Abstract
Purpose
We aimed to compare the expression of phospholipase C ζ (PLCζ), as one of the main sperm factors involved in oocyte activation, at both RNA and protein levels in fertile men and those with varicocele.
Methods
This study included 35 individuals with male factor infertility presenting primary infertility with grade II and III unilateral varicocele and 20 fertile men without varicocele. Semen parameters were assessed according to WHO 2010. Sperm DNA fragmentation, relative expression of PLCζ at messenger RNA, and protein levels were evaluated by sperm chromatin structure assay (SCSA), real-time PCR, and Western blot analysis, respectively.
Results
The results of this study reveal that the mean relative expression of PLCζ was significantly lower in individuals with varicocele compared to fertile men at both transcription and translation levels. In addition, the percentage of DNA fragmentation was significantly higher in infertile men with varicocele compared to fertile men.
Conclusions
The findings of the present study illustrate that one of the etiologies of reduced fertility associated with varicocele is the low expression of PLCζ. This effect could subsequently reduce the sperm ability to induce oocyte activation. Therefore, these results hold promise to modify our understanding of reproductive physiology of varicocele state.
Keywords: PLCζ, Varicocele, DNA fragmentation, Sperm parameters
Introduction
Expansion of spermatic veins in the scrotum is termed “varicocele” and considered as a common cause of male infertility. The incidence of varicocele was reported from 4 to 30 % in the general population and from 17 to 41 % among infertile men. Although the association between the pathophysiology of varicocele and infertility remains unclear, some studies have considered varicocele as a possible defense mechanism for repairing testicular dysfunction, while others have considered varicocele as a cause of male infertility due to high incidence of varicocele among infertile men with low semen quality, reduction of testicular size, and improvement of semen parameters and pregnancy rates after surgical repair [1, 2]. At the physiological level, several hypotheses including hyperthermia and retrograde blood flow have been explored but at the molecular level, very little is known [3, 4].
One of the events playing a key role in the initiation of development is oocyte activation. This phenomenon is mediated by sperm-born oocyte-activating factors (SOAFs) [5, 6]. Recently, scientists and clinicians have focused on the identification and introduction of sperm factors involved in oocyte activation. So far, several factors have been investigated in different species, and phospholipase C ζ (PLCζ) and post-acrosomal sheath WW domain-binding protein (PAWP) have gained the most attention in the mammals. In this regard, several in vitro studies have revealed that microinjection of recombinant PLCζ and PAWP into oocytes induces meiotic resumption, pronuclear formation, and subsequent early embryonic development in mammalian oocytes [7, 8].
Upon oocyte-sperm membrane fusion, PLCζ is released into the oocyte and interacts with intracellular vesicular phosphoinositide 4,5-bisphosphate (PIP2). Subsequently, PIP2 is hydrolyzed to form inositol triphosphate (IP3) and diacylglycerol (DAG). Then, IP3 interacts with its receptor on intracellular stores to induce Ca2+ oscillations. Unlike PLCζ, the exact mechanism of PAWP signaling pathway is not well known. It is proposed that following release of PAWP into the oocyte cytosol, it activates PLCγ through yes-associated proteins (YAPs). It is likely that PLCγ cleaves membrane PIP2 and thereby induces Ca2+ oscillations [9, 10]. Recent study by Satouh et al. [11] shows that PAWP null mice present abnormal morphology but normal Ca2+ oscillations post insemination and thereby concluded that PAWP does not play an essential role in mouse fertilization. Considering the fact that PAWP is a testis-specific gene, further studies are needed to confirm its role in spermatogenesis [11] or its possible co-interaction with PLCζ.
Reduced expression and/or mutations in PLCζ have been associated with low or failed fertilization in infertile men following intra-cytoplasmic sperm injection (ICSI) [12–18]. In addition, Knott et al. [19] showed that sperm derived from transgenic mice express short hairpin RNAs targeting PLCζ messenger RNA (mRNA) and present perturbed patterns of Ca2+ oscillations with lower rate of egg activation [19]. In the light of these considerations, we also showed that expression level of PLCζ mRNA were significantly lower in infertile men with previous failed fertilization and globozoospermia than fertile individuals [12]. Other researchers also observed the absence or low expression of PLCζ protein in infertile men with globozoospermia and concluded that this factor could be considered as a prognostic marker of oocyte activation [12, 18, 20]. Recent studies have demonstrated that sperm factors involved in oocyte activation are sensitive to heat treatment or cold shock during freeze-thawing [21, 22]. Considering varicocele is a chronic genital heat stress condition, and this state is associated with low semen quality and fertility potential, the aim of this study was to compare PLCζ as one of the main sperm factors involved in oocyte activation at both transcription and translation levels in men with varicocele and fertile men. In addition, the effect of varicocele on DNA fragmentation was assessed.
Materials and methods
Semen collection
This study received the approval of the Institutional Review Board of Royan Institute. Informed written consent was obtained from each participant. This study included 35 individuals with primary male infertility presenting grade II or III varicocele (left side) upon palpation which was confirmed by Doppler duplex ultrasound. Twenty fertile individuals without any clinical presentation of varicocele, who requested pre-implantation genetic diagnosis (PGD) for gender selection for family balancing, were considered as control group.
Semen samples were collected by masturbation into a sterile specimen container following 3–5 days of sexual abstinence and allowed to liquefy for a period of 15–30 min at room temperature. One portion of semen was used for evaluation of sperm concentration, total motility, and morphology according to WHO [23]. The remaining portion of the semen sample was washed twice in phosphate buffer saline (PBS; pH 7.4) and used for evaluation of DNA fragmentation, relative expression of PLCζ at mRNA, and protein levels by sperm chromatin structure assay (SCSA), real-time PCR, and Western blot analysis, respectively. All the samples had somatic cell count of less than 1 million per milliliter.
Exclusion criteria
Leucocytospermia, azoospermia, seminal sperm antibodies, fever within 90 days prior to the seminal analysis, abnormal hormonal profile, anatomical disorders, testicular size discrepancy, Klinefelter’s syndrome, cancer, grade I varicocele, recurrent varicocele, urogenital infections, previous history of scrotal trauma or surgery, excessive alcohol, drug consumption, and occupational exposure to heat were excluded from this study.
Assessment of DNA fragmentation by sperm chromatin structure assay
Briefly, one to two million sperm were separated from semen and the final volume was adjusted to 1 ml TNE (Tris HCl (Merck, Darmstadt, Germany)/NaCl (Merck, Darmstadt, Germany)/EDTA (Merck, Darmstadt, Germany)) buffer. For control tube, 1200 μl of acridine orange (Sigma, St. Louis, USA) staining solution was added to 200 μl of diluted semen sample, while for test tube, 400 μl acid-detergent solution was firstly mixed with 200 μl of diluted semen sample and after 30 s, 1200 μl of acridine orange staining solution was added to this mixture. Finally, the percentage of DNA fragmentation was assessed by a FACSCalibur flow cytometry (Becton Dickinson, San Jose, CA, USA). A minimum of 10,000 sperm were examined and analyzed using BD CellQuest Pro software [24].
Assessment of PLCζ expression by real-time PCR
RNA extraction and complementary DNA synthesis
The procedure of RNA extraction and complementary DNA (cDNA) synthesis was according to Aghajanpour et al. [12]. Total RNA from washed samples in both fertile and infertile individuals was extracted using Trizol (Ambion, Burlington, Canada). The integrity of extracted RNA was evaluated by agarose electrophoresis, and final concentration was assessed by measuring absorbance at 260 nm. To eliminate possible contamination of genomic DNA, RNA-containing samples were treated with DNase I (Fermentas, Burlington, Canada). First-strand cDNA synthesis was carried out using 2 μg of total RNA with the RevertAid First Strand cDNA Synthesis kit (Takara, Otsu, Japan) according to the manufacturer’s protocol [12].
Quantitative real-time PCR analysis
Real-time PCR was carried out according to the manufacturer’s protocol (Takara, Otsu, Japan) in a thermal cycler step one plus applied Biosystems (ABi). The PCR mixture for each reaction contained 10 μl SYBR premix Ex Taq II (Takara, Otsu, Japan), 1 μl of each primer (5 pmol/μl), and 50 ng cDNA adjusted to a final volume of 20 μl using dH2O. All reactions were carried out in triplicate. Real-time specific primer pairs were designed by the Beacon designer 7.5. The primers used were previously designed as PLCζ (forward: 5′-CAGATGCCTTGTTCAGTTATTGTC-3′; reverse: 5′-GCCTTCATTTCCTACGGGTTG-3′) and GAPDH (forward: 5′-CCACTCCTCCACCTTTGACG-3′; reverse: 5′-CCACCACCCTGTTGCTGTAG-3′). The real-time PCR protocol composed of the following: 30 s at 95 °C followed by 40 repetitive cycles for 5 s at 95 °C, 10 s at 60 °C for PLCζ, and GAPDH, and then 30 s at 72 °C. The expression level of PLCζ mRNA was normalized to GAPDH expression level as a housekeeping gene. Calculation of relative expression was demonstrated as 2−ΔΔCt as previously reported [12].
Assessment of PLCζ expression by Western blot
Protein was extracted from washed samples using TRI Reagent (Sigma-Aldrich; USA). Protein concentration of each sample was estimated by Bradford assay (Bio-Rad; USA) to determine total protein load per each lane. Equal amounts of each sample containing 40 μg of protein were subjected to 12 % SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred to PVDF membrane (Bio-Rad; USA). The membranes were blocked with 5 % skim milk (Merck, USA) and polyclonal anti-phospholipase C zeta (PLCζ) antibody (1:32,000, Covalab, France) and monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH), clone 6C5 (1:5000, Millipore, USA) were used as specific primary antibodies. After washing three times, secondary antibody used for PLCζ was horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Dako, Japan) and for GAPDH was anti-rabbit IgG (Dako, Japan). After washing three times, target protein bands were detected with an Amersham ECL Advance Western Blotting Detection Kit (GE Healthcare, Germany). The fire reader (Uvitec, Cambridge) was used for recording chemiluminescence images. Densitometric analysis of the images was performed by Quantity One Software v 4.6.9 (Bio-Rad, Munchen, Germany). Results were expressed as mean relative intensity [20].
Results
Routine semen analyses were performed on 20 fertile men and 35 individuals with varicocele according to the World Health Organization [23]. Table 1 shows the descriptive of semen parameters in individuals with varicocele and fertile men. The mean of sperm concentration (38.82 ± 6.87 vs. 86.16 ± 9.10), total sperm count (106.97 ± 14.38 vs. 498.99 ± 74.16), percentage of sperm motility (46.99 ± 3.45 vs. 58.61 ± 4.27), and semen volume (3.20 ± 0.31 vs. 5.71 ± 0.53) were significantly lower in infertile men with varicocele compared to fertile men. In addition, the percentage of abnormal sperm morphology (96.66 ± 0.29 vs. 95.65 ± 0.29) was significantly higher in infertile men with varicocele compared to fertile men.
Table 1.
Descriptive of semen parameters in fertile individuals and men with varicocele
| Parameters | Fertile group (n = 20) | Varicocele group (n = 35) | p value |
|---|---|---|---|
| Mean + SE | |||
| Sperm concentration (106/ml) | 86.16 ± 9.10 | 38.82 ± 6.87 | 0.00 |
| Sperm motility (%) | 58.61 ± 4.27 | 46.99 ± 3.45 | 0.048 |
| Progressive motility (%) | 2.97 ± 38.47 | 23.80 ± 2.01 | 0.00 |
| Abnormal sperm morphology (%) | 95.65 ± 0.29 | 96.66 ± 0.29 | 0.02 |
| Volume (ml) | 5.71 ± 0.53 | 3.20 ± 0.31 | 0.00 |
| Total count (106/ejaculate) | 498.99 ± 74.16 | 106.97 ± 14.38 | 0.00 |
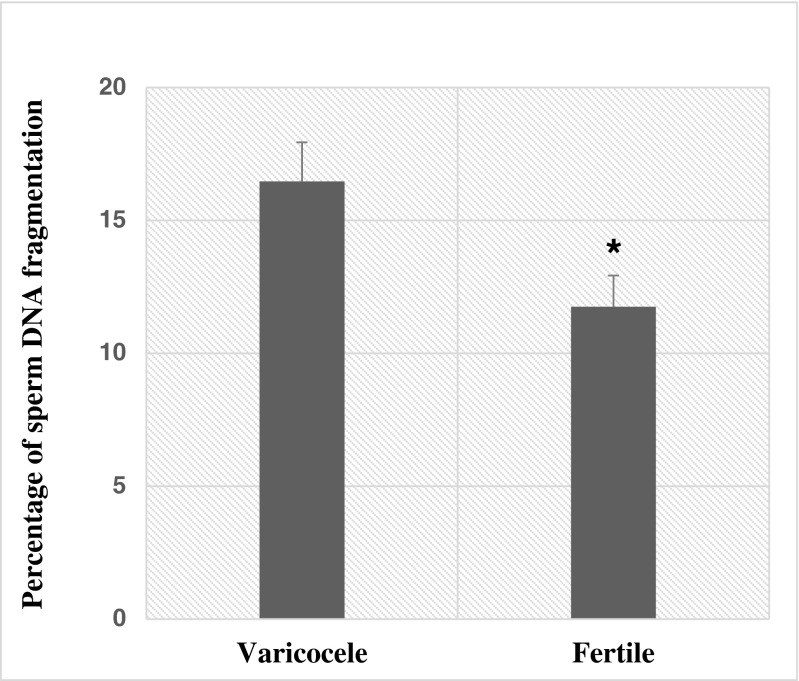
Sperm DNA fragmentation was assessed by the SCSA technique and compared between two groups. As shown in Fig. 1, the percentage of sperm DNA fragmentation was significantly higher in individuals with varicocele compared to fertile men (16.46 ± 1.47 vs. 11.75 ± 1.17; p = 0.02).
Fig. 1.
Comparison of percentage of sperm DNA fragmentation between fertile individuals and men with varicocele. *p < 0.05, significant differences
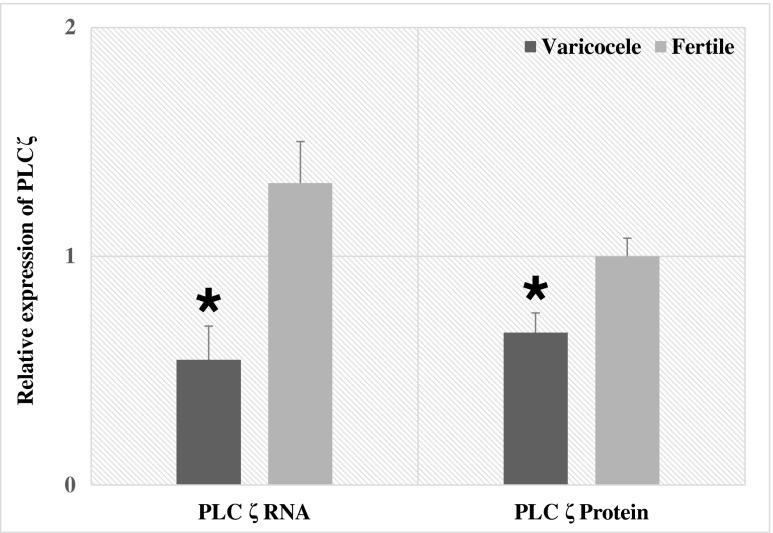
PLCζ was detected by real-time quantitative PCR (qPCR) and Western blot techniques at RNA and protein levels, respectively. Figure 2 shows that the mean relative expression of PLCζ was significantly lower in individuals with varicocele (0.54 ± 0.14 (p = 0.002)) compared to fertile men (1.32 ± 0.18) at RNA level. Western blot for sperm PLCζ is shown in Fig. 3. Mean relative expression of PLCζ at protein level was significantly lower in individuals with varicocele (0.66 ± 0.08 (p = 0.01)) compared to fertile men (1.00 ± 0.07). We analyzed the association between the percentage of DNA fragmentation and the relative expression of PLCζ in individuals with varicocele and fertile men. Significant correlation was not observed between these parameters.
Fig. 2.
Comparison of relative expression of PLCζ at both the RNA and protein levels between fertile individuals and men with varicocele. *p < 0.05, significant differences
Fig. 3.
Western blot of PLCζ and GAPDH in four fertile individuals and five men with varicocele
Discussion
Maintaining the temperature difference between the body and testes is essential for normal spermatogenesis process, and multiple human studies have confirmed that thermoregulatory failure is one of the major factors that can disturb sperm quality and increase the risk of infertility in individuals with varicocele [25, 26]. In addition to thermoregulatory failure, hypoxia due to venous stasis, high levels of oxidants, anti-sperm antibodies, reflux of renal and adrenal metabolites down the spermatic vein, and dilution of intra-testicular substrates, such as testosterone, were proposed to explain impairments in testicular function resulting from varicocele [27, 28]. However, to gain more insight into molecular pathology of varicocele, evaluation of various metabolites, transcripts, and proteins in sperm may pave the way for better understanding the molecular pathology of varicocele.
Numerous studies have shown that heat stress decreases testicular DNA polymerase activity, Sertoli cell function, and testosterone production while increases reactive oxygen species (ROS) and apoptosis in individuals with varicocele [28]. In addition, it alters the expression profiles of many genes [4, 29, 30]. In this regard, we previously showed high expression of heat shock 70 kDa protein 2 (HSPA2) in testicular tissue and caudal segment of epididymides in rat varicocele model [31]. In addition, heat stress in a form of cold shock during freeze-thawing also can reduce concentrations of some sperm proteins such as glutathione [32] and PLCζ [22]. PLCζ is predominantly localized in equatorial, acrosomal, and post-acrosomal segments of the sperm [13, 33, 34]. It is likely that loss of integrity of sperm membranes may result in loss and altered distribution of PLCζ [21]. Considering the role of PLCζ in oocyte activation, we assessed the expression of PLCζ in individuals with varicocele. The results of the current study show that mean relative expression of PLCζ at both RNA and protein levels was significantly lower in individuals with varicocele compared to fertile individuals. Our results were in agreement with research from Swann’s group that showed PLCζ is sensitive to relatively mild heat treatment and heat-treated sperm induce lower mean number of Ca2+ oscillations. This phenomenon is likely to account for lower rate of oocyte activation and poor embryo development following intra-cytoplasmic sperm injection (ICSI). They further showed that this effect can be rescued by co-insemination of heat-treated sperm with recombinant human PLCζ protein [22]. This is consistent with our finding that reduction of PLCζ may be due to chronic heat stress condition in individuals with varicocele which results in lower transcription and translation of this PLCζ.
In this study, the relative expression of PLCζ was assessed in relation to reference gene GAPDH at both mRNA and protein levels. A study of background literature using quantitative PCR or microarray analysis reveals that testis-specific genes or genes with enhanced expression in the testis, such as HSPA2, SPAG11, protamines 1 and 2, MTIM, PHLDA1, INSL3, and CCIN, are upregulated or downregulated upon exposure to heat stress, as in varicocele condition [31, 35].
One reason for this altered expression might be related to common transcription factors, such as CREM required for the expression of these genes during spermatogenesis [35, 36]. In regard to this, Kimmins et al. stated that CREM knockout mice are infertile and spermatogenesis is arrested at round spermatid stage. They concluded that “infertility in these mice is attributed to the lack of expression of key post-meiotic genes required for differentiation such as protamine 1 and 2, transition proteins 1 and 2, proacrosin and calspermin among others” [36]. Therefore, reduced expression of PLCζ could be the consequence of a similar phenomenon. Another reason for reduced expression of PLCζ could be related to oxidative stress. DNA and RNA are common targets of reactive oxygen species [37] which might have a critical effect on embryo quality and pregnancy rates in IVF/ICSI treatments [38–40] and increased upon heat stress or in varicocele condition [30, 41].
Therefore, in order to evaluate the relation between PLCζ expression and ROS, we concomitantly assessed the degree of DNA damage in these individuals. As expected and in agreement with background literature [42–44], the mean percentage of sperm DNA fragmentation was higher in individuals with varicocele compared to fertile men. In addition, Park et al. [45] demonstrated a significant correlation between the expression of PLCζ and the DNA oxidation status assessed using 8-hydroxy-2-deoxyguanosine in human spermatozoa [45]. Unlike Park et al., we did not observe a correlation between the sperm DNA fragmentation assessed by the SCSA technique and PLCζ expression. The difference may be related to the bases of the two assays, as one directly measured DNA oxidation while the other one assessed the ability of sperm to undergo denaturation. Furthermore, ROS, in addition to reducing the expression of PLCζ, may also modify the functionality of enzymes.
Similar to previous studies, we observed that mean sperm parameters such as sperm concentration, count, motility, semen volume, and morphology were significantly lower in individuals with varicocele compared to fertile men. It is important to note that for the assessment of PLCζ at RNA and protein level, we needed high number of sperm; therefore, one limitation of our study was the exclusion of individuals with varicocele with poor semen analysis. This may indicate that reduced expression of PLCζ may be even more severe in varicocele individuals with higher rate of sperm anomalies.
Considering the fact that the expression of PLCζ is reduced in varicocele state, therefore, in these individuals, if spermatozoa find the chance of penetrating an oocyte, it has a lower chance to induce oocyte activation. Therefore, varicocelectomy which improves sperm quality may also improve the expression of PLCζ. From this study and previous studies, we concluded that if couples with advance varicocele do not want to undergo surgery and aim for ICSI procedure, they may face lower fertilization rate, since the degree of expression of PLCζ is reduced in these individuals. Therefore, it would be better for these couples to opt for ICSI along with artificial oocyte activation [12, 18]. In this regard, some studies have demonstrated that fertilization rates were significantly lower in individuals with varicocele candidate for ICSI compared to individuals with varicocele that underwent varicocelectomy [46, 47].
Conclusion
The results of this associative study show that the expression of PLCζ is lower in individuals with varicocele and may account for lower reported rate of fertilization in these individuals but we could not differentiate whether this effect is related to infertility state or state of varicocele. However, a clinical conclusion which can be derived from this study is that couples whose partner presents clinical grade I or III varicocele and does not want to undergo surgery and want to undergo ICSI may benefit from artificial oocyte activation and antioxidant therapy.
Acknowledgments
This study was supported by the Royan Institute, and we would like to express our gratitude to the staff members of Isfahan Fertility and Infertility for their full support.
Compliance with ethical standards
This study received the approval of the Institutional Review Board of Royan Institute. Informed written consent was obtained from each participant.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by the Royan Institute and Isfahan fertility.
Footnotes
Capsule
The findings of the present study illustrate that one of the etiologies of reduced fertility associated with varicocele is the low expression of PLCζ.
Elham Janghorban-Laricheh and Nasim Ghazavi-Khorasgani contributed equally to this work.
References
- 1.Kantartzi PD, Goulis CD, Goulis GD, Papadimas I. Male infertility and varicocele: myths and reality. Hippokratia. 2007;11(3):99–104. [PMC free article] [PubMed] [Google Scholar]
- 2.Homade A, Esteves S, Agawal A. Varicocele and male infertility: current concepts, controversies and consensus. Springer brief in reproductive biology. Springer; 2016. ISBN: 978-3-319-24934-6.
- 3.Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology. 2007;70(3):532–538. doi: 10.1016/j.urology.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Pastuszak AW, Wang R. Varicocele and testicular function. Asian J Androl. 2015;17(4):659–667. doi: 10.4103/1008-682X.153539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amdani SN, Yeste M, Jones C, Coward K. Sperm factors and oocyte activation: current controversies and considerations. Biol Reprod. 2015;93(2):50. doi: 10.1095/biolreprod.115.130609. [DOI] [PubMed] [Google Scholar]
- 6.Anifandis G, Messini CI, Dafopoulos K, Daponte A, Messinis IE. Sperm contributions to oocyte activation: more that meets the eye. J Assist Reprod Genet. 2016;33(3):313–316. doi: 10.1007/s10815-016-0653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nomikos M, Swann K, Lai FA. Is PAWP the “real” sperm factor? Asian J Androl. 2015;17(3):444–446. doi: 10.4103/1008-682X.142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aarabi M, Sutovsky P, Oko R. Re: is PAWP the ‘real’ sperm factor? Asian J Androl. 2015;17(3):446–449. doi: 10.4103/1008-682X.145071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashir J, Nomikos M, Swann K, Lai FA. PLCζ or PAWP: revisiting the putative mammalian sperm factor that triggers egg activation and embryogenesis. Mol Hum Reprod. 2015;21(5):383–388. doi: 10.1093/molehr/gav009. [DOI] [PubMed] [Google Scholar]
- 10.Tosti E, Ménézo Y. Gamete activation: basic knowledge and clinical applications. Hum Reprod Update. 2016;22(4):420–439. doi: 10.1093/humupd/dmw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satouh Y, Nozawa K, Ikawa M. Sperm postacrosomal WW domain-binding protein is not required for mouse egg activation. Biol Reprod. 2015;93(4):94. doi: 10.1095/biolreprod.115.131441. [DOI] [PubMed] [Google Scholar]
- 12.Aghajanpour S, Ghaedi K, Salamian A, Deemeh MR, Tavalaee M, Moshtaghian J. Quantitative expression of phospholipase C zeta, as an index to assess fertilization potential of a semen sample. Hum Reprod. 2011;26(11):2950–2956. doi: 10.1093/humrep/der285. [DOI] [PubMed] [Google Scholar]
- 13.Yelumalai S, Yeste M, Jones C, Amdani SN, Kashir J, Mounce G, et al. Total levels, localization patterns, and proportions of sperm exhibiting phospholipase C zeta are significantly correlated with fertilization rates after intracytoplasmic sperm injection. Fertil Steril. 2015;104(3):561–8.e4. doi: 10.1016/j.fertnstert.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Kashir J, Jones C, Lee HC, Rietdorf K, Nikiforaki D, Durrans C, et al. Loss of activity mutations in phospholipase C zeta (PLCζ) abolishes calcium oscillatory ability of human recombinant protein in mouse oocytes. Hum Reprod. 2011;26(12):3372–3387. doi: 10.1093/humrep/der336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashir J, Konstantinidis M, Jones C, Lemmon B, Lee HC, Hamer R, et al. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum Reprod. 2012;27(1):222–231. doi: 10.1093/humrep/der384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashir J, Konstantinidis M, Jones C, Heindryckx B, De Sutter P, Parrington J, et al. Characterization of two heterozygous mutations of the oocyte activation factor phospholipase C zeta (PLCζ) from an infertile man by use of minisequencing of individual sperm and expression in somatic cells. Fertil Steril. 2012;98(2):423–431. doi: 10.1016/j.fertnstert.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Kashir J, Jones C, Mounce G, Ramadan WM, Lemmon B, Heindryckx B, et al. Variance in total levels of phospholipase C zeta (PLC-ζ) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertil Steril. 2013;99(1):107–117. doi: 10.1016/j.fertnstert.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod. 2009;24(10):2417–2428. doi: 10.1093/humrep/dep207. [DOI] [PubMed] [Google Scholar]
- 19.Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ. Transgenic RNA interference reveals role for mouse sperm phospholipase Czeta in triggering Ca2+ oscillations during fertilization. Biol Reprod. 2005;72(4):992–996. doi: 10.1095/biolreprod.104.036244. [DOI] [PubMed] [Google Scholar]
- 20.Tavalaee M, Nasr-Esfahani MH. Expression profile of PLCf, PAWP, and TR-KIT in association with fertilization potential, embryo development, and pregnancy outcomes in globozoospermic candidates for intra-cytoplasmic sperm injection and artificial oocyte activation. Andrology. 2016; 1–7. [DOI] [PubMed]
- 21.Kashir J, Heynen A, Jones C, Durrans C, Craig J, Gadea J, et al. Effects of cryopreservation and density-gradient washing on phospholipase C zeta concentrations in human spermatozoa. Reprod Biomed Online. 2011;23(2):263–267. doi: 10.1016/j.rbmo.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Sanusi R, Yu Y, Nomikos M, Lai FA, Swann K. Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Cζ. Mol Hum Reprod. 2015;21(10):783–791. doi: 10.1093/molehr/gav042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Examination and processing human semen. 5th ed. Cambridge: Cambridge University Press; 2010.
- 24.Evenson DP. Sperm chromatin structure assay (SCSA) Methods Mol Biol. 2013;927:147–164. doi: 10.1007/978-1-62703-038-0_14. [DOI] [PubMed] [Google Scholar]
- 25.Shiraishi K, Takihara H, Matsuyama H. Elevated scrotal temperature, but not varicocele grade, reflects testicular oxidative stress-mediated apoptosis. World J Urol. 2010;28(3):359–364. doi: 10.1007/s00345-009-0462-5. [DOI] [PubMed] [Google Scholar]
- 26.Shiraishi K, Matsuyama H, Takihara H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int J Urol. 2012;19(6):538–550. doi: 10.1111/j.1442-2042.2012.02982.x. [DOI] [PubMed] [Google Scholar]
- 27.Bahreinian M, Tavalaee M, Abbasi H, Kiani-Esfahani A, Shiravi AH, Nasr-Esfahani MH. DNA hypomethylation predisposes sperm to DNA damage in individuals with varicocele. Syst Biol Reprod Med. 2015;61(4):179–186. doi: 10.3109/19396368.2015.1020116. [DOI] [PubMed] [Google Scholar]
- 28.Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001;7(5):473–481. doi: 10.1093/humupd/7.5.473. [DOI] [PubMed] [Google Scholar]
- 29.Setchell BP. The Parkes lecture. Heat and the testis. J Reprod Fertil. 1998;114(2):179–194. doi: 10.1530/jrf.0.1140179. [DOI] [PubMed] [Google Scholar]
- 30.Durairajanayagam D, Agarwal A, Ong C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod Biomed Online. 2015;30(1):14–27. doi: 10.1016/j.rbmo.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Afiyani AA, Deemeh MR, Tavalaee M, Razi M, Bahadorani M, Shokrollahi B, et al. Evaluation of heat-shock protein A2 (HSPA2) in male rats before and after varicocele induction. Mol Reprod Dev. 2014;81(8):766–776. doi: 10.1002/mrd.22345. [DOI] [PubMed] [Google Scholar]
- 32.Gadea J, Molla M, Selles E, Marco MA, Garcia-Vazquez FA, Gardon JC. Reduced glutathione content in human sperm is decreased after cryopreservation: effect of to the addition of reduced glutathione the freezing and thawing extenders. Cryobiology. 2011;62(1):40–46. doi: 10.1016/j.cryobiol.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Grasa P, Coward K, Young C, Parrington J. The pattern of localization of the putative oocyte activation factor, phospholipase Czeta, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod. 2008;23(11):2513–2522. doi: 10.1093/humrep/den280. [DOI] [PubMed] [Google Scholar]
- 34.Young C, Grasa P, Coward K, Davis LC, Parrington J. Phospholipase C zeta undergoes dynamic changes in its pattern of localization in sperm during capacitation and the acrosome reaction. Fertil Steril. 2009;91(5):2230–2242. doi: 10.1016/j.fertnstert.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira A, Neto A, Almeida C, Silva-Ramos M, Versos R, Barros A, et al. Comparative study of gene expression in patients with varicocele by microarray technology. Andrologia. 2012;44:260–265. doi: 10.1111/j.1439-0272.2011.01173.x. [DOI] [PubMed] [Google Scholar]
- 36.Kimmins S, Kotaja N, Davidson I, Sassone-Corsi P. Testis-specific transcription mechanisms promoting male germ-cell differentiation. Reproduction. 2004;128(1):5–12. doi: 10.1530/rep.1.00170. [DOI] [PubMed] [Google Scholar]
- 37.Cui J, Holmes EH, Greene TG, Liu PK. Oxidative DNA damage precedes DNA fragmentation after experimental stroke in rat brain. FASEB J. 2000;14(7):955–967. doi: 10.1096/fasebj.14.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon L, Proutski I, Stevenson M, Jennings D, McManus J, Lutton D, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod Biomed Online. 2013;26(1):68–78. doi: 10.1016/j.rbmo.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Simon L, Liu L, Murphy K, Ge S, Hotaling J, Aston KI, et al. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum Reprod. 2014;29(5):904–917. doi: 10.1093/humrep/deu040. [DOI] [PubMed] [Google Scholar]
- 40.Anifandis G, Bounartzi T, Messini CI, Dafopoulos K, Markandona R, Sotiriou S, et al. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia. 2015;47(3):295–302. doi: 10.1111/and.12259. [DOI] [PubMed] [Google Scholar]
- 41.Tunc O, Tremellen K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J Assist Reprod Genet. 2009;26(9–10):537–544. doi: 10.1007/s10815-009-9346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nasr-Esfahani MH, Abasi H, Razavi S, Ashrafi S, Tavalaee M. Varicocelectomy: semen parameters and protamine deficiency. Int J Androl. 2009;32(2):115–122. doi: 10.1111/j.1365-2605.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 43.Li F, Yamaguchi K, Okada K, Matsushita K, Ando M, Chiba K, et al. Significant improvement of sperm DNA quality after microsurgical repair of varicocele. Syst Biol Reprod Med. 2012;58(5):274–277. doi: 10.3109/19396368.2012.692431. [DOI] [PubMed] [Google Scholar]
- 44.Tavalaee M, Bahreinian M, Barekat F, Abbasi H, Nasr-Esfahani MH. Effect of varicocelectomy on sperm functional characteristics and DNA methylation. Andrologia. 2015;47(8):904–909. doi: 10.1111/and.12345. [DOI] [PubMed] [Google Scholar]
- 45.Park JH, Kim SK, Kim J, Kim JH, Chang JH, Jee BC. Relationship between phospholipase C zeta immunoreactivity and DNA fragmentation and oxidation in human sperm. Obstet Gynecol Sci. 2015;58(3):232–238. doi: 10.5468/ogs.2015.58.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasqualotto FF, Braga DP, Figueira RC, Setti AS, Iaconelli A, Jr, Borges E., Jr Varicocelectomy does not impact pregnancy outcomes following intracytoplasmic sperm injection procedures. J Androl. 2012;33(2):239–243. doi: 10.2164/jandrol.110.011932. [DOI] [PubMed] [Google Scholar]
- 47.Pathak P, Chandrashekar A, Hakky TS, Pastuszak AW. Varicocele management in the era of in vitro fertilization/intracytoplasmic sperm injection. Asian J Androl. 2016;18(3):343–348. doi: 10.4103/1008-682X.178482. [DOI] [PMC free article] [PubMed] [Google Scholar]