gonadotropin administration. When adequate follicular growth and serum 17β estradiol levels were observed, recombinant hCG (Ovidrel; Serono, Geneva, Switzerland) was administered to trigger the final follicular maturation. The oocytes were collected 35 h after hCG administration through transvaginal ultrasound ovum pick-up.
Sample collection, preparation of oocytes, and morphological assessment
Immediately after follicle aspiration, the CCs were collected by using two 18-G needles coupled to 5-mL syringes under a stereomicroscope. The samples were stored at −20 °C, and oocytes were maintained in culture medium (Global® for Fertilization, LifeGlobal, Connecticut, USA) supplemented with 10 % human synthetic albumin (HSA, Irvine Scientific, Santa Ana, USA), which was covered with mineral oil (Ovoil™, Vitrolife, Kungsbacka, Sweden) and stored at 37 °C and 6 % CO2 for 5 h.
The surrounding CCs that could not be manually removed were removed by exposure to a HEPES-buffered medium containing hyaluronidase (80 IU/mL, Irvine Scientific, Santa Ana, USA). The remaining CCs were then mechanically removed by gentle pipetting with a hand-drawn Pasteur pipette (Humagen Fertility Diagnostics, Charlottesville, Virginia, USA). Oocyte morphology was assessed immediately prior to sperm injection using an inverted Nikon Diaphot microscope (Eclipse TE 300; Nikon®, Tokyo, Japan) with a Hoffmann modulation contrast system under ×400 magnification. Oocytes that had released their first polar body were considered to be matured and were used for ICSI.
Intracytoplasmic sperm injection
Intracytoplasmic sperm injection was performed on all MII oocytes using the technique described by Palermo et al. [35]. The oocytes were individually placed in 4-μL droplets of buffered medium (Global® w/HEPES, LifeGlobal, Connecticut, USA), and the sperm was placed in a central 4-μL droplet of polyvinylpyrrolidone solution (PVP, Irvine Scientific, Santa Ana, USA) in a 50 × 40-mm glass culture dish (WillCo-dish®, New Jersey, USA) covered with warm mineral oil (Ovoil™, Vitrolife, Kungsbacka, Sweden), on a heated stage (37.0 ± 0.5 °C) of an inverted microscope.
Assessment of fertilization, embryo quality, and embryo transfer
After the ICSI procedure, the presumptive embryos were individually maintained in a 50-μL drop of culture medium (Global®, LifeGlobal, Connecticut, USA) supplemented with 10 % human serum albumin (HAS) and covered with mineral oil in a humidified atmosphere with 6 % CO2 at 37 °C until transfer, which occurred on the fifth day of development.
Approximately 18 h after ICSI, fertilization was confirmed by the presence of two pronuclei and the extrusion of the second polar body. Subsequently, embryos were transferred to new drops of culture medium to be individually cultured for 48 h. The quality of the embryos was evaluated under an inverted microscope. High-quality embryos were defined as having the following characteristics: 8–10 cells on the third day of development, less than 15 % fragmentation, symmetric blastomeres, and the absence of multinucleation and zona pellucida dysmorphisms. Embryos lacking any of the above characteristics were considered to be of medium or low quality.
Up to two embryos from each couple were transferred to the patient. In special cases of low morphological quality, three embryos were transferred. Embryo selection for transfer was based on embryo and oocyte morphology.
Clinical follow-up
Ten days after embryo transfer, the quantitative measurement of serum levels of the beta subunit of human chorionic gonadotropin (β-hCG), which is indicative of positive pregnancy, was performed. A clinical pregnancy was defined by the detection of a gestational sac and fetal heartbeat by pelvic transvaginal ultrasonography, performed between 3 and 4 weeks after embryo transfer.
Sample preparation for mass spectrometry
To obtain the minimum amount of material for the study, CC samples from each group were pooled. Each pool was centrifuged at 3000×g for 15 min in order to discard the supernatant and concentrate the cells on the microtube wall, by specific positioning of microtubes in the centrifuge. Then, the cells were subjected to lysis by adding 100 μL of sample loading buffer (0.5 M Tris-HCL [pH 6.8], 10 % SDS, Glycerol, Bromophenol blue, DTT, Water—GE Healthcare, Piscataway, NJ) to each sample, followed by manual pipetting to obtain the lysates. The resulting lysates in the sample loading buffer were homogenized for 1 min and heated at 100 °C for 5 min for subsequent electrophoresis for protein concentration in the gels.
The proteins were concentrated in the gels in duplicate using 10 % (v/v) sodium dodecyl sulfate−polyacrylamide gel electrophoresis, and protein detection in gel was performed using Coomassie Brilliant Blue staining.
For protein band excision from the gels, the bands with a minimum size of 2 mm × 5 mm and intensively stained by Coomassie Brilliant Blue were cut into cubes (1 × 1 mm) and transferred to microtubes, according to the respective samples. For each sample, one of the replicates had the albumin removed by band excision, guided by Amersham ECL full-range rainbow molecular weight marker (GE Healthcare, Piscataway, NJ). Gel piece destaining, reduction and alkylation, digestion of band pieces, and excision of peptides were performed as described by the Lamond Laboratory protocol (see http://www.lamondlab.com/pdf/LLingeldigestion.pdf).
The peptide quantification was carried out using the Bicinchoninic acid assay, after protein digestion and MS. For this purpose, 2 μL of sample containing the peptides was used in order to evaluate the efficiency of the protein extraction method.
During all the process of sample preparation, the following cautions were taken in order to prevent keratin contamination: (i) the glassware was washed with Extran and milliQ water followed by rinse with methanol; (ii) all the solutions were prepared right before using; (iii) the staining tray was washed and covered during gel staining; (iv) personnel working on the sample preparation were properly dresses with lab coat and gloves.
Liquid chromatography and mass spectrometry
LC-MS/MS analyses were performed with digested protein samples (15 μL) in an Agilent 1290 Infinity LC System coupled to 6550 iFunnel Q-TOF LC/MS. The chromatographic conditions were as follows: POROSHELL 120 EC, C-18 column (Agilent 100 mm × 2.1 mm × 2.7 μM) maintained at 60 °C, with a flow rate of 0.5 μL/min with water, 0.1 % formic acid in water (A), and 0.1 % formic acid in acetonitrile (B). The elution gradients were as follows: 0 to 20 min—10 to 35 % B; 20 to 28 min—35 to 90 % B; and 28 to 30 min—90 % B in isocratic mode, resulting in 30 min of time acquisition. After chromatographic separation, the samples were subjected to MS in the Agilent Dual JetStream ionization source, in positive ion mode at 35 psi of nebulizer gas and 3.5 kV of capillary voltage. Full scan mass spectra were acquired with a scan range of 300–1700 m/z (8 spectra) and for MS/MS spectra with a scan range of 50–1700 m/z (4 spectra). The data obtained from LC/MS were analyzed using the Agilent MassHunter Qualitative Analysis Software B.06.
Data analysis
The Agilent Mass Hunter Qualitative raw data were saved in .mgf format and processed using Mascot (Matrix Science, London, UK). Spectra were processed using Mascot Distiller, under conditions optimized for the Agilent Mass Spectrometer, and processed results were used in database searches using a reviewed SwissProt Human database, considering the use of trypsin, and a maximum of two missed cleavages. Carbamidomethylation was set as a fixed modification, and methionine oxidation as a variable modification. A maximum mass error of 30 ppm was allowed for MS and of 1 Da for MS/MS. Proteins were considered identified if they presented a maximum p value of less than 5 %. A reverse database was used for estimation of false-positive rates. Finally, quantification was achieved using Exponentially Modified Protein Abundance Index (emPAI), which is a function based on the number of observed and observable peptides for any given protein [36].
Results
Blastocyst formation chance
The comparison of patients and cycles’ characteristics between the blastocyst and non-blastocyst groups is described in Table 1.
Table 1.
Comparison of patients and cycles’ characteristics between the blastocyst and non-blastocyst groups
| Variables | Blastocyst group | Non-blastocyst group | p value |
|---|---|---|---|
| Maternal age (year) | 34.1 ± 1.8 | 33.3 ± 4.3 | 0.485 |
| Paternal age (year) | 38.1 ± 3.3 | 36.5 ± 3.8 | 0.325 |
| FSH administered (IU) | 2244 ± 612 | 2128 ± 425 | 0.771 |
| Estradiol level (pg/mL) | 1654 ± 1675 | 1354 ± 1354 | 0.548 |
| Number of aspirated follicles | 18.4 ± 8.1 | 20.3 ± 10.0 | 0.451 |
| Number of retrieved oocytes | 14.3 ± 7.5 | 13.8 ± 4.0 | 0.683 |
| Number of MII oocytes | 11.8 ± 4.9 | 9.7 ± 5.4 | 0.532 |
| Fertilisation rate % | 83.2 ± 15.1 | 78.3 ± 18.4 | 0.913 |
Note: values are mean + SD, unless otherwise noted. NS
Analysis of samples from the blastocyst and non-blastocyst groups revealed 87 different proteins. Most of the detected proteins were binding proteins, followed by enzymes. However, structural, transport, construction, and DNA repair proteins were also identified (Fig. 1).
Fig. 1.
Distribution of groups of proteins detected from analysis of cumulus cell samples from blastocyst and non-blastocyst groups
Among the 87 detected proteins, 30 were exclusively expressed in the blastocyst group, and 17 were exclusively expressed in the non-blastocyst group. Forty proteins were expressed in both groups, and six of these were equally expressed and 34 were differentially expressed (Tables 2 and 3). From the proteins differentially expressed between the blastocyst and non-blastocyst groups, 23 proteins were highly expressed in the blastocyst group, and 11 proteins were highly expressed in the non-blastocyst group (Fig. 2).
Table 2.
Proteins exclusively detected in cumulus cell samples from the blastocyst and non-blastocyst groups
| Proteins exclusively expressed in the blastocyst group | Proteins exclusively expressed in the non-blastocyst group |
|---|---|
| NADPH/adrenodoxin oxidoreductase | 7-methylguanosine phosphate-specific 5′-nucleotidase |
| Protein AF-17 | Beta-2-glycoprotein 1 |
| Annexin A2 | Arf-GAP with Rho-GAP domain, ANK repeat and PH domain-containing protein 2 |
| Rho guanine nucleotide exchange factor 1 | Band 3 anion transport protein |
| Rho guanine nucleotide exchange factor 2 | Biogenesis of lysosome-related organelles complex 1 subunit 1 |
| ATP synthase subunit alpha | Cytochrome c oxidase subunit 2 |
| CDK5 and ABL1 enzyme substrate 1 | Protocadherin Fat 2 |
| Cardiomyopathy-associated protein 5 | Histone H2A type 1-B/E |
| Delta(24)-sterol reductase | Histone H3.3C |
| Corticosteroid 11-beta-dehydrogenase isozyme 1 | HEAT repeat-containing protein 1 |
| Alpha-enolase | Hemopexin |
| Endoplasmic reticulum-Golgi intermediate compartment protein 1 | Ig gamma-2 chain C region |
| Alpha-2-HS-glycoprotein | Interleukin enhancer-binding factor 2 |
| Glyceraldehyde-3-phosphate dehydrogenase | Malate dehydrogenase |
| Stress-70 protein | Serine/threonine-protein kinase MRCK gamma |
| 78 kDa glucose-regulated protein | Protein disulfide-isomerase |
| Glutathione S-transferase A1 | Testicular haploid expressed gene protein-like |
| Histone H2A type 1-D | |
| Histone H2A.J | |
| Histone H2AX | |
| Histone H2B type 1-C/E/F/G/I | |
| Heterogeneous nuclear ribonucleoprotein K | |
| Ig heavy chain V-III region TUR | |
| Proto-oncogene serine/threonine-protein kinase mos | |
| Protein disulfide-isomerase A3 | |
| Peptidyl-prolyl cis-trans isomerase B | |
| 40S ribosomal protein S16 | |
| Scavenger receptor class B member 1 | |
| Tubulin beta chain | |
| Serotransferrin |
Table 3.
Proteins equally and differentially represented in cumulus cell samples from the blastocyst and non-blastocyst groups
| Proteins equally expressed among groups | Proteins differentially expressed among groups |
|---|---|
| ATP-binding cassette sub-family B member 6 | 3 beta-hydroxysteroid dehydrogenase/Delta 5-- > 4-isomerase type 2 |
| AP-5 complex subunit zeta-1 | Alpha-1-antitrypsin |
| Protein broad-minded | Alpha-1B-glycoprotein |
| Aromatase | Alpha-1-antichymotrypsin |
| Elongation factor 1-alpha 1 | Actin, aortic smooth muscle |
| Ig alpha-1 chain C region | Actin, cytoplasmic 1 |
| ADP/ATP translocase 2 | |
| Serum albumin | |
| Angiotensinogen | |
| Annexin A5 | |
| Annexin A6 | |
| Apolipoprotein A-I | |
| N-acetylserotonin O-methyltransferase-like protein | |
| ATP synthase subunit beta | |
| Calreticulin | |
| 60 kDa heat shock protein | |
| Complement C3 | |
| Cholesterol side-chain cleavage enzyme | |
| Endoplasmin | |
| Fibrinogen alpha chain | |
| Fibrinogen beta chain | |
| Fibrinogen gamma chain | |
| Neutral alpha-glucosidase AB | |
| Histone H2B type 1-A | |
| Histone H3.1 t | |
| Histone H4 | |
| Hemoglobin subunit alpha | |
| Hemoglobin subunit beta | |
| Haptoglobin | |
| Ig gamma-1 chain C region | |
| Ig kappa chain C region | |
| Protein disulfide-isomerase A6 | |
| Tubulin alpha-1B chain | |
| Vimentin |
Fig. 2.
Description of signal intensities, expressed as the emPAI value for each differentially expressed in the blastocyst and non-blastocyst groups
Pregnancy outcome
The comparison of patients and cycles’ characteristics between the positive-pregnancy and negative-pregnancy groups are described in Table 4.
Table 4.
Comparison of patients and cycles’ characteristics between the positive-pregnancy and negative-pregnancy groups
| Variables | Positive-pregnancy | Negative-pregnancy | p value |
|---|---|---|---|
| Maternal age (year) | 33.4.1 ± 3.5 | 34.8 ± 5.8 | 0.456 |
| Paternal age (year) | 36.8 ± 2.1 | 37.3 ± 4.2 | 0.467 |
| FSH administered (IU) | 2145 ± 513 | 2250 ± 435 | 0.669 |
| Estradiol level (pg/mL) | 1598 ± 1348 | 1652 ± 1254 | 0.652 |
| Number of aspirated follicles | 20.3 ± 7.4 | 21.8 ± 9.1 | 0.465 |
| Number of retrieved oocytes | 15.5 ± 8.3 | 14.6 ± 3.8 | 0.562 |
| Number of MII oocytes | 12.3 ± 4.1 | 10,6 ± 5.6 | 0.546 |
| Fertilization rate % | 85.1 ± 10.8 | 83.4 ± 17.3 | 0.256 |
Note: values are mean + SD, unless otherwise noted. NS
Analysis of samples from the positive-pregnancy and negative-pregnancy groups revealed 72 proteins. Similar to the embryo quality groups, most of the detected proteins were binding proteins, followed by enzymes. Construction, growth factors, anticoagulant, and DNA repair proteins were also detected (Fig. 3).
Fig. 3.
Distribution of groups of proteins detected in cumulus cell samples of positive-pregnancy and negative-pregnancy groups
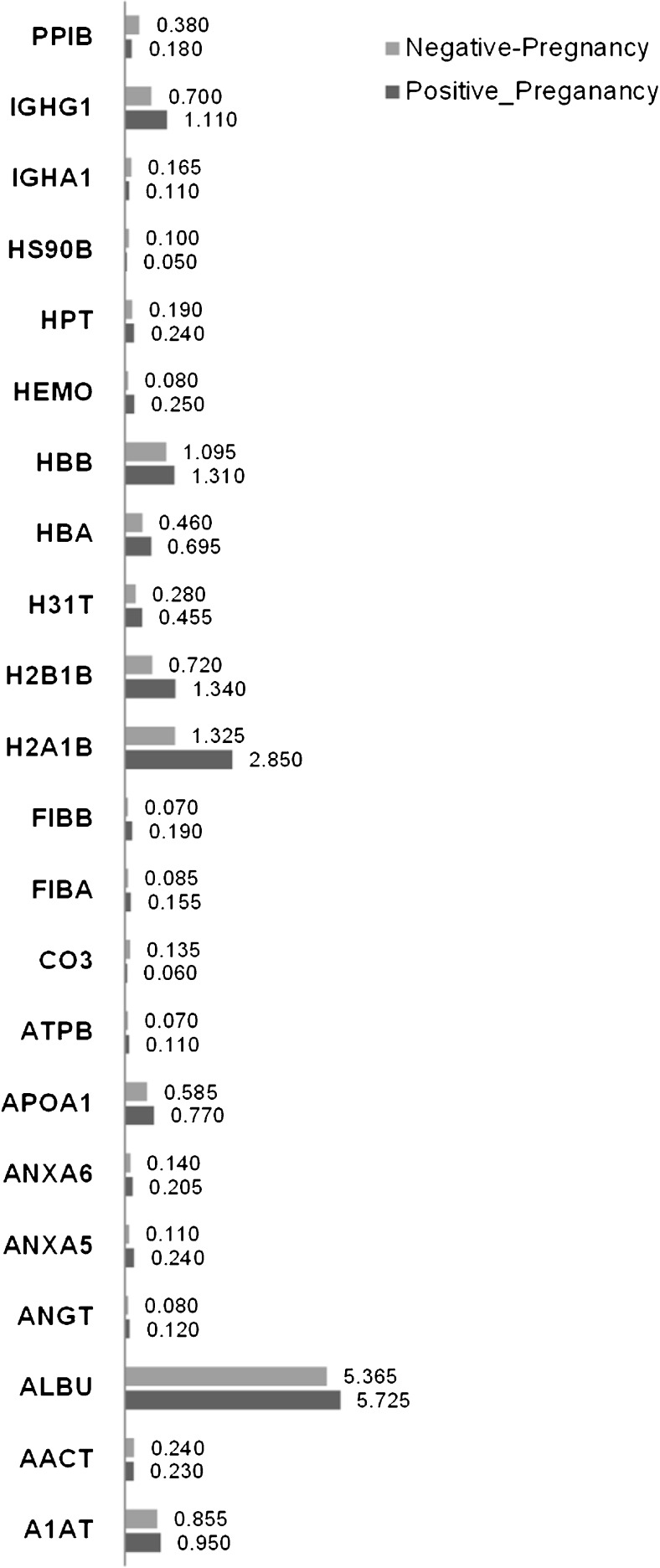
Among the 72 detected proteins, 19 were exclusively expressed in the positive-pregnancy group, and 16 were exclusively expressed in the negative-pregnancy group. Thirty-eight proteins were expressed in both groups, and 16 of these were equally expressed and 22 were differentially expressed (Tables 5 and 6). Among the proteins expressed among the positive- and negative-pregnancy groups, 16 proteins were highly expressed in the positive-pregnancy group and six proteins were highly expressed in the negative-pregnancy group (Fig. 4).
Table 5.
Proteins detected in cumulus cell samples from the positive-pregnancy and negative-pregnancy groups
| Proteins exclusively expressed in the positive-pregnancy group | Proteins exclusively expressed in the negative-pregnancy group |
|---|---|
| ATP-binding cassette sub-family B member 6 | 3 beta-hydroxysteroid dehydrogenase/Delta 5-- > 4-isomerase type 2 |
| Actin | Alpha-2-macroglobulin |
| Ceruloplasmin | 2-phosphoxylose phosphatase 1 |
| 60 kDa heat shock protein | Actin |
| Ubiquinone biosynthesis monooxygenase COQ6 | Annexin A2 |
| Carnosine synthase 1 | Clathrin heavy chain 1 |
| Uncharacterized protein C19orf57 | Guanylate-binding protein 4 |
| Uncharacterized protein CXorf66 | Histone H2B type 1-A |
| Elongation factor 1-alpha 1 | Histone H3.3C |
| Alpha-enolase | Heterogeneous nuclear ribonucleoprotein K |
| Protocadherin Fat 2 | Ig kappa chain V-I region Lay |
| Glutathione S-transferase A1 | La-related protein 1 |
| Histone H2A type 2-A | L-lactate dehydrogenase A chain |
| Ig heavy chain V-III region TUR | Nck-associated protein 1 |
| Ig gamma-2 chain C region | Scavenger receptor class B member 1 |
| Immunoglobulin lambda-like polypeptide 5 | Serotransferrin |
| Proto-oncogene serine/threonine-protein kinase mos | |
| Protein disulfide-isomerase A3 | |
| Paired mesoderm homeobox protein 2B |
Table 6.
Proteins equally and differentially represented in cumulus cell samples from the positive-pregnancy and negative-pregnancy groups
| Proteins equally expressed among groups | Proteins differentially expressed among groups |
|---|---|
| Alpha-1-acid glycoprotein 1 | Alpha-1-antitrypsin |
| Alpha-1B-glycoprotein | Alpha-1-antichymotrypsin |
| AP-5 complex subunit zeta-1 | Serum albumin |
| Protein broad-minded | Angiotensinogen |
| Complement C4-A | Annexin A5 |
| Cholesterol side-chain cleavage enzyme | Annexin A6 |
| Endoplasmin | Apolipoprotein A-I |
| Fibrinogen gamma chain | ATP synthase subunit beta |
| Glyceraldehyde-3-phosphate dehydrogenase | Complement C3 |
| Neutral alpha-glucosidase AB | Fibrinogen alpha chain |
| Histone H4 | Fibrinogen beta chain |
| Plasma protease C1 inhibitor | Histone H2A type 1-B/E |
| Ig kappa chain C region | Histone H2B type 1-B |
| Ig lambda-2 chain C regions | Histone H3.1 t |
| Tubulin alpha-1A chain | Hemoglobin subunit alpha |
| Tubulin alpha-1B chain | Hemoglobin subunit beta |
| Hemopexin | |
| Haptoglobin | |
| Heat shock protein HSP 90-beta | |
| Ig alpha-1 chain C region | |
| Ig gamma-1 chain C region | |
| Peptidyl-prolyl cis-trans isomerase B |
Fig. 4.
Description of signal intensities, expressed as the emPAI value for each differentially expressed in the positive-pregnancy and negative-pregnancy groups.
Discussion
In the present study, a number of proteins were detected in the human CCs by LC-MS. Some proteins were differently expressed in the CCs of patients in which all the embryos achieved the blastocyst stage when compared with those in which no embryo achieved this stage, and some proteins were differently expressed in the CCs of patients that achieved pregnancy when compared with patients that did not achieve the same outcome.
CCs are in direct contact with the oocyte, and an intense bi-directional communication occurs between them during folliculogenesis, which is essential for oocyte development. Therefore, the majority of investigations have focused on the analysis of CC gene expression to identify non-invasive predictors of embryo development and treatment outcome [14, 24–28]. However, few experiments have focused on protein expression [37], which is a more reliable indicator of the cellular phenotype.
In a previous experiment, Hamamah et al. [37] compared the protein expression profile of human CCs in relation to oocyte fertilization and ovarian stimulation protocol by 2D polyacrylamide gel electrophoresis. The study showed that less than 20 proteins differed between CCs surrounding fertilized oocytes and those surrounding non-fertilized oocytes; however, the proteins were not identified in this procedure.
A significant implication of the present study is the identification of potential biomarker candidates for predicting embryo quality and especially for blastocyst formation competence. The identification of patients able to benefit from extended embryo culture programs would be a valuable approach for assisted reproduction success. In fact, although it is known that prolonging the embryo culture period allows for a better selection of embryos for transfer leading to increased implantation rates and reduced risk of twin and higher order pregnancies [38], there is concern that a strategy of blastocyst culture may result in higher cycle cancellation rates [39].
Potential CC biomarkers correlated to oocyte quality, embryo competence, or pregnancy outcome have been identified by microarray analysis [14, 24, 26, 40], RT–PCR [41, 42], or quantitative RT–PCR [25, 43, 44]. Although genomics can supply valuable information regarding expected functions, the proteome is the entire complement of proteins expressed by a genome, and therefore, it is more indicative of the phenotype and can provide important information that is complementary to the data obtained from gene expression studies. Moreover, gene expression studies of RNA transcripts do not often predict protein abundance or function [45].
A recently published transcriptomic analysis and meta-analysis of human CCs tried to identify differentially expressed genes and analyze biological processes in human CCs. The authors concluded that transcriptomes of CCs as well as biological functions are distinctive for each cell subpopulation [46]. We also did not find any correlation between the proteins identified here and those from genomic studies. Indeed, changes in gene expression are not necessarily reflected in changes in translated proteins, nor does gene analysis take into account posttranscriptional, translational, or posttranslational changes that relate to cyclical transitions. Of the hundreds of gene expression changes typically identified by microarray, relatively few are common to more than two studies [47, 48]. Comparison of proteomic data with published gene expression data in similar cohorts of women [47, 49, 50] also have revealed an overall lack of correlation between the two, suggesting that posttranscriptional or translational regulation is an important feature in human biology.
Among the proteins identified here, some were exclusively represented in both the blastocyst and the positive pregnancy groups: glutathione S-transferase A4 (GST A4), Ig heavy chain V-III region TUR, protein disulfide-isomerase (PDI) A3, and proto-oncogene serine/threonine-protein kinase mos.
The expression of glutathione S-transferases (GST) in human CCs has already been reported by previous studies [51]. Overexpression of the sub-class GSTA4 has been shown to protect cells from apoptosis [52], and GSTP has also been associated with JNK and protects cells from death signals or oxidative stress [53].
Some GSTs have been shown to be upregulated through the MAPK pathways as self-defense responses to toxins and growth factors [54, 55]. In reproductive cells, p38 MAPK plays a pivotal role in oocyte maturation [56–58] and steroidogenesis [59, 60]. Therefore, it is plausible that GSTs have a strong link to embryo development and implantation.
Immunoglobulins are heterodimeric proteins composed of two heavy (H) and two light (L) chains. They can be separated functionally into variable (V) domains that binds antigens and constant (C) domains that specify effector functions such as activation of complement or binding to Fc receptors [61]. The Ig heavy chain V-III region TUR has already been identified in the human follicular fluid by LC/MS/MS [62], and it has been hyper-represented in samples from fertilized oocytes [63].
Our results suggest a possibility that these proteins identified in human CCs might not only be involved in folliculogenesis, but also as part of immune response pathways. The importance of the immunologic system during embryo development and reproduction biology is unclear, but a role during embryo implantation had been suggested [64].
Previous reports have identified PDIA3 by MS in the mammalian ovary tissue and [65] in embryo culture media [66]. Its function in the reproductive systems has not yet been elucidated; however, it could be suggested that it includes cell growth and death. The endoplasmic reticulum contains a host of proteins involved in co- and post-translational modifications of newly synthesized polypeptides. The most abundant members of this group of proteins are the PDI [67]. Although it seems clear that proteins contain all the information required for proper folding in the absence of enzyme, PDI may be essential for this process, and it has been suggested that PDI does, in fact, catalyze protein folding within the endoplasmic reticulum [68].
Proto-oncogene serine/threonine-protein kinase mos is essential for the initiation of oocyte maturation [69], for the progression of meiosis I to meiosis II [70], and for the second meiotic metaphase arrest [71]. And together with our findings, these studies suggest that it is also important for oocyte quality, embryo development, and implantation.
It could be argued that the value of a cumulus-based diagnostic test in determining blastocyst formation is limited, as blastocyst formation is determined after in vitro embryo culture, since embryonic gene activation occurs after the second cleavage. However, the oocyte machinery guides the early embryo development and influences in blastocyst formation, and consequently implantation. Considering that the communication between the oocyte and CC is essential for the acquisition of oocyte competence, the proteins identified here may be potential therapeutic targets and may be used, in the future, as supplementation in culture media. Additionally, this study helps to understand the physiology of in vivo maturation in controlled stimulated cycles.
In a previous study [72], the protein profile of individual human embryos was obtained by LC-MS, and it was correlated with morphology; however, the method used in this study resulted in embryo death, limiting or prohibiting its clinical use. In an additional study, Katz-Jaffe et al. [73] non-invasively identified protein biomarkers in the surrounding embryo culture medium and correlated these findings with embryo development. For this study, the protein profile was obtained from pooled samples from thawed embryos cultured to the blastocyst stage. Although it is a promising approach, the analyses of samples obtained from embryos at the blastocyst stage may clutter the use of the obtained information for clinical propose, since embryo transfer is to be performed in a limited time after sample collection.
For this reason, in the present study, the proteomic analysis was performed in CCs rather than in the culture media. Indeed, because of its close contact with the oocyte, CCs reflect the biology and competence of both oocytes and embryos.
An important limitation of this study is the requirement of large amounts of material for proteomic analysis [74]. Indeed, MS requires a large quantity of starting material. This combined with limited template, low protein expression and the lack of sensitivity of proteomic platforms are some of the foremost obstacles [13]. For the present study, the protein concentrations obtained from CCs collected from a single patient were insufficient for LC-MS; therefore, samples from the same group were pooled together in order to achieve the required concentration. For this preliminary study, we aimed to determine which proteins are potential biomarkers for blastocyst formation and pregnancy. Our results provide a rationale for conducting further research aimed to determine evaluating diagnostic and to therapeutic targets for oocyte in vitro maturation and embryo culture.
Although a different protein profile could be identified among patients in different groups, it is of paramount importance to develop a technique to identify and to differentiate the protein profile of a single patient’s sample or even from CCs of a single complex cumulus oophorus.
Although the LC/MS technique has emerged with promising results in the omics field, it is not able to detect the proteins that are really expressed in cells due to some limitations (peptide mass, efficiency of digestion, volatility, LC resolution etc.); therefore, the data described here are limited to protein detection technique by LC/MC approach and protein abundance in CCs.
In the present study, potential biomarkers for blastocyst formation potential and pregnancy outcome were identified. The next step is to individually identify these proteins and to determine their frequency in subjects. Therefore, CC proteomics may be useful for the prediction of pregnancy success and the identification of patients that should be included in extended embryo culture programs or patients who would benefit from cleavage stage embryo transfers.
Acknowledgments
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP, a Brazilian founding agency: 2013/50052-7.
Footnotes
Capsule
CC proteomics may be useful for predicting pregnancy success and the identification of patients that should be included in extended embryo culture programs.
References
- 1.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. doi: 10.1016/S0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 2.Andersen AN, Goossens V, Ferraretti AP, Bhattacharya S, Felberbaum R, de Mouzon J, et al. Assisted reproductive technology in Europe, 2004: results generated from European registers by ESHRE. Hum Reprod. 2008;23(4):756–71. doi: 10.1093/humrep/den014. [DOI] [PubMed] [Google Scholar]
- 3.Pandian Z, Bhattacharya S, Ozturk O, Serour G, Templeton A. Number of embryos for transfer following in-vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev. 2009;2:CD003416. doi: 10.1002/14651858.CD003416.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Setti PE, Bulletti C. Strategies to improve embryo implantation to supraphysiological rates. Ann N Y Acad Sci. 2011;1221:75–9. doi: 10.1111/j.1749-6632.2011.05950.x. [DOI] [PubMed] [Google Scholar]
- 5.Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. 2007;4:CD002118. doi: 10.1002/14651858.CD002118.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23(1):91–9. doi: 10.1093/humrep/dem339. [DOI] [PubMed] [Google Scholar]
- 7.Tesarik J, Kopecny V, Plachot M, Mandelbaum J. Early morphological signs of embryonic genome expression in human preimplantation development as revealed by quantitative electron microscopy. Dev Biol. 1988;128(1):15–20. doi: 10.1016/0012-1606(88)90261-8. [DOI] [PubMed] [Google Scholar]
- 8.Langley MT, Marek DM, Gardner DK, Doody KM, Doody KJ. Extended embryo culture in human assisted reproduction treatments. Hum Reprod. 2001;16(5):902–8. doi: 10.1093/humrep/16.5.902. [DOI] [PubMed] [Google Scholar]
- 9.Wilson M, Hartke K, Kiehl M, Rodgers J, Brabec C, Lyles R. Integration of blastocyst transfer for all patients. Fertil Steril. 2002;77(4):693–6. doi: 10.1016/S0015-0282(01)03235-6. [DOI] [PubMed] [Google Scholar]
- 10.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril. 2004;81(3):551–5. doi: 10.1016/j.fertnstert.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Ryan GL, Sparks AE, Sipe CS, Syrop CH, Dokras A, Van Voorhis BJ. A mandatory single blastocyst transfer policy with educational campaign in a United States IVF program reduces multiple gestation rates without sacrificing pregnancy rates. Fertil Steril. 2007;88(2):354–60. doi: 10.1016/j.fertnstert.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Braga DP, Setti AS, de Cassia SFR, Machado RB, Iaconelli A, Jr, Borges E., Jr Patient selection criteria for blastocyst transfers in extended embryo culture programs. J Assist Reprod Genet. 2012;29(12):1357–62. doi: 10.1007/s10815-012-9875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz-Jaffe MG, McReynolds S, Gardner DK, Schoolcraft WB. The role of proteomics in defining the human embryonic secretome. Mol Hum Reprod. 2009;15(5):271–7. doi: 10.1093/molehr/gap012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamel M, Dufort I, Robert C, Gravel C, Leveille MC, Leader A, et al. Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum Reprod. 2008;23(5):1118–27. doi: 10.1093/humrep/den048. [DOI] [PubMed] [Google Scholar]
- 15.Botros L, Sakkas D, Seli E. Metabolomics and its application for non-invasive embryo assessment in IVF. Mol Hum Reprod. 2008;14(12):679–90. doi: 10.1093/molehr/gan066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bromer JG, Seli E. Assessment of embryo viability in assisted reproductive technology: shortcomings of current approaches and the emerging role of metabolomics. Curr Opin Obstet Gynecol. 2008;20(3):234–41. doi: 10.1097/GCO.0b013e3282fe723d. [DOI] [PubMed] [Google Scholar]
- 17.Aydiner F, Yetkin CE, Seli E. Perspectives on emerging biomarkers for non-invasive assessment of embryo viability in assisted reproduction. Curr Mol Med. 2010;10(2):206–15. doi: 10.2174/156652410790963349. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira CR, Turco EGL, Saraiva SA, Bertolla RP, Perecin F, Souza GHFM, et al. Proteomics, Metabolomis and Lipidomics in Reproductive Biotechnologies: The MS Solutions. Acta Sci Vet. 2010;38:s277–s289. [Google Scholar]
- 19.Seli E, Vergouw CG, Morita H, Botros L, Roos P, Lambalk CB, et al. Noninvasive metabolomic profiling as an adjunct to morphology for noninvasive embryo assessment in women undergoing single embryo transfer. Fertil Steril. 2010;94(2):535–42. doi: 10.1016/j.fertnstert.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 20.Cortezzi SS, Garcia JS, Ferreira CR, Braga DP, Figueira RC, Iaconelli A, Jr, et al. Secretome of the preimplantation human embryo by bottom-up label-free proteomics. Anal Bioanal Chem. 2011;401(4):1331–9. doi: 10.1007/s00216-011-5202-1. [DOI] [PubMed] [Google Scholar]
- 21.Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol. 2010;88(4):399–413. doi: 10.1139/Y10-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patrizio P, Fragouli E, Bianchi V, Borini A, Wells D. Molecular methods for selection of the ideal oocyte. Reprod Biomed Online. 2007;15(3):346–53. doi: 10.1016/S1472-6483(10)60349-5. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16(10):715–25. doi: 10.1093/molehr/gaq031. [DOI] [PubMed] [Google Scholar]
- 24.van Montfoort AP, Geraedts JP, Dumoulin JC, Stassen AP, Evers JL, Ayoubi TA. Differential gene expression in cumulus cells as a prognostic indicator of embryo viability: a microarray analysis. Mol Hum Reprod. 2008;14(3):157–68. doi: 10.1093/molehr/gam088. [DOI] [PubMed] [Google Scholar]
- 25.Hamel M, Dufort I, Robert C, Leveille MC, Leader A, Sirard MA. Genomic assessment of follicular marker genes as pregnancy predictors for human IVF. Mol Hum Reprod. 2010;16(2):87–96. doi: 10.1093/molehr/gap079. [DOI] [PubMed] [Google Scholar]
- 26.Assou S, Haouzi D, Mahmoud K, Aouacheria A, Guillemin Y, Pantesco V, et al. A non-invasive test for assessing embryo potential by gene expression profiles of human cumulus cells: a proof of concept study. Mol Hum Reprod. 2008;14(12):711–9. doi: 10.1093/molehr/gan067. [DOI] [PubMed] [Google Scholar]
- 27.Assou S, Boumela I, Haouzi D, Anahory T, Dechaud H, De Vos J, et al. Dynamic changes in gene expression during human early embryo development: from fundamental aspects to clinical applications. Hum Reprod Update. 2011;17(2):272–90. doi: 10.1093/humupd/dmq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasca S, Pellestor F, Assou S, Loup V, Anahory T, Dechaud H, et al. Identifying new human oocyte marker genes: a microarray approach. Reprod Biomed Online. 2007;14(2):175–83. doi: 10.1016/S1472-6483(10)60785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latham KE, Garrels JI, Chang C, Solter D. Analysis of embryonic mouse development: construction of a high-resolution, two-dimensional gel protein database. Appl Theor Electrophor. 1992;2(6):163–70. [PubMed] [Google Scholar]
- 30.Shi CZ, Collins HW, Garside WT, Buettger CW, Matschinsky FM, Heyner S. Protein databases for compacted eight-cell and blastocyst-stage mouse embryos. Mol Reprod Dev. 1994;37(1):34–47. doi: 10.1002/mrd.1080370106. [DOI] [PubMed] [Google Scholar]
- 31.Navarrete Santos A, Tonack S, Kirstein M, Kietz S, Fischer B. Two insulin-responsive glucose transporter isoforms and the insulin receptor are developmentally expressed in rabbit preimplantation embryos. Reproduction. 2004;128(5):503–16. doi: 10.1530/rep.1.00203. [DOI] [PubMed] [Google Scholar]
- 32.Wang HM, Zhang X, Qian D, Lin HY, Li QL, Liu DL, et al. Effect of ubiquitin-proteasome pathway on mouse blastocyst implantation and expression of matrix metalloproteinases-2 and -9. Biol Reprod. 2004;70(2):481–7. doi: 10.1095/biolreprod.103.021634. [DOI] [PubMed] [Google Scholar]
- 33.Gutstein HB, Morris JS, Annangudi SP, Sweedler JV. Microproteomics: analysis of protein diversity in small samples. Mass Spectrom Rev. 2008;27(4):316–30. doi: 10.1002/mas.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen C, Hebeda KM, Linkels M, Grefte JM, Raemaekers JM, van Krieken JH, et al. Protein profiling of B-cell lymphomas using tissue biopsies: A potential tool for small samples in pathology. Cell Oncol. 2008;30(1):27–38. doi: 10.1155/2008/898356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 36.Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, et al. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics. 2005;4(9):1265–72. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Hamamah S, Matha V, Berthenet C, Anahory T, Loup V, Dechaud H, et al. Comparative protein expression profiling in human cumulus cells in relation to oocyte fertilization and ovarian stimulation protocol. Reprod Biomed Online. 2006;13(6):807–14. doi: 10.1016/S1472-6483(10)61028-0. [DOI] [PubMed] [Google Scholar]
- 38.Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100(1):100–7. doi: 10.1016/j.fertnstert.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 39.Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7:CD002118. doi: 10.1002/14651858.CD002118.pub4. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Jafari N, Barnes RB, Confino E, Milad M, Kazer RR. Studies of gene expression in human cumulus cells indicate pentraxin 3 as a possible marker for oocyte quality. Fertil Steril. 2005;83(Suppl 1):1169–79. doi: 10.1016/j.fertnstert.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 41.Cillo F, Brevini TA, Antonini S, Paffoni A, Ragni G, Gandolfi F. Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction. 2007;134(5):645–50. doi: 10.1530/REP-07-0182. [DOI] [PubMed] [Google Scholar]
- 42.Feuerstein P, Cadoret V, Dalbies-Tran R, Guerif F, Bidault R, Royere D. Gene expression in human cumulus cells: one approach to oocyte competence. Hum Reprod. 2007;22(12):3069–77. doi: 10.1093/humrep/dem336. [DOI] [PubMed] [Google Scholar]
- 43.McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19(12):2869–74. doi: 10.1093/humrep/deh535. [DOI] [PubMed] [Google Scholar]
- 44.Anderson RA, Sciorio R, Kinnell H, Bayne RA, Thong KJ, de Sousa PA, et al. Cumulus gene expression as a predictor of human oocyte fertilisation, embryo development and competence to establish a pregnancy. Reproduction. 2009;138(4):629–37. doi: 10.1530/REP-09-0144. [DOI] [PubMed] [Google Scholar]
- 45.Williamson AJ, Smith DL, Blinco D, Unwin RD, Pearson S, Wilson C, et al. Quantitative proteomics analysis demonstrates post-transcriptional regulation of embryonic stem cell differentiation to hematopoiesis. Mol Cell Proteomics. 2008;7(3):459–72. doi: 10.1074/mcp.M700370-MCP200. [DOI] [PubMed] [Google Scholar]
- 46.Burnik Papler T, Vrtacnik Bokal E, Maver A, Kopitar AN, Lovrecic L. Transcriptomic Analysis and Meta-Analysis of Human Granulosa and Cumulus Cells. PLoS One. 2015;10(8):e0136473. doi: 10.1371/journal.pone.0136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haouzi D, Dechaud H, Assou S, De Vos J, Hamamah S. Insights into human endometrial receptivity from transcriptomic and proteomic data. Reprod Biomed Online. 2012;24(1):23–34. doi: 10.1016/j.rbmo.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Horcajadas JA, Sharkey AM, Catalano RD, Sherwin JR, Dominguez F, Burgos LA, et al. Effect of an intrauterine device on the gene expression profile of the endometrium. J Clin Endocrinol Metab. 2006;91(8):3199–207. doi: 10.1210/jc.2006-0430. [DOI] [PubMed] [Google Scholar]
- 49.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 50.Chen JI, Hannan NJ, Mak Y, Nicholls PK, Zhang J, Rainczuk A, et al. Proteomic characterization of midproliferative and midsecretory human endometrium. J Proteome Res. 2009;8(4):2032–44. doi: 10.1021/pr801024g. [DOI] [PubMed] [Google Scholar]
- 51.Ito M, Imai M, Muraki M, Miyado K, Qin J, Kyuwa S, et al. GSTT1 is upregulated by oxidative stress through p38-MK2 signaling pathway in human granulosa cells: possible association with mitochondrial activity. Aging (Albany NY) 2011;3(12):1213–23. doi: 10.18632/aging.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng JZ, Singhal SS, Sharma A, Saini M, Yang Y, Awasthi S, et al. Transfection of mGSTA4 in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis by inhibiting JNK-mediated signaling. Arch Biochem Biophys. 2001;392(2):197–207. doi: 10.1006/abbi.2001.2452. [DOI] [PubMed] [Google Scholar]
- 53.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18(5):1321–34. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desmots F, Rissel M, Gilot D, Lagadic-Gossmann D, Morel F, Guguen-Guillouzo C, et al. Pro-inflammatory cytokines tumor necrosis factor alpha and interleukin-6 and survival factor epidermal growth factor positively regulate the murine GSTA4 enzyme in hepatocytes. J Biol Chem. 2002;277(20):17892–900. doi: 10.1074/jbc.M112351200. [DOI] [PubMed] [Google Scholar]
- 55.Kuo WH, Chou FP, Young SC, Chang YC, Wang CJ. Geniposide activates GSH S-transferase by the induction of GST M1 and GST M2 subunits involving the transcription and phosphorylation of MEK-1 signaling in rat hepatocytes. Toxicol Appl Pharmacol. 2005;208(2):155–62. doi: 10.1016/j.taap.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Yamashita Y, Hishinuma M, Shimada M. Activation of PKA, p38 MAPK and ERK1/2 by gonadotropins in cumulus cells is critical for induction of EGF-like factor and TACE/ADAM17 gene expression during in vitro maturation of porcine COCs. J Ovarian Res. 2009;2:20. doi: 10.1186/1757-2215-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villa-Diaz LG, Miyano T. Activation of p38 MAPK during porcine oocyte maturation. Biol Reprod. 2004;71(2):691–6. doi: 10.1095/biolreprod.103.026310. [DOI] [PubMed] [Google Scholar]
- 58.Salhab M, Tosca L, Cabau C, Papillier P, Perreau C, Dupont J, et al. Kinetics of gene expression and signaling in bovine cumulus cells throughout IVM in different mediums in relation to oocyte developmental competence, cumulus apoptosis and progesterone secretion. Theriogenology. 2011;75(1):90–104. doi: 10.1016/j.theriogenology.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Manna PR, Stocco DM. The role of specific mitogen-activated protein kinase signaling cascades in the regulation of steroidogenesis. J Signal Transduct. 2011;2011:821615. doi: 10.1155/2011/821615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inagaki K, Otsuka F, Miyoshi T, Yamashita M, Takahashi M, Goto J, et al. p38-Mitogen-activated protein kinase stimulated steroidogenesis in granulosa cell-oocyte cocultures: role of bone morphogenetic proteins 2 and 4. Endocrinology. 2009;150(4):1921–30. doi: 10.1210/en.2008-0851. [DOI] [PubMed] [Google Scholar]
- 61.Schroeder HW, Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S41–52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Twigt J, Steegers-Theunissen RP, Bezstarosti K, Demmers JA. Proteomic analysis of the microenvironment of developing oocytes. Proteomics. 2012;12(9):1463–71. doi: 10.1002/pmic.201100240. [DOI] [PubMed] [Google Scholar]
- 63.Bayasula, Iwase A, Kobayashi H, Goto M, Nakahara T, Nakamura T, et al. A proteomic analysis of human follicular fluid: comparison between fertilized oocytes and non-fertilized oocytes in the same patient. J Assist Reprod Genet. 2013;30(9):1231–8. doi: 10.1007/s10815-013-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim YS, Kim MS, Lee SH, Choi BC, Lim JM, Cha KY, et al. Proteomic analysis of recurrent spontaneous abortion: Identification of an inadequately expressed set of proteins in human follicular fluid. Proteomics. 2006;6(11):3445–54. doi: 10.1002/pmic.200500775. [DOI] [PubMed] [Google Scholar]
- 65.Edassery SL, Shatavi SV, Kunkel JP, Hauer C, Brucker C, Penumatsa K, et al. Autoantigens in ovarian autoimmunity associated with unexplained infertility and premature ovarian failure. Fertil Steril. 2010;94(7):2636–41. doi: 10.1016/j.fertnstert.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beardsley AJ, Li Y, O’Neill C. Characterization of a diverse secretome generated by the mouse preimplantation embryo in vitro. Reprod Biol Endocrinol. 2010;8:71. doi: 10.1186/1477-7827-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noiva R, Lennarz WJ. Protein disulfide isomerase. A multifunctional protein resident in the lumen of the endoplasmic reticulum. J Biol Chem. 1992;267(6):3553–6. [PubMed] [Google Scholar]
- 68.Bulleid NJ, Freedman RB. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 1988;335(6191):649–51. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- 69.Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335(6190):519–25. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- 70.Daar I, Paules RS, Vande Woude GF. A characterization of cytostatic factor activity from Xenopus eggs and c-mos-transformed cells. J Cell Biol. 1991;114(2):329–35. doi: 10.1083/jcb.114.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;370(6484):68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- 72.Katz-Jaffe MG, Gardner DK, Schoolcraft WB. Proteomic analysis of individual human embryos to identify novel biomarkers of development and viability. Fertil Steril. 2006;85(1):101–7. doi: 10.1016/j.fertnstert.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Katz-Jaffe MG, Schoolcraft WB, Gardner DK. Analysis of protein expression (secretome) by human and mouse preimplantation embryos. Fertil Steril. 2006;86(3):678–85. doi: 10.1016/j.fertnstert.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 74.Seli E, Robert C, Sirard MA. OMICS in assisted reproduction: possibilities and pitfalls. Mol Hum Reprod. 2010;16(8):513–30. doi: 10.1093/molehr/gaq041. [DOI] [PubMed] [Google Scholar]