Abstract
Purpose
To verify if the presence of varicocele (grades II and III) with and without seminal alterations, using the 5th centile cutoff values in table A1.1 of the World Health Organization (WHO, 2010) manual, alters the seminal plasma levels of proteins DNASE1 (deoxyribonuclease-1) and IGFBP7 (Insulin-like growth factor-binding protein 7), which are related to apoptosis regulation and cell proliferation, respectively, demonstrating that these proteins are important for correct spermatogenesis.
Methods
This cross sectional study was performed at the Sao Paulo Federal University Paulo between May 2014 and April 2016. A total of 61 male adolescents were included in this study, of which 20 controls without varicocele (C), 22 with varicocele and normal semen analysis (VNS) and 19 with varicocele and altered semen analysis (VAS). Seminal plasma from each patient was used for Western blotting analysis of individual protein levels. Values of each protein were normalized to a testicular housekeeping protein (PARK7—protein deglycase DJ-1).
Results
Levels of IGFBP7 protein are increased in varicocele. Levels of DNASE1 are progressively decreased in varicocele (lower in varicocele and normal semen analysis, lowest in varicocele and altered semen analysis) when compared to adolescents without varicocele. DNASE1 levels are positively correlated with sperm concentration and morphology (correlation values of 0.400 and 0.404, respectively; p values of 0.001 and 0.001, respectively).
Conclusion
In conclusion, in adolescents, seminal plasma levels of IGFBP7, responsible for proliferative activity, are increased in varicocele grades II and III, and DNASE1, responsible for apoptosis regulation, are lower in varicocele, lowest in varicocele and low semen quality. These proteins demonstrate molecular alterations brought upon by varicocele. Moreover, DNASE1 is capable of discriminating a varicocele that causes alterations to semen quality from one that does not. We propose that the initial response of varicocele is to increase proliferative activity which, if followed by regulation of apoptosis, may lead to the ejaculation of a population of sperm that are in accordance with WHO cutoff values but, in the presence of dysregulated apoptosis, leads to lower sperm concentration and morphology.
Keywords: Adolescent, Apoptosis, Seminal plasma, Spermatogenesis, Varicocele
Introduction
Varicocele, defined by the presence of dilated veins in the pampiniform plexus with blood reflux [1], is considered the main treatable cause of male infertility [2, 3]. Varicoceles are detected in 15 % of the general adolescent and adult male population. However, 35 % of men with primary infertility, and up to 80 % of men with secondary infertility present varicoceles [4–6], which has led to the suggestion that varicocele causes a progressive decline in male fertility [7–9].
Conversely, 80 % of adolescents with varicocele will not present infertility in adulthood [10, 11], which has led to a suggestion of a mainly expectant approach, unless there is evidence of testicular and/or seminal alterations [12]. However, previous reports have demonstrated that varicocele may lead to alterations not usually detected in conventional semen analysis, such as increased sperm DNA fragmentation [13] and decreased sperm mitochondrial activity [14]. Moreover, treatment of varicoceles through a microsurgical approach (microsurgical varicocelectomy) has been shown to improve semen quality [12, 14, 15], testicular volume [16, 17], and sperm DNA integrity and mitochondrial activity [14], but this is not the case for all treated adolescents [18]. Therefore, there is a need to indicate, with improved sensitivity, which adolescents should be treated for varicoceles, so that they do not become infertile men in the future [11].
In this context, we have previously determined, using a proteomics pipeline, the seminal plasma proteome of adolescents with varicocele [19–21]. In one of this studies, using a two-dimensional gel electrophoresis (2D-GE) approach, we identified that adolescents with varicocele and seminal alterations present a seminal plasma proteome shifted towards apoptosis, while adolescents with varicocele but normal semen analysis present a seminal plasma proteome associated with normal spermatogenesis [20]. In a follow-up study, using a gel-free shotgun proteomics approach, we observed a number of different proteomics pathways which demonstrate a shift away from homeostasis in the seminal plasma of adolescents with varicocele, more so pronounced in those with altered semen analysis [21]. Among the proteins found by the authors, we can highlight the DNASE1 (deoxyribonuclease-1), which participates in apoptosis [22], and IGFBP7 (insulin-like growth factor-binding protein 7), which regulates cell proliferation [23].
In this study, we hypothesized that DNASE1 and IGFBP7 may be useful in discriminating a varicocele that is from a varicocele that is not causing alteration in semen parameters. Our hypothesis is that the equilibrium between apoptosis and proliferation will influence the seminal phenotype. Therefore, we aimed to evaluate the expression levels of DNASE1 and IGFBP7 in seminal plasma of adolescents with varicocele and normal and altered semen parameters, and adolescents without varicocele.
Material and methods
Patients and study group
A cross-sectional study was carried out including 61 adolescents who were recruited from the National Industrial Education Service in São Paulo, Brazil. All adolescents and their legal representatives were informed of the study conditions and those that agreed provided a signed consent form. Institutional Review Board approval was obtained from the Sao Paulo Federal University Research Ethics Committee (CAAE: 31701614.4.0000.5505). Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
All adolescents included in this study were aged between 15 and 17 years with full sexual maturity (Tanner V) [24]. Physical examination was performed by a same physician in a room with adequate illumination and a controlled temperature of 23 °C. Varicocele was diagnosed and graded by the method proposed by Dubin and Amelar [25], by a physical examination, as per the Practice Committee of the American Society for Reproductive Medicine and the Society for Male Reproduction and Urology recommendations [12] A Prader orchidometer was utilized to assess testicular volume. Exclusion criteria were presence of systemic diseases (such as cancer and endocrinopathies and their treatments), endocrine disorders, obesity, congenital malformation of the genitalia, genetic syndromes, prior history of inguinoscrotal surgery, orchitis or epididymitis, testicular torsion, testicular dystopia, absence of masturbation, and other conditions that could affect fertility. Adolescents with uni- or bilateral grade I varicoceles were also excluded from the study, because there are reports that, in adolescents, a grade I varicocele may not lead to alterations in testicular function [26].
Semen processing
Semen collections were performed after 2 to 5 days of ejaculatory abstinence and, after liquefaction, an aliquot was utilized for semen analysis [27]. After semen analysis, the 5th centile cutoff values in table A1.1 of the World Health Organization manual [27] were used to define a “normal” sample, and the patients were grouped into three experimental groups: controls without varicocele and normal semen analysis (C, n = 20), with uni- or bilateral grade II or III varicocele and normal semen analysis (VNS, n = 22), and with uni- or bilateral grade II or III varicocele and altered semen analysis (VAS, n = 19). The remainder of each sample was centrifuged at 800g, in order to separate the sperm from the supernatant seminal plasma, which was frozen and kept at −20 °C.
Protein expression analysis by Western blotting
For Western blotting analysis, samples were thawed and centrifuged at 16,100g at 4 °C for 30 min, in order to remove cellular debris. Total protein concentration in each sample was measured using a modified Lowry - Bicinchoninic Acid (BCA) assay [28]. Briefly, samples were diluted (1:80) in milli-Q water and measured in triplicate and standard curve points (0, 200, 400, 600, 800, and 1000 mg/mL of bovine serum albumin in water) were measured in duplicate in a 96-well plate. Absorbance was measured using a microplate reader at 540 nm. Samples with a coefficient of variation during quantification of over 5 % were re-quantified, to ensure greater accuracy.
For Western blotting experiments, a volume corresponding to 50 μg of seminal plasma total protein was suspended to a final volume of 7.5 μL in milli-Q water for each sample. This was then diluted 1:1 (v:v) in sample buffer (0.125 M Tris–HCl, pH 6.8, 4 % [w:v] SDS, 20 % [v:v] glycerol, 5 % (v:v) Beta-mercaptoethanol, 0.02 % bromophenol blue) and boiled in a dry bath for 5 min. Proteins were loaded onto 10 % polyacrylamide gels under denaturing conditions, and the Full Range Rainbow markers was used as molecular mass marker (GE Healthcare, Amersham Place, UK). . Following protein separation by one-dimensional electrophoresis, proteins were transferred to 0.45 μm nitrocellulose membranes through a Wet transfer system (MiniVE, GE Healthcare, Amersham Place, UK).
Following transfer, nitrocellulose membranes were incubated in blocking buffer (3 % bovine serum albumin [BSA] in Tris-buffered saline with 0.1 % tween-20 [TTBS]) for 1 h, washed in TTBS, and incubated with primary antibodies against the proteins of interest (DNASE1—anti-human DNASE1 polyclonal antibody produced in mouse [SAB1405721, Sigma-Aldrich, Saint Louis, USA], IGFBP7—anti-human IGFBP7 polyclonal antibody produced in goat [SAB2501563, Sigma-Aldrich, Saint Louis, USA]) for 2 h at room temperature. Membranes were then washed with TTBS and incubated with horseradish peroxidase (HRP)-conjugated appropriate secondary antibodies for 1 h at room temperature.
For signal detection, membranes were incubated with an enhanced chemiluminescence (ECL) reagent (GE Healthcare, Amersham Place, UK), and chemiluminescent images were acquired using a LAS-4000 system and processed using ImageQuant TL 7.0 Software (GE Healthcare, Amersham Place, UK). Background intensity was subtracted from each lane in order to calculate signal intensity, using the “image rectangle” option in the software. Following detection, membranes were incubated in mild stripping buffer (0.2 M glycine, 0.1 % [w:v] SDS, 1 % (v:v) Tween 20, pH 2.2) and probed with a primary anti-human DJ-1 (Protein deglycase DJ-1, gene name: PARK7; UniProt accession Q99497) goat polyclonal antibody as a testicular housekeeping protein (SAB2500750, Sigma-Aldrich, Saint Louis, USA) [21]. A HRP labelled anti-goat secondary antibody was then used, and membranes were submitted to ECL and image detection as described above. Protein quantities for each protein (IGFBP7 and DNASE1) from each sample were then normalized to PARK7 levels, in order to correct for loading variations.
Statistical analysis
For statistical analysis the SPSS software (PASW) 18.0 was utilized. To compare the frequency of varicocele between groups (VNS and VAS), a Pearson’s chi-square test was performed. Semen analysis and Western blotting data were tested for normality of distribution using a Kolmogorov-Smirnov test, and for homogeneity of variance using a Levene test. Normally distributed and homoscedastic variables were compared between groups (Control, Varicocele and normal semen analysis, and Varicocele and altered semen analysis) with one-way analysis of variance (ANOVA), followed by a Sidak post-hoc test. For non-parametric variables, a Kruskal-Wallis test, followed by a Games Howell post-hoc test, was used. To verify correlations between semen variables and the expression levels of each protein, a Spearman’s Rank Test correlation analysis was performed. For multivariate data analysis, we applied a binary logistic regression model to verify the predictive value of DNASE1 and IGFBP7 in discriminating the VNS from the VAS group. These results were utilized to construct a receiver operating characteristics (ROC) curve. Results were considered significant when p < 0.05.
Results
Distribution of varicocele grades is presented in Table 1. Frequencies of different varicocele grades (only varicocele grades II and III were included) were not different between the varicocele groups. Clinical characteristics and semen quality results are presented in Table 2. The VAS group was characterized as of lower sperm concentration and morphology when compared to the VNS and control groups.
Table 1.
Varicocele grades and laterality in adolescents with varicocele and normal semen analysis (VNS) and in adolescents with varicocele and altered semen analysis (VAS). Frequencies were compared using a Pearson’s chi-square test
| VNS (n = 22) | VAS (n = 19) | |
|---|---|---|
| Left I, right II | 2 (9.09 %) | |
| Left II | 3 (13.63 %) | 1 (5.26 %) |
| Left II, right I | 6 (27.27 %) | 5 (26.31 %) |
| Left II, right II | 6 (27.27 %) | 4 (21.05 %) |
| Left III, right I | 1 (4.54 %) | 3 (15.78 %) |
| Left III, right II | 4 (18.18 %) | 4 (21.05 %) |
| Left III | 1 (5.26 %) | |
| Left III, right III | 1 (5.26 %) |
Pearson’s chi-square test – p = 0.493
Table 2.
Individual traits and semen analysis results in adolescents without varicocele (control group) in adolescents with varicocele and normal semen (VNS), and adolescents with varicocele and altered semen (VAS). The groups were compared using ANOVA parametric test with post hoc Sidak, unless otherwise specified
| Control (n = 20) |
VNS (n = 22) |
VAS (n = 19) |
p | |
|---|---|---|---|---|
| Age (years) | ||||
| Median; IR | 16.5; 2.00 | 16.0; 2.00 | 16.0; 1.00 | Ω 0.595 |
| Q1 - Q3 | 15.0–17.0 | 15.0–17.0 | 16.0–17.0 | |
| Abstinence (days) | ||||
| Median; IR | 3.5; 1.40 | 4.0; 2.00 | 3.0; 2.00 | Ω 0.289 |
| Q1 - Q3 | 2.9–4.0 | 3.0–5.0 | 2.3–4.0 | |
| Volume (mL) | ||||
| Median; IR | 2.7; 1.15 | 2.7; 1.00 | 2.7; 1.40 | Ω 0.937 |
| Q1 - Q3 | 2.1–3.1 | 2.2–3.1 | 1.9–3.3 | |
| Sperm concentration (million/mL) | ||||
| Mean; DP | 80.5; 50.49 a | 96.1; 60.94 a | 13.4; 11.25 b | <0.00001* |
| CI (95 %) | 56.9–104.2 | 69.8–122.5 | 8.1–18.9 | |
| Progressive motility (%) | ||||
| Median; IR | 64.5; 10.50 | 58.0; 10.00 | 56.0; 16.00 | Ω 0.122 |
| Q1 - Q3 | 59.3–66.8 | 54–63.5 | 51.5–66 | |
| Total motility (%) | ||||
| Mean; SD | 65.5; 7.25 a | 60.1; 12.18 a | 60; 12.65 a | 0.201 |
| CI (95 %) | 62.1–68.9 | 54.9–65.4 | 53.9–66.1 | |
| Sperm morphology (% normal) | ||||
| Median; IR | 9.5; 3.80 a | 9.0; 4.00 a | 2;.0 4.00 b | Ω <0.00001* |
| Q1 - Q3 | 7.8–11.0 | 6.0–10.0 | 1.5–4.5 | |
| Left testicular volume (mL) | ||||
| Median; IR | 20.0; 4.50 a | 17.0; 3.00 ab | 15.0; 6.00 b | Ω 0.046* |
| Q1 - Q3 | 15.5–20.0 | 15–18.0 | 12.0–18.0 | |
| Right testicular volume (mL) | ||||
| Median; IR | 20.0; 4.25 | 18.0; 3.00 | 15.0; 3.00 | Ω 0.186 |
| Q1 - Q3 | 17.2–21.5 | 17.0–20.0 | 15.0–18.0 | |
VNS – Varicocele and normal semen group
VAS – Varicocele and altered semen group
SD – standard derivation
CI (95 %) – 95 % confidence interval of the mean
Ω - a non-parametric Kruskal-Wallis test was used, followed by a Games Howell post hoc test, when applicable. Values expressed in median; interquartile range (IR)
Q1-Q3 –first and third quartile, respectively
a,b – different superscript letters in a same row indicate statistical difference between groups in a post hoc test (p < 0.05)
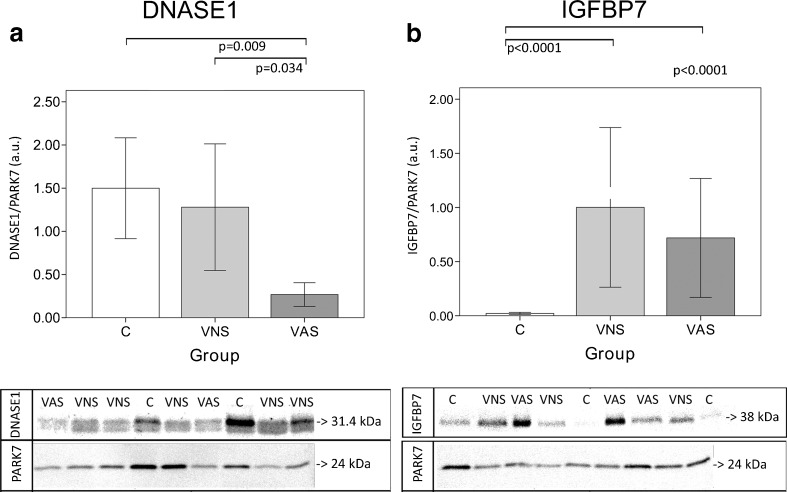
Typical Western blotting images and graphs with the expression levels of the analyzed proteins are presented in Fig. 1. DNASE1 was significantly under-represented in the VAS group when compared to both other groups (C and VNS), and IGFBP7 was over-represented in both varicocele groups (VNS and VAS) when compared to the control group (C). When data from the three groups was pooled for correlation analysis, positive correlations between DNASE1 and sperm concentration (correlation value = 0.400; 95 % CI 0.164–0.592; p = 0.01), and between DNASE1 and morphology were observed (correlation value = 0.404; 95 % CI 0.16–0.595; p = 0.01). This correlation was not observed in individual groups.
Fig. 1.
Bar graphs of seminal plasma DNASE1 (deoxyribonuclease-1, UniProt accession P24855) (a) and IGFBP7 (insulin-like growth factor-binding protein 7, UniProt accession Q16270) (b) levels, and example images of Western blot results in adolescents without varicocele (controls, [C]), with varicocele and normal semen analysis as per the World Health Organization 2010 manual 5th centile cutoff values (VNS) and with varicocele and altered semen analysis (VAS). Error bars in the graphs indicate the 95 % confidence interval of the mean
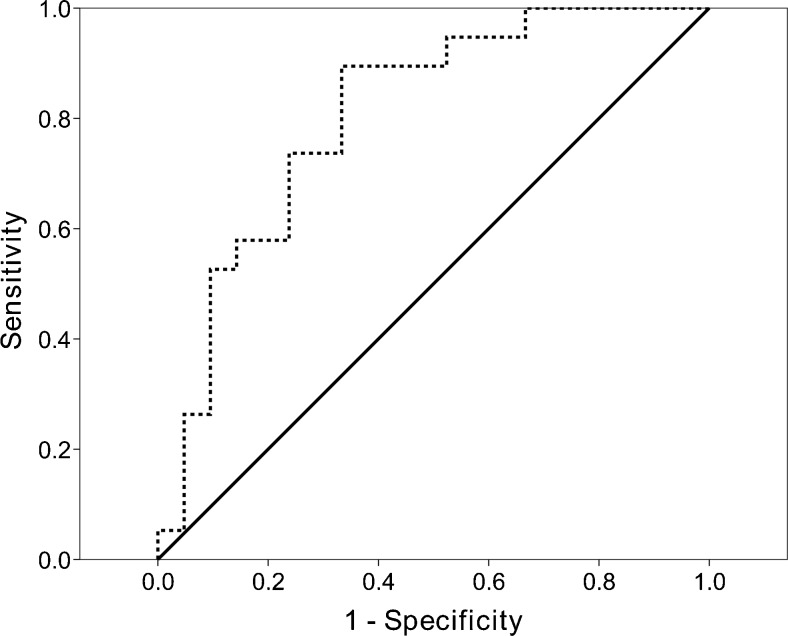
The binary logistic regression model was significant in discriminating the VNS from the VAS group (p = 0.001) with a negative predictive value of 66.7 %, a positive predictive value of 89.5 %, and an overall predictive value of 77.5 %. Although the logistic regression model with the highest predictive values included both proteins, only the odds-ratio for DNASE1 was significant in that model (p = 0.013), with a value of 0.054 (CI 95 % 0.005–0.540) (increasing values of DNASE1 decrease the odds of belonging to the VAS group). The generated ROC curve presented an area under the curve of 0.805 (p = 0.001) (Fig. 2).
Fig. 2.
Receiver operating characteristic (ROC) curve showing the discerning value of a logistic regression model including DNASE1 and IGFBP7 as independent variables in separating varicocele and normal semen from varicocele and altered semen. The total area under the curve was 0.805 (p = 0.001)
Discussion
The adolescent varicocele is often of a complex nature to be approached. In the first place, although many of these adolescents may present with impaired spermatogenesis [20], not all adolescents with varicocele will present a decrease in fertile potential when they achieve adulthood [10, 11]. Furthermore, spermatogenesis is not yet mature in the adolescence, and the alterations observed in a sample may be transient [29]. Therefore, conventional semen analysis is not suitable for a decisive report of the changes in fertile potential caused by varicocele [30]. We have previously shown, for example, that sperm functional alterations may be present even in the absence of semen analysis alterations [13]. Moreover, we have also observed that a number of sperm functional alterations [14, 19] and alterations to seminal plasma protein expression are associated to the adolescent varicocele [19–21]. We need, however, to better understand which molecular mechanisms are associated with the adolescent varicocele, especially to one that will determine infertility in the future [31]. Bearing this in mind, our study design was to observe molecular differences in seminal plasma of adolescents with a varicocele grade II or III that is already defining alterations to semen quality, and to see how this compares to a adolescents with varicocele which is not causing alteration in semen parameters (according to the 5th centile cutoff values of the World Health Organization manual [32]), or to samples from healthy controls.
Seminal plasma is constituted of proteins from the seminiferous tubules lumen [33], from the epididymal fluid containing secreted proteins and specific microvesicles—epididymosomes—with the function of transferring proteins to sperm [34], and from seminal vesicle and the prostate [35, 36]. In varicocele, these proteins are associated to maintenance of the homeostatic functions, when semen quality is still maintained [20]. Moreover, varicocelectomy is associated to a return to seminal plasma homeostasis in adolescents [19], which indicates monitoring these proteins may be of interest in understanding the underlying mechanisms of varicocele as it initiates.
In a previous study, we identified a number of differentially expressed proteins when comparing adolescents with varicocele and altered semen analysis, with varicocele and normal semen analysis and controls without varicocele [21]. In that study, multivariate data processing and filtering led to the suggestion of some proteins that could potentially demonstrate molecular mechanisms of seminal plasma in the adolescent varicocele [21]. This present study was thus designed to verify the levels of two proteins indicated by this previous article (IGFBP7 and DNASE1) that can help us to understand the role of seminal plasma proteins in male fertility.
In this study, semen quality analysis was performed in order to classify the varicocele (grades II and III) groups as those with altered semen (VAS group) or normal semen (VNS group). The differences observed between the VAS group and the VNS and control (C) groups were in sperm concentration and morphology. These demonstrate that the decreased semen quality in our study group occurred due to a testicular and/or epididymal alteration. It should be pointed out, however, that this was not an epidemiologic study on semen quality in the adolescent varicocele, and these results should only be considered in order to better understand our protein expression results.
Insulin-like growth factor-binding protein 7 (IGFBP7) was increased in both groups with varicocele, indistinguishably. This protein belongs to the group of growth factors binding proteins, which are related to cell growth, differentiation and proliferation in mammals [23]. This protein is also related to cell adhesion and acts as a stimulus for the production of prostacyclin (PGI2) [37, 38], which is the main product of endothelial cyclooxygenase and is responsible for vasodilation [39]. Because varicocele leads to testicular hypoxia [40, 41], this is a likely response mechanism to increase oxygen supply to the testes, which can be an important mechanism related to decreased oxidative stress. Oxidative stress has been demonstrated to affect sperm physiology and can impair sperm function and lead to increased sperm DNA fragmentation [42–44]. Thus, our results indicate that varicocele leads to a proliferative response in the seminiferous tubules, likely in order to balance an altered oxidative environment.
Conversely, deoxyribonuclease-1 (DNASE1) was under-represented in both groups of varicocele, and this under-representation is progressive—highest in controls without varicocele, lower in VNS, and lowest in VAS. DNASE1 is a calcium- and magnesium-dependent endonuclease responsible for digestion of DNA residues [45], as well as an autonomous nuclease in cells that are subjected to drug-induced apoptosis [46], demonstrating that this protein is related to regulation of apoptosis. Probably due to the lower action of this protein, adolescents with varicocele and altered semen analysis present failure in germ cell apoptosis regulation. This is especially important because it suggests a testicular origin of sperm DNA alteration. It has been suggested that peptides with a DNASE domain in seminal plasma from bovines and equines are fertility markers [47]. With our study, we demonstrate that alterations seminal levels of DNASE1 and IGFBP7 can identify testicular alterations in varicocele; however, it remains to be verified if this is true only for varicocele, or if these proteins identify alterations in testicular function in other conditions related to male infertility as well.
When our pooled data from the three groups was utilized for correlation analysis, we observed that DNASE1 was positively correlated to sperm concentration and to sperm morphology. Therefore, alteration of apoptosis regulation may be a possible mechanism by which varicocele leads to increased DNA fragmentation in adolescents, as observed in other studies in adolescents [13, 14, 19]. This is important because it suggests a testicular (and not a post-testicular) origin to alterations to sperm DNA quality in these patients, which in turn renders an immediate surgical approach more important, in order to revert initial testicular damage. Moreover, it is interesting that up-regulation of a protein responsible for apoptosis leads to an improved seminal phenotype, in patients with increased testicular proliferative activity.
As was observed in the logistic regression, the proposed model that included the proteins DNASE1 and IFGBP7 was significant, with an area under a ROC curve of 0.805, and with negative, positive, and overall predictive values of 66.7, 89.5, and 77.5 %. These results indicate that monitoring of these two proteins highly increases the chance of detecting alterations of semen quality. We propose that, alongside other routine clinical exams, assessing seminal plasma levels of DNASE1 and IGFBP7 may determine an optimal point in which intervention is indicated in the adolescent varicocele.
One of the limitations of this study was the inclusion of a relatively small cohort of adolescents (n = 61). Moreover, our endpoint in this study was seminal alteration per se, which makes it necessary to perform a confirmatory prospective multicentric cohort study in order to validate our findings, as well as to better define cutoff values. Finally, it is important to better understand the role of seminal plasma proteins in different causes of infertility, because this is a promising field that can indicate potential biomarkers, and because the regulatory activity of extracellular proteins in seminal plasma is fundamental for proper sperm function after ejaculation [48].
In conclusion, in adolescents, seminal plasma levels of IGFBP7, responsible for proliferative activity, are increased in varicocele grades II and III, and DNASE1, responsible for apoptosis regulation, are lower in varicocele, lowest in varicocele and low semen quality. These proteins demonstrate molecular alterations brought upon by varicocele. Moreover, DNASE1 is capable of discriminating a varicocele that causes alterations to semen quality from one that does not. We propose that the initial response of varicocele is to increase proliferative activity which, if followed by regulation of apoptosis, may lead to the ejaculation of a population of sperm that are in accordance with WHO cutoff values but, in the presence of dysregulated apoptosis, leads to lower sperm concentration and morphology.
Compliance with ethical standards
Conflicts of interest
None declared.
Authors’ roles
L.B.B. was responsible for conception and design of the study, acquisition of samples for analysis, acquisition of confirmatory proteomics data, interpretation of data, drafting of the article, revision of the article, and final approval for submission. P.T.D.G. participated in the acquisition of samples for analysis, interpretation of data, revision of the article, and final approval for submission. M.C. participated in the acquisition of samples for analysis, interpretation of data, revision of the article and final approval for submission. P.I. participated in the interpretation of data, revision of the article, and final approval for submission. M.P.A. participated in the interpretation of data, revision of the article, and final approval for submission. R.P.B. participated in conception and design of the study, analysis and interpretation of data, drafting of the article, and final approval for submission. A.P.C. participated in the conception and design of the study, clinical discussion of all the patients included in the study, discussion of results, drafting of the article, and final approval for submission.
Funding
L.B.B. was a recipient of a Masters scholarship from the Sao Paulo Research Foundation - FAPESP (process number 2014/11636-6). A.P.C. and R.P.B. are recipients of a Scientific Productivity scholarship from the Brazilian National Council for Scientific and Technological Development – CNPq (processes numbers 313077/2014-2 and 306616/2013-0, respectively). This work was supported by a Research Grant from the Sao Paulo Research Foundation – FAPESP (process number 2014/17185-6) and from the Brazilian National Council for Scientific and Technological Development (process number 454514/2014-0).
Ethical approval
Institutional Review Board approval was obtained from the Sao Paulo Federal University Research Ethics Committee (CAAE: 31701614.4.0000.5505).
Footnotes
Capsule
In adolescents, seminal plasma levels of IGFBP7 are higher in varicocele, and of DNASE1 are lower in varicocele, lowest in varicocele in the presence of low semen quality.
References
- 1.Gat Y, Bachar GN, Zukerman Z, Belenky A, Gornish M. Varicocele: a bilateral disease. Fertil Steril. 2004;81:424–9. doi: 10.1016/j.fertnstert.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Benoff S, Gilbert BR. Varicocele and male infertility: part I. Preface Hum Reprod Update. 2001;7:47–54. doi: 10.1093/humupd/7.1.47. [DOI] [PubMed] [Google Scholar]
- 3.Hauser R, Paz G, Botchan A, Yogev L, Yavetz H. Varicocele: effect on sperm functions. Hum Reprod Update. 2001;7:482–5. doi: 10.1093/humupd/7.5.482. [DOI] [PubMed] [Google Scholar]
- 4.Smith R, Kaune H, Parodi D, Madariaga M, Rios R, Morales I, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod Oxf Engl. 2006;21:986–93. doi: 10.1093/humrep/dei429. [DOI] [PubMed] [Google Scholar]
- 5.Witt MA, Lipshultz LI. Varicocele: a progressive or static lesion? Urology. 1993;42:541–3. doi: 10.1016/0090-4295(93)90268-F. [DOI] [PubMed] [Google Scholar]
- 6.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–6. doi: 10.1016/S0015-0282(16)55809-9. [DOI] [PubMed] [Google Scholar]
- 7.Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80:1431–6. doi: 10.1016/S0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 8.Zini A, Blumenfeld A, Libman J, Willis J. Beneficial effect of microsurgical varicocelectomy on human sperm DNA integrity. Hum Reprod Oxf Engl. 2005;20:1018–21. doi: 10.1093/humrep/deh701. [DOI] [PubMed] [Google Scholar]
- 9.Cozzolino DJ, Lipshultz LI. Varicocele as a progressive lesion: positive effect of varicocele repair. Hum Reprod Update. 2001;7:55–8. doi: 10.1093/humupd/7.1.55. [DOI] [PubMed] [Google Scholar]
- 10.The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. World Health Organization. Fertil Steril 1992;57:1289–93. [PubMed]
- 11.Diamond D. Adolescent versus adult varicoceles—how do evaluation and management differ? J Urol. 2009;181:2418–9. doi: 10.1016/j.juro.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Practice Committee of the American Society for Reproductive Medicine, Society for Male Reproduction and Urology. Report on varicocele and infertility: a committee opinion. Fertil Steril 2014;102:1556–60. [DOI] [PubMed]
- 13.Bertolla RP, Cedenho AP, Hassun Filho PA, Lima SB, Ortiz V, Srougi M. Sperm nuclear DNA fragmentation in adolescents with varicocele. Fertil Steril. 2006;85:625–8. doi: 10.1016/j.fertnstert.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 14.Lacerda JI, Del Giudice PT, da Silva BF, Nichi M, Fariello RM, Fraietta R, et al. Adolescent varicocele: improved sperm function after varicocelectomy. Fertil Steril. 2011;95:994–9. doi: 10.1016/j.fertnstert.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology. 2007;70:532–8. doi: 10.1016/j.urology.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Gentile DP, Cockett AT. The effect of varicocelectomy on testicular volume in 89 infertile adult males with varicoceles. Fertil Steril. 1992;58:209–11. doi: 10.1016/S0015-0282(16)55165-6. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M, Hibi H, Katsuno S, Miyake K. Effects of varicocelectomy on testis volume and semen parameters in adolescents: a randomized prospective study. Nagoya J Med Sci. 1995;58:127–32. [PubMed] [Google Scholar]
- 18.Cocuzza M, Cocuzza MA, Bragais FMP, Agarwal A. The role of varicocele repair in the new era of assisted reproductive technology. Clin São Paulo Braz. 2008;63:395–404. doi: 10.1590/S1807-59322008000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Giudice PT, da Silva BF, Lo Turco EG, Fraietta R, Spaine DM, Santos LFA, et al. Changes in the seminal plasma proteome of adolescents before and after varicocelectomy. Fertil Steril. 2013;100:667–72. doi: 10.1016/j.fertnstert.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Zylbersztejn DS, Andreoni C, Del Giudice PT, Spaine DM, Borsari L, Souza GHMF, et al. Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil Steril. 2013;99:92–8. doi: 10.1016/j.fertnstert.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 21.Del Giudice PT, Belardin LB, Camargo M, Zylbersztejn DS, Carvalho VM, Cardozo KHM, et al. Determination of testicular function in adolescents with varicocoele—a proteomics approach. Andrology. 2016;4:447–55. doi: 10.1111/andr.12174. [DOI] [PubMed] [Google Scholar]
- 22.Samejima K, Earnshaw WC. Trashing the genome: the role of nucleases during apoptosis. Nat Rev Mol Cell Biol. 2005;6:677–88. doi: 10.1038/nrm1715. [DOI] [PubMed] [Google Scholar]
- 23.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 24.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–9. doi: 10.1016/S0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 26.Mori MM, Bertolla RP, Fraietta R, Ortiz V, Cedenho AP. Does varicocele grade determine extent of alteration to spermatogenesis in adolescents? Fertil Steril. 2008;90:1769–73. doi: 10.1016/j.fertnstert.2007.09.052. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambrige University Press; 2010.
- 28.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 29.Mori MM, Cedenho AP, Koifman S, Srougi M. Sperm characteristics in a sample of healthy adolescents in São Paulo. Brazil Cad Saúde Pública. 2002;18:525–30. doi: 10.1590/S0102-311X2002000200018. [DOI] [PubMed] [Google Scholar]
- 30.Milardi D, Grande G, Sacchini D, Astorri AL, Pompa G, Giampietro A, et al. Male fertility and reduction in semen parameters: a single tertiary-care center experience. Int J Endocrinol. 2012;2012:649149. doi: 10.1155/2012/649149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camargo M, Intasqui P, Bertolla RP. Proteomic profile of seminal plasma in adolescents and adults with treated and untreated varicocele. Asian J Androl. 2016;18:194–201. doi: 10.4103/1008-682X.168788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. 2010.
- 33.Gerena RL, Irikura D, Urade Y, Eguchi N, Chapman DA, Killian GJ. Identification of a fertility-associated protein in bull seminal plasma as lipocalin-type prostaglandin D synthase. Biol Reprod. 1998;58:826–33. doi: 10.1095/biolreprod58.3.826. [DOI] [PubMed] [Google Scholar]
- 34.D’Amours O, Frenette G, Bordeleau L-J, Allard N, Leclerc P, Blondin P, et al. Epididymosomes transfer epididymal sperm binding protein 1 (ELSPBP1) to dead spermatozoa during epididymal transit in bovine. Biol Reprod. 2012;87:94. doi: 10.1095/biolreprod.112.100990. [DOI] [PubMed] [Google Scholar]
- 35.Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Utleg AG, Yi EC, Xie T, Shannon P, White JT, Goodlett DR, et al. Proteomic analysis of human prostasomes. Prostate. 2003;56:150–61. doi: 10.1002/pros.10255. [DOI] [PubMed] [Google Scholar]
- 37.Akaogi K, Okabe Y, Funahashi K, Yoshitake Y, Nishikawa K, Yasumitsu H, et al. Cell adhesion activity of a 30-kDa major secreted protein from human bladder carcinoma cells. Biochem Biophys Res Commun. 1994;198:1046–53. doi: 10.1006/bbrc.1994.1149. [DOI] [PubMed] [Google Scholar]
- 38.Oh Y, Nagalla SR, Yamanaka Y, Kim HS, Wilson E, Rosenfeld RG. Synthesis and characterization of insulin-like growth factor-binding protein (IGFBP)-7. Recombinant human mac25 protein specifically binds IGF-I and -II. J Biol Chem. 1996;271:30322–5. doi: 10.1074/jbc.271.48.30322. [DOI] [PubMed] [Google Scholar]
- 39.Yamauchi T, Umeda F, Masakado M, Isaji M, Mizushima S, Nawata H. Purification and molecular cloning of prostacyclin-stimulating factor from serum-free conditioned medium of human diploid fibroblast cells. Biochem J. 1994;303:591–8. doi: 10.1042/bj3030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9:678–90. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 41.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 42.Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987;81:459–69. doi: 10.1530/jrf.0.0810459. [DOI] [PubMed] [Google Scholar]
- 43.Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg H-R, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–42. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol N Y N 1989. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 45.Liu QY, Ribecco M, Pandey S, Walker PR, Sikorska M. Apoptosis-related functional features of the DNaseI-like family of nucleases. Ann N Y Acad Sci. 1999;887:60–76. doi: 10.1111/j.1749-6632.1999.tb07922.x. [DOI] [PubMed] [Google Scholar]
- 46.Oliveri M, Daga A, Cantoni C, Lunardi C, Millo R, Puccetti A. DNase I mediates internucleosomal DNA degradation in human cells undergoing drug-induced apoptosis. Eur J Immunol. 2001;31:743–51. doi: 10.1002/1521-4141(200103)31:3<743::AID-IMMU743>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 47.Alghamdi AS, Funnell BJ, Bird SL, Lamb GC, Rendahl AK, Taube PC, et al. Comparative studies on bull and stallion seminal DNase activity and interaction with semen extender and spermatozoa. Anim Reprod Sci. 2010;121:249–58. doi: 10.1016/j.anireprosci.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Ding Z, Qu F, Guo W, Ying X, Wu M, Zhang Y. Identification of sperm forward motility-related proteins in human seminal plasma. Mol Reprod Dev. 2007;74:1124–31. doi: 10.1002/mrd.20624. [DOI] [PubMed] [Google Scholar]