Abstract
The prototypic β-herpesvirus human cytomegalovirus (CMV) establishes life-long persistence within its human host. The CMV envelope consists of various protein complexes that enable wide viral tropism. More specifically, the glycoprotein complex gH/gL/gO (gH-trimer) is required for infection of all cell types, while the gH/gL/UL128/130/131a (gH-pentamer) complex imparts specificity in infecting epithelial, endothelial and myeloid cells. Here we utilize state-of-the-art robotics and a high-throughput neutralization assay to screen and identify monoclonal antibodies (mAbs) targeting the gH glycoproteins that display broad-spectrum properties to inhibit virus infection and dissemination. Subsequent biochemical characterization reveals that the mAbs bind to gH-trimer and gH-pentamer complexes and identify the antibodies' epitope as an ‘antigenic hot spot' critical for virus entry. The mAbs inhibit CMV infection at a post-attachment step by interacting with a highly conserved central alpha helix-rich domain. The platform described here provides the framework for development of effective CMV biologics and vaccine design strategies.
Human cytomegalovirus (CMV) poses a risk for immunosuppressed patients and newborns, with limited treatment options available. Here, Gardner et al. use a high-throughput approach and identify monoclonal antibodies that bind a highly conserved domain in the viral glycoprotein gH as potent inhibitors of CMV infection.
Human cytomegalovirus (CMV) is a betaherpesvirus with a seroprevalance of 60–90% that can cause morbidity and mortality in susceptible individuals1,2. At particular risk for CMV disease are newborns, organ transplant recipients, AIDS patients and the elderly3. CMV exhibits a broad cell tropism and utilizes multiple envelope protein complexes to attach and fuse with host cell membranes. There is limited structural information available for the fusogenic CMV glycoprotein(s) comprised of gB, gH and gL; and their functional interactions are poorly understood4,5,6,7. gB catalyses membrane fusion during viral entry, and gH and gL likely serve as factors which activate gB to permit pH-independent fusion at the cellular membrane or within macropinocytic vesicles7,8,9. gH and gL are present in the trimeric gH/gL/gO complex that contributes to viral entry into diverse cell types8,10,11,12. The pentameric complex (PC) also contains gH/gL together with three additional proteins UL128, UL130 and UL131a. The PC is required for viral entry into epithelial, endothelial and myeloid cells where the virion enters through endocytosis or macropinocytosis followed by a low pH-dependent fusion event9,13,14,15.
Existing CMV drugs exhibit some efficacy in treating infection, although toxicity, drug–drug interactions and the development of drug-resistant viral strains are common limitations16. Immunotherapeutics, in contrast, offer a safe strategy for prevention or mitigation of CMV infection including foetal transmission. CMV-hyperimmune globulin has proven somewhat effective for transplant patients17,18,19 and treatment of congenital CMV infection20,21,22,23, though it may not completely protect the CMV-vulnerable foetus24. A number of drawbacks exist for immunoglobulin preparations, including batch-to-batch variability, low concentrations of neutralizing antibodies, as well as risks associated with blood-derived products25. gB-specific antibodies can impart some protection from infection26,27,28,29, and recent work has revealed that monoclonal antibodies (mAbs) which target the PC30,31,32 are only effective at preventing CMV infection of non-fibroblast cells in vitro31,33,34.
In this study, we identified various novel CMV mAbs that block virus infection. Utilizing the high-throughput capabilities of automated hybridoma colony picking coupled with a high-throughput neutralization (HTN) assay, we generated a panel of potent, broad-spectrum neutralizing mAbs targeting conformational epitopes of gH. These mAbs revealed a highly conserved domain on the CMV-gH protein that is susceptible to neutralization, thus validating this rapid and effective framework for the development of potent and broadly specific antiviral biologics.
Results
Generation of neutralizing α-CMV mAbs
Our goal was to generate broad-spectrum α-CMV mAbs using a HTN assay35,36. Neutralization of the reporter CMV strain AD169 encoding for a chimeric IE2 protein coupled to yellow fluorescent protein (YFP; AD169IE2-YFP) was examined using human CMV-positive serum, α-gH mAb 14-4b and controls (CMV-negative serum, α-MHC class I heavy chain (HC-10) and α-CD44). Fluorescence intensity from infected MRC5 fibroblasts was measured with a fluorescent cytometer35. Using cells infected with mock-treated virus as 100% infection, only the α-gH mAb (14-4b) and CMV-positive serum neutralized a CMV infection providing proof-of-concept for the AD169IE2-YFP-neutralization assay (Fig. 1a).
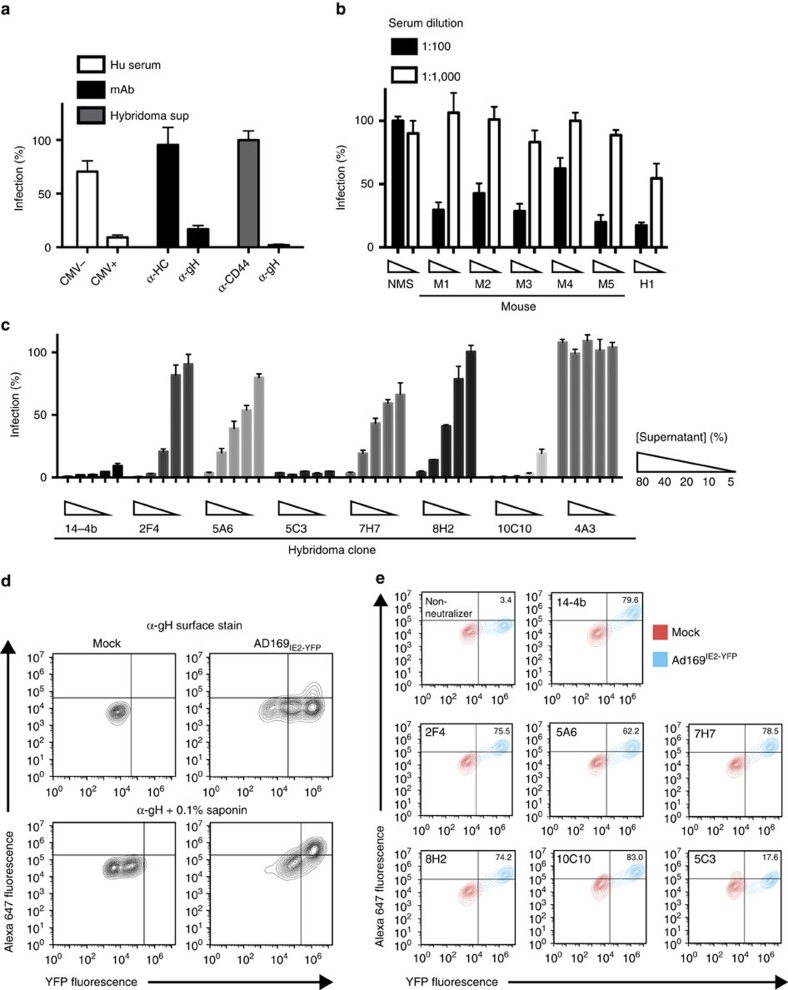
Figure 1. Identification of CMV-neutralizing mAbs using a HTN assay.
(a) Human serum, purified mAb (anti-MHCI heavy chain (HC) mAb (HC-10), anti-gH mAb 14-4b) and hybridoma supernatant were tested in a HTN assay for their ability to block infection of fibroblasts by AD169IE2-YFP. (b) Serum from mice inoculated with AD169 was tested for its ability to neutralize AD169IE2-YFP infection at 1:100 (black bars) or 1:1000 (white bars). Normal mouse serum (NMS) or CMV+ human serum (H1) were used as controls. (c) Hybridoma supernatant from 6 CMV-neutralizing clones was tested in 5-point dilutions (5–80%) for their ability to inhibit AD169IE2-YFP infection. Supernatant from the neutralizing anti-gH mAb 14-4b and supernatant from the non-neutralizing hybridoma clone 4A3 were utilized as controls. (d) MRC5 cells infected with AD169IE2-YFP were exposed to anti-gH (14-4b) flow cytometry staining without (top row) or with (bottom row) permeabilization with saponin. (e) Mock-infected MRC5 cells (red) or infected with AD169IE2-YFP (blue) were permeabilized and then exposed to hybridoma supernatant from the CMV-neutralizing clones followed by detection with flow cytometry. Experiments for (a–c) were performed in technical triplicate and s.d. is depicted.
We hypothesized that antibodies targeting the core binding and fusion envelope proteins (for example, gM/gN, gB and gH/gL) would be ideal targets for broad-spectrum mAbs. To that end, mice were immunized with AD169 because it does not incorporate an intact pentamer complex in the virion13 and thus would not elicit a humoral response to the immunodominant proteins UL128, UL130 and UL131a (refs 29, 31). A HTN assay using serum from the immunized mice (Fig. 1b) identified the animals with the highest neutralizing capacity and ∼2,000 hybridoma clones were generated from two animals and subjected to the AD169IE2-YFP -HTN assay (Supplementary Fig. 1). Of the clones that decreased infection by >50%, six clones neutralized infection in a dose-dependent manner. These clones were subjected to a 5-point dose-dependence HTN assay and demonstrated the capacity to neutralize infection (Fig. 1c). To determine whether the neutralizing clones recognize infected cells, AD169IE2-YFP-infected fibroblasts were subjected to flow cytometry with the hybridoma clones following assay optimization with the α-gH mAb 14-4b (Fig. 1d,e). The neutralizing hybridoma clones exclusively bound to AD169IE2-YFP-infected cells (Fig. 1e) suggesting they target viral envelope proteins.
CMV-neutralizing mAbs target envelope proteins gB and gH
What are the protein target(s) of the CMV-neutralizing antibodies? Proteins recovered from metabolically labelled AD169IE2-YFP-infected cells following immunoprecipitation with the neutralizing mAbs were resolved by SDS–polyacrylamide gel electrophoresis (SDS–PAGE; Fig. 2a). The neutralizing mAbs recovered polypeptides segregating into two distinct groups. Group I mAbs recovered three major protein species (∼130, 100 and 60 kDa (Fig. 2a, left panel)), a result consistent with immature and mature forms of gB37. The neutralizing mAbs 10C10 and 5C3 (group II) recovered distinct proteins (∼100, 37 and 12 kDa (Fig. 2a, right panel)): a pattern consistent with gH, gL and UL128 proteins. This would be expected because although AD169 virions do not incorporate an intact PC due to a truncated UL131 protein38,39,40, UL128 is expressed in the endoplasmic reticulum (ER) of infected cells. Additionally, the absence of UL130 from an immunoprecipitation targeting a gH complex is consistent with previous findings13. Further validation that the mAbs targeted glycoproteins was based on the faster migration pattern upon peptide-N-glycosidase F (PNGase) digestion (Fig. 2b). The predominant polypeptides recovered by the neutralizing mAbs 2F4 (group I) and 10C10 (group II) from infected fibroblasts were confirmed by mass spectrometry analysis to be gB, gH and gL, respectively (Supplementary Table 1). Collectively, the neutralizing mAbs recognized the core envelope proteins gB and gH/gL.
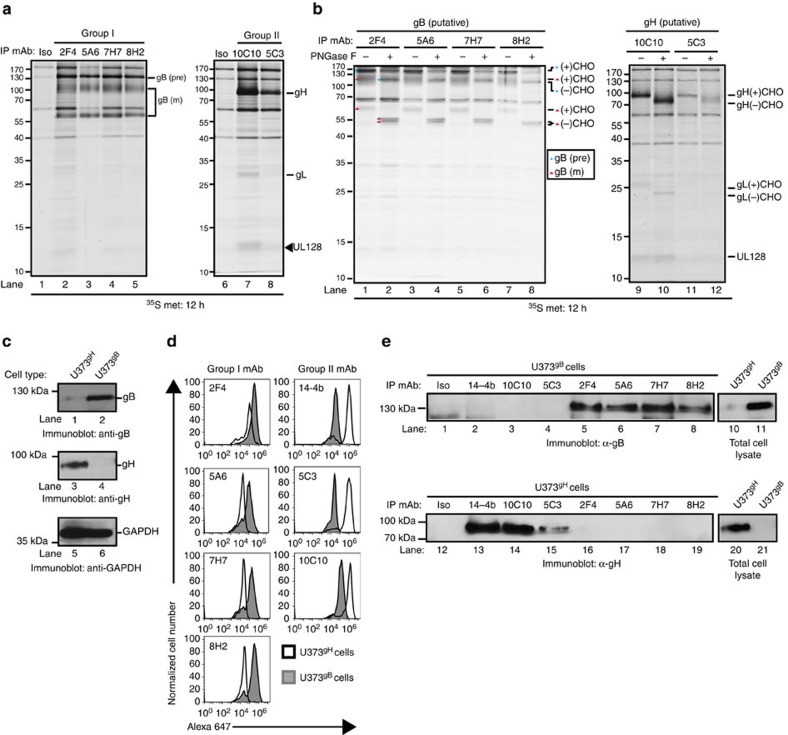
Figure 2. Identification of CMV-neutralizing mAb targets.
(a) Lysates from metabolically labelled AD169IE2-YFP-infected MRC5 cells were exposed to the CMV-neutralizing mAbs and recovered immune complexes were resolved by SDS–PAGE. Arrows denote the suspected peptide identity. (b) Immune complexes recovered from the metabolically labelled cell lysates were exposed to PNGase treatment and resolved by SDS–PAGE. Arrows denote the suspected peptide identity and glycosylation state. (c) Total cell lysates from U373 glioblastoma cells stably expressing gH (U373gH) or gB (U373gB) were resolved by SDS–PAGE and exposed to immunoblot for gB (lanes 1–2), gH (lanes 3–4) and GAPDH (lanes 5–6) proteins. (d) U373gH (white peaks) and U373gB (grey peaks) cells were permeabilized and exposed to the neutralizing CMV mAbs followed by detection with flow cytometry. mAb 14-4b was used as a positive control for labelling of the gH protein. (e) Total cell lysates from U373gB (lanes 1–8) and U373gH (lanes 12–19) were exposed to the CMV-neutralizing mAbs. The recovered immune complexes were resolved by SDS–PAGE and exposed to anti-gB (lanes 1–8) or anti-gH (lanes 12–19) antibody. Total cell lysates from U373gB (lanes 10–11) and U373gH (lanes 20–21) validated expression of the respective CMV protein in each cell type. Relative molecular mass markers are indicated in all relevant figures.
To confirm the exact protein target of the neutralizing mAbs, U373 cells expressing gH or gB (Fig. 2c) were subjected to flow cytometry (Fig. 2d) or immunoprecipitation studies (Fig. 2e). An α-gB and α-gH immunoblot confirmed expression of the envelope proteins (Fig. 2c, lanes 1–2 and 3–4). Following the establishment of the flow cytometry assay (Supplementary Fig. 2), group I mAbs 2F4, 5A6, 7H7 and 8H2 bound exclusively to U373gB cells (Fig. 2d, left column), while the α-gH antibody 14-4b, and the group II antibodies 5C3 and 10C10 bound only to U373gH cells (Fig. 2d, right column). The mAbs' specificity for gB or gH was validated by immunoprecipitation (Fig. 2e). Taken together with the inability of the mAbs to bind denatured protein in an immunoblot assay (Supplementary Fig. 2), the collective set of data indicates that the neutralizing mAbs bind to conformational epitopes of CMV gB and gH.
CMV-neutralizing mAbs selectivity inhibit virus infection
To examine the specificity of the CMV-neutralizing antibodies, we utilized a CMV TB40/E strain that expresses FLAG-YFP (TB40/EFLAG-YFP) in a HTN assay36,41 (Fig. 3a). The α-gH mAbs 14-4b, 10C10 and 5C3 significantly blocked infection; yet, the α-gB mAbs did not reduce infection levels implying that the α-gB mAbs displayed selective inhibition of CMV strains. To quantify the potency of the neutralizing mAbs, IC50 values were determined using AD169IE2-YFP or TB40/EFLAG-YFP-HTN assays (Fig. 3b,c). The neutralizing mAbs potently inhibited an AD169IE2-YFP infection (IC50 values: 0.24 μg ml−1 (5C3) to 4.2 μg ml−1 (7H7), while only the α-gH mAbs 10C10 and 5C3 neutralized TB40/EFLAG-YFP (IC50 values: 0.38 μg ml−1 and 0.07 μg ml−1 (Fig. 3b)). Consistent with Fig. 3a, the purified α-gB mAbs were unable to reduce infection levels of TB40/EFLAG-YFP (Fig. 3c), despite recognition of CMV gB by the α-gB mAbs as demonstrated by immunoprecipitation studies from TB40/E WT-infected cells (Fig. 3d). The data demonstrate that the α-gH mAbs are capable of neutralizing multiple CMV strains.
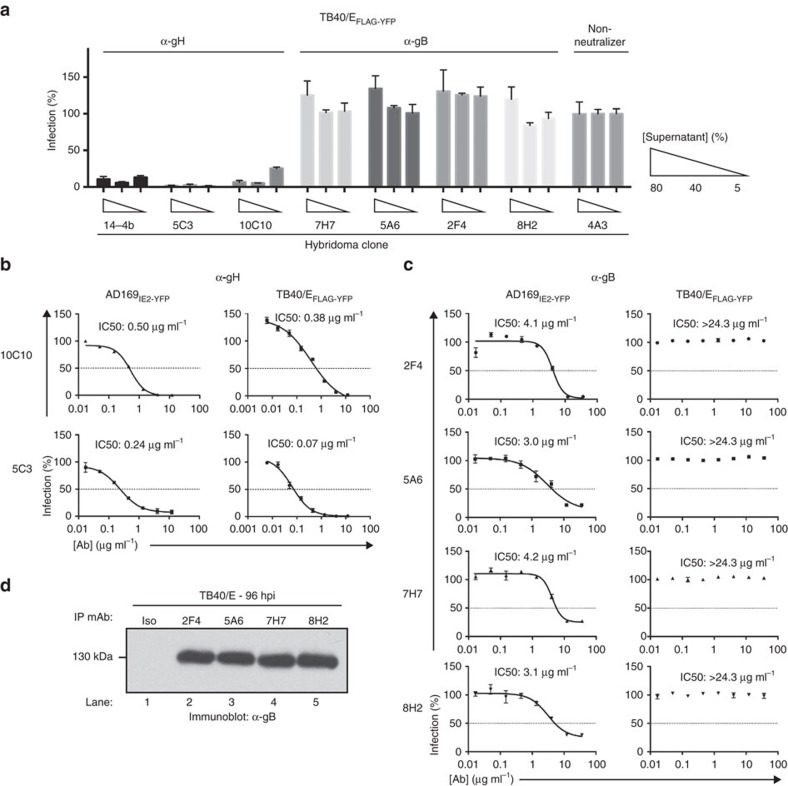
Figure 3. Examination of the neutralizing capacity of anti-CMV mAbs.
(a) Hybridoma supernatant from the 6 CMV-neutralizing mAbs was tested at 3 concentrations (5, 40 and 80%) for their ability to inhibit TB40/EFLAG-YFP infection of MRC5 cells. Supernatant from the neutralizing anti-gH mAb 14-4b and supernatant from the non-neutralizing hybridoma clone 4A3 were utilized as controls. Experiments were performed in technical triplicates and s.d. is depicted. (b) Anti-gH mAbs 10C10 and 5C3 were pre-incubated with AD169IE2-YFP (left panel) and TB40/EFLAG-YFP (right panel) at 8 concentrations (0.01–12 μg ml−1) and infection levels of MRC5 cells was subsequently measured. (c) Anti-gB mAbs 2F4, 5A6, 7H7 and 8H2 were pre-incubated with AD169IE2-YFP (left panel) and TB40/EFLAG-YFP (right panel) at 8 concentrations (0.01–12 μg ml−1) and infection levels of MRC5 cells was subsequently measured. Non-linear regression analysis was performed and the half maximal inhibitory concentration (IC50) was calculated for all antibodies. (d) MRC5 cells infected with TB40/E were harvested at 96 hpi and total cell lysates were exposed to the anti-gB antibodies. Recovered immune complexes were resolved by SDS–PAGE and exposed to anti-gB immunoblot (lanes 1–5). Relative molecular mass markers are indicated. Experiments for (a–c) were performed in technical triplicate and s.d. is depicted. Respective virus neutralization experiments were replicated four times each.
α-gH mAbs block virus infection and dissemination
To determine the broad-spectrum neutralization capacity of α-gH mAbs, we developed a HTN assay using a fluorescently labelled α-Immediate Early (IE) mAb (α-IEAlexa488) (Fig. 4a). AD169-WT, TB40/E, VHL/E and TR strains pre-incubated with α-gH mAbs or Cytogam, a polyclonal IgG preparation from CMV seropositive individuals42, were analyzed for virus infection in MRC5 fibroblasts. Both 10C10 and 5C3 demonstrated enhanced neutralization of all strains (IC50: 0.28–4.18 μg ml−1) when compared to Cytogam, which was ineffective at blocking virus infection (Fig. 4a and Supplementary Table 2). Additionally, analysis of TB40/E, DAVIS, VHL/E and TR neutralization by 10C10 and 5C3 via immunoblot analysis demonstrated complete reduction of Late Antigen expression (Supplementary Fig. 3).
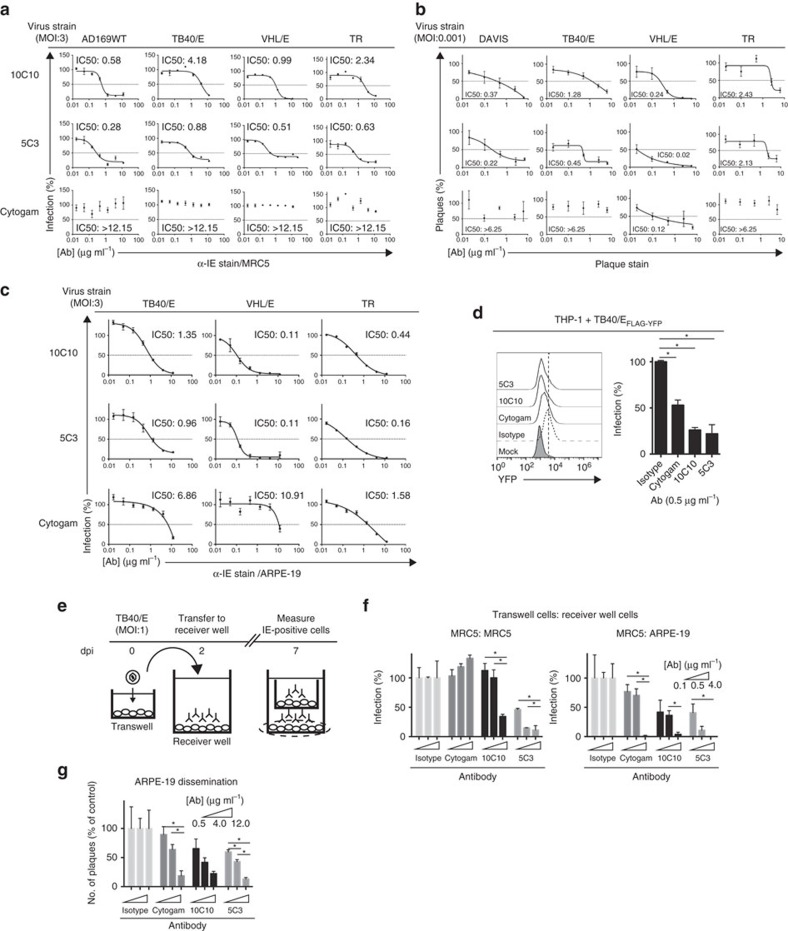
Figure 4. Examination of the potency and versatility of anti-gH mAbs in epithelial cells.
(a) Cytogam and mAbs 10C10 and 5C3 were pre-incubated with CMV strains AD169, TB40/E, VHL/E and TR (0.01–12 μg ml−1), and subsequent infection levels of MRC5 cells was analyzed by immunostaining with anti-Immediate Early (IE) gene product (α-IEAlexa488) antibody. Experiments were performed in technical triplicate. (b) Cytogam and mAbs 10C10 and 5C3 were pre-incubated with CMV strains DAVIS, TB40/E, VHL/E and TR (0.025–6.25 μg ml−1), and subsequent infection levels of MRC5 cells was analyzed by plaque assay. Data represents averages from three experiments performed in duplicate. (c) Cytogam and mAbs 10C10 and 5C3 were pre-incubated with CMV strains TB40/E, VHL/E and TR (0.01–12 μg ml−1), and subsequent infection levels of human retinal pigment epithelial cells (ARPE-19) was measured by anti-IE staining. Experiments were performed in technical triplicate. Non-linear regression analysis was performed and the half maximal inhibitory concentration (IC50) was calculated for all antibodies under all conditions. (d) Cytogam and mAbs 10C10 and 5C3 were pre-incubated with the fluorescent CMV strain TB40/EFLAG-YFP (0.5 μg ml−1) before infection of THP-1 cells (MOI: 3) and fluorescence levels were analyzed by flow cytometry at 4 dpi. Experiments were performed in technical triplicate. (e) MRC5 cells were seeded on a transwell insert with 3 μm pore and infected (MOI:1) with TB40/E. At 2 dpi the transwell insert was transferred to a receiver well containing an isotype control, Cytogam or mAbs 10C10 and 5C3 (0.1–4 μg ml−1) and at 7 dpi the cells from the receiver layer were analyzed by α-IEAlexa488 immunostain. (f) MRC5 (left panel) and ARPE-19 (right panel) cells from the transwell infection experiment were analyzed for infection levels by α-IEAlexa488 immunostain. Experiments were performed in technical triplicate. (g) Infection levels of ARPE-19 cells infected with TB40/E (MOI:0.1) and exposed to an isotype control, Cytogam or mAbs 10C10 and 5C3 (0.5–12 μg ml−1) over a 10 day period were analyzed by α-IEAlexa488 immunostain. Experiments were performed in technical triplicate. S.d. is depicted for all experiments. *P<0.05 (Student's two-tailed t test).
Potential variation in affinity of the fluorescent α-IE antibody between virus strains may complicate the comparison of IC50 values. Thus, traditional plaque reduction assays were performed in MRC5 cells infected with clinical-like strains DAVIS, TB40/E, VHL/E and TR (Fig. 4b). The α-gH mAbs reduced plaque numbers with IC50 values between 0.02 and 2.43 μg ml−1, whereas Cytogam was unable to limit plaque numbers of the clinical-like strains with the exception of VHL/E (IC50: 0.12 μg ml−1; Fig. 4b and Supplementary Table 2). These results demonstrate the effectiveness of α-gH neutralizing mAbs in blocking virus infection.
α-gH mAbs broadly inhibit infection of non-fibroblast cells
To further analyse the efficacy of the α-gH mAbs as broad-spectrum antivirals, we examined their potency in blocking virus infection of human retinal pigment epithelial cells (ARPE-19) using TB40/EFLAG-YFP (Supplementary Fig. 3). The mAbs 10C10 and 5C3 inhibited infection with IC50 values of 0.13 μg ml−1 (10C10) and 0.04 μg ml−1 (5C3) (Supplementary Table 2). Additionally, 10C10 and 5C3 inhibited infection of TB40/E, VHL/E and TR with IC50 values from 0.11 to 1.35 μg ml−1 (Fig. 4c, top 2 rows and Supplementary Table 2) and Cytogam exhibited variable neutralizing capacity with IC50 values of >6 μg ml−1 for TB40/E and VHL/E infection, and 1.58 μg ml−1 for the clinical strain TR (Fig. 4c bottom row and Supplementary Table 2). Finally, 10C10 and 5C3 (0.5 μg ml−1) reduced infection by >50% with Cytogam decreasing infection by ∼50% of TB40/EFLAG-YFP-infected monocytes (THP-1; Fig. 4d). We conclude that the α-gH mAbs are effective neutralizing mAbs of various viral strains and cell types.
α-gH neutralizing mAbs block viral dissemination
To assess the capability of the α-gH mAbs to block nascent infection, we next examined the ability of the mAbs to limit cell-free virus spread in a transwell assay (Fig. 4e). TB40/E-infected fibroblasts in the top chamber of a transwell dish were positioned above a receiver well of MRC5 fibroblasts or ARPE-19 epithelial cells containing α-gH mAbs, Cytogam, or an isotype control (0.1–4 μg ml−1) followed by analysis of virus infection 7 days post-infection (dpi; Fig. 4f). The α-gH mAbs decreased infection in a dose-dependent manner in both cell types with an enhanced inhibition in ARPE-19 cells, whereas Cytogam prevented virus infection of only ARPE-19 cells. Further, we examined the effectiveness of the α-gH mAbs to block viral dissemination through multiple infection cycles in a monolayer. ARPE-19 cells infected with TB40/E (MOI: 0.1) were treated with α-gH mAb, Cytogam, or an isotype control (0.5–12 μg ml−1) at 48 h post infection (hpi) and analyzed for virus-infected cells at 10 dpi (Fig. 4g). The α-gH mAbs and Cytogam limited virus dissemination based on the decreased number of IE-positive plaques. Additionally, infection of primary CD14+ monocytes followed by coculture with MRC5 cells in the presence of the α-gH mAbs demonstrated their ability to block dissemination from fibroblasts to monocytes (Supplementary Fig. 4).
To further characterize the efficacy of the anti-gH antibodies 5C3 and 10C10 to limit viral dissemination, AD169IE2-YFP-infected (MOI: 0.1) MRC5 cells were incubated with isotype, Cytogam, 5C3 and 10C10 at 24 hpi without replenishing the antibodies and analyzed for cytopathogenicity and viral plaques (Supplementary Fig. 5). Analysis of the infected cells indicated that 5C3 and 10C10 were more effective at limiting cytopathic effects, plaque number and the number of IE2-YFP-positive cells/plaque. Collectively, our data support the model that the α-gH mAbs are quite effective at limiting viral dissemination.
α-gH mAbs bind to multiple envelope protein complexes
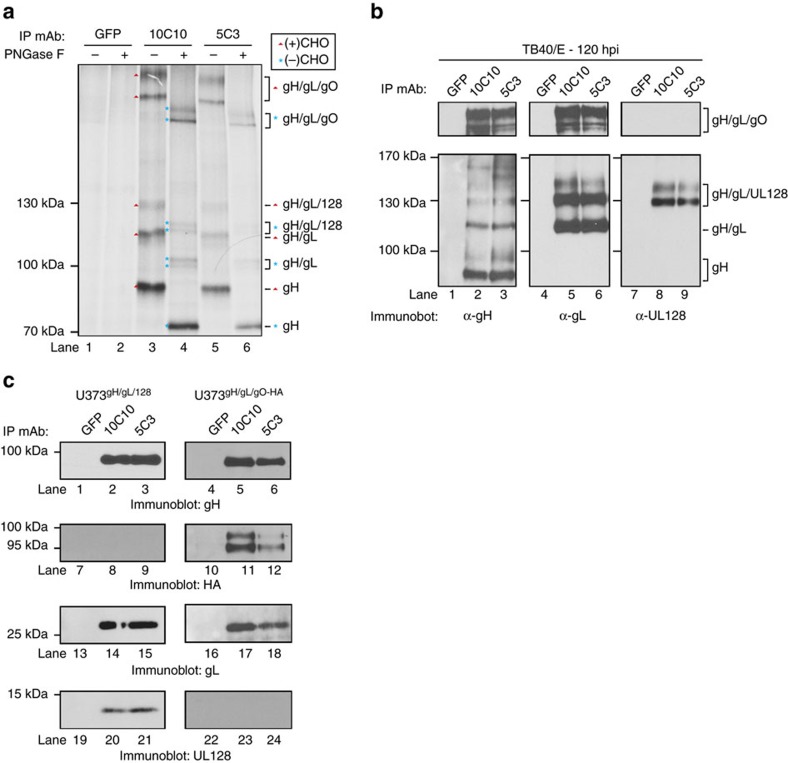
To determine if the α-gH mAbs recognize both the gH/gL-trimer and gH/gL-pentamer complexes, proteins recovered by 10C10 and 5C3 from 35S-methionine-labelled, TB40/E-infected cells were subjected to PNGase treatment and resolved by non-reducing SDS–PAGE (Fig. 5a). Based on the relative molecular weights of the proteins, we observed large complexes (>250 kDa) likely consisting of covalently linked multimers of glycosylated gH/gL/gO trimers43, ∼130 kDa species comprised of the gH/gL/UL128 trimer, ∼100–130 kDa proteins made of gH/gL dimers and polypeptides <100 kDa that represent the nascent gH protein (Fig. 5a, lanes 3 and 5). PNGase treatment confirmed the glycosylation status of the recovered proteins (Fig. 5a, lanes 4 and 6). These data were validated by immunoblot analysis for gH, gL and UL128 (Fig. 5b). The detection of UL128 covalently bound to gH/gL was used as a proxy for the PC. As expected, gH and gL exist within diverse complexes ranging from ∼90 kDa to >250 kDa (Fig. 5b, lanes 1–3 and 4–6) and the gH/gL/UL128 species, which migrated at ∼130 kDa (Fig. 5b, lanes 7–9). Further, U373 cells expressing gH/gL/hemagglutinin (HA)-tagged gO (U373gH/gL/gO-HA) or gH/gL/UL128 (U373gH/gL/UL128) were used to validate 10C10 and 5C3 binding to UL128- (Fig. 5c, lanes 1–3, 13–15 and 19–21) and gO-containing gH/gL complexes (Fig. 5c, lanes 4–6, 10–12 and 16–18). Additionally, mass spectrometry analysis of the viral proteins recovered from TB40/E-infected cells (72 hpi) with the anti-gH mAb 10C10 identified gH, gL, gO, UL128, UL130 and UL131a (Supplementary Table 3). These findings further support the model that the anti-gH mAbs recover both the gH/gL-trimer and pentamer complexes. Additional biochemical studies defined that 5C3 binds preferentially to the gH/gL complex, whereas 10C10 bound equally well to gH or gH/gL (Supplementary Fig. 6).
Figure 5. α-gH mAbs bind to multiple glycoprotein complexes.
(a) Lysates from metabolically labelled TB40/E-infected MRC5 cells were exposed to an isotype control mAb recognizing GFP, or mAb 10C10 and 5C3. Recovered immune complexes were split and treated with PNGase F, then resolved by non-reducing SDS–PAGE. Arrows denote the identity of glycosylated protein complexes and asterisks denote the identity of de-glycgosylated protein complexes. (b) Lysates from TB40/E-infected MRC5 cells were exposed to a GFP mAb, 10C10 or 5C3. Immune complexes were resolved by non-reducing SDS–PAGE followed by immunoblot for gH (lanes 1–3), gL (lanes 4–6) and UL128 (lanes 7–9). Lines denote the glycoprotein complexes. (c) Lysates from U373 cells stably expressing gH/gL/UL128 (U373gH/gL/UL128) and gH/gL/gO-HA (U373gH/gL/gO-HA) were exposed to GFP, 10C10 or 5C3 mAbs and the recovered immune complexes were resolved by SDS–PAGE and subjected to immunoblot for gH (lanes 1–6), HA (lanes 7–12), gL (lanes 13–18) and UL128 (lanes 19–24).
10C10 and 5C3 block a post-attachment entry step
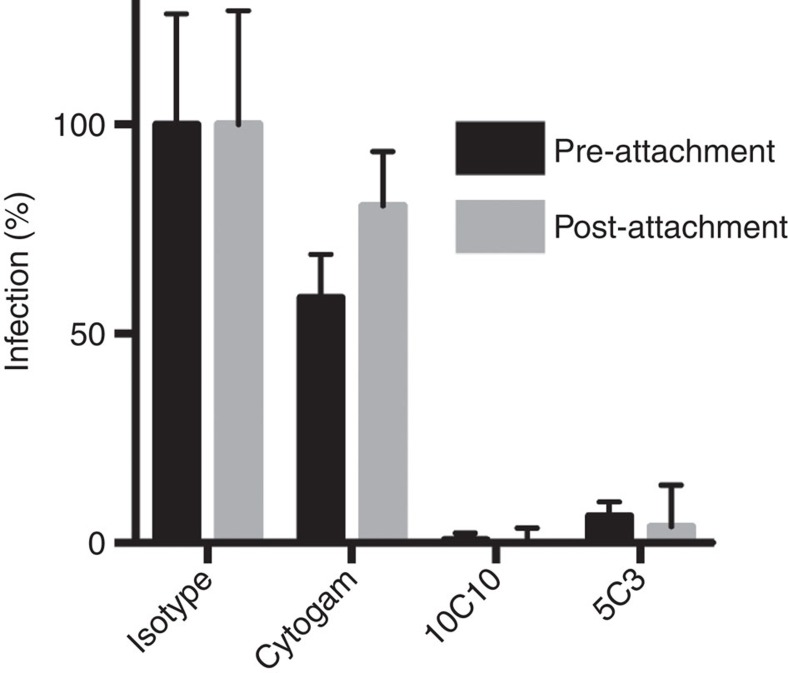
Which step of the entry process do mAbs 10C10 and 5C3 block? To address this question, AD169IE2-YFP was pre-incubated with 10C10, 5C3, Cytogam or an isotype control to allow for viral binding by the mAbs, and added to MRC5 fibroblasts (pre-attachment; Fig. 6). Alternatively, AD169IE2-YFP was added to MRC5 cells at 4 °C to prevent virus internalization, followed by addition of 10C10, 5C3, Cytogam and an isotype control (post-attachment); then returned to 37 °C. Virus infection was measured by YFP-positive cells and normalized to isotype-treated cells. The mAbs 10C10 and 5C3 blocked infection pre- and post-viral attachment suggesting inhibition of a step downstream of viral binding.
Figure 6. The anti-gH mAbs 10C10 and 5C3 neutralize infection at a post-attachment step.
AD169IE2-YFP was incubated with MRC5 cells at 4 °C followed by addition of Cytogam, an isotype control or anti-gH mAbs 10C10 and 5C3. Following an additional incubation at 4 °C, cell/virus/antibody were transferred to 37 °C. Infection levels (grey bars) were measured at 16 hpi and compared with virus prep that had been pre-incubated with mAbs before exposure to cells (black bars). Experiment was performed in technical triplicate and was replicated four times. S.d. is depicted for all experiments.
Epitope mapping of 5C3 and 10C10
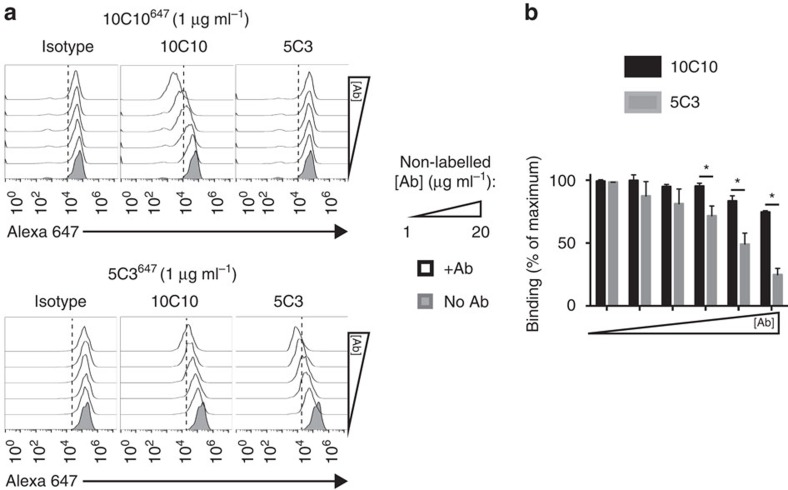
A binding competition assay was performed to define whether 10C10 and 5C3 recognize the same epitope (Fig. 7). U373gH/gL cells incubated with a fluorescent-labelled Alexa647 10C10 antibody (10C10647, 1 μg ml−1) and competed with non-fluorescent 10C10, 5C3 or an isotype control (1–20 μg ml−1; Fig. 7a, top row) were analyzed by flow cytometry. Only incubation with 10C10 resulted in a dose-dependent fluorescent decrease indicating displacement of 10C10647 (centre panel). Intriguingly, in competition experiments with a fluorescent-labelled 5C3 antibody (5C3647; Fig. 7a, bottom row), both 5C3 and 10C10 caused a decrease in 5C3647 binding, although a more pronounced reduction was observed following incubation with 5C3 (compare center and right panels). Quantification of percentage fluorescence change revealed that 10C10 (black bars) only displaces 5C3647 at high mAb concentrations (Fig. 7b). These data suggest that 10C10 and 5C3 bind to a similar region and 10C10 may have a slightly higher affinity for the gH polypeptide.
Figure 7. mAbs 10C10 and 5C3 bind to a similar region within gH.
(a)U373gH/gL cells were left unstained (grey peak) or were labelled with mAbs 10C10 (top row) and 5C3 (bottom row) conjugated to an Alexa647 fluorophore (10C10647 and 5C3647), together with increasing concentrations of non-conjugated 10C10 or 5C3 (1–20 μg ml−1; white peaks). (b) The percentage of cells stained by 5C3647 in the presence of non-labelled 10C10 (black bars) or non-labelled 5C3 (grey bars) is depicted for all concentrations. Experiment was performed in technical duplicate and was replicated four times. S.d. is depicted for all experiments. *P<0.05 (Student's two-tailed t test).
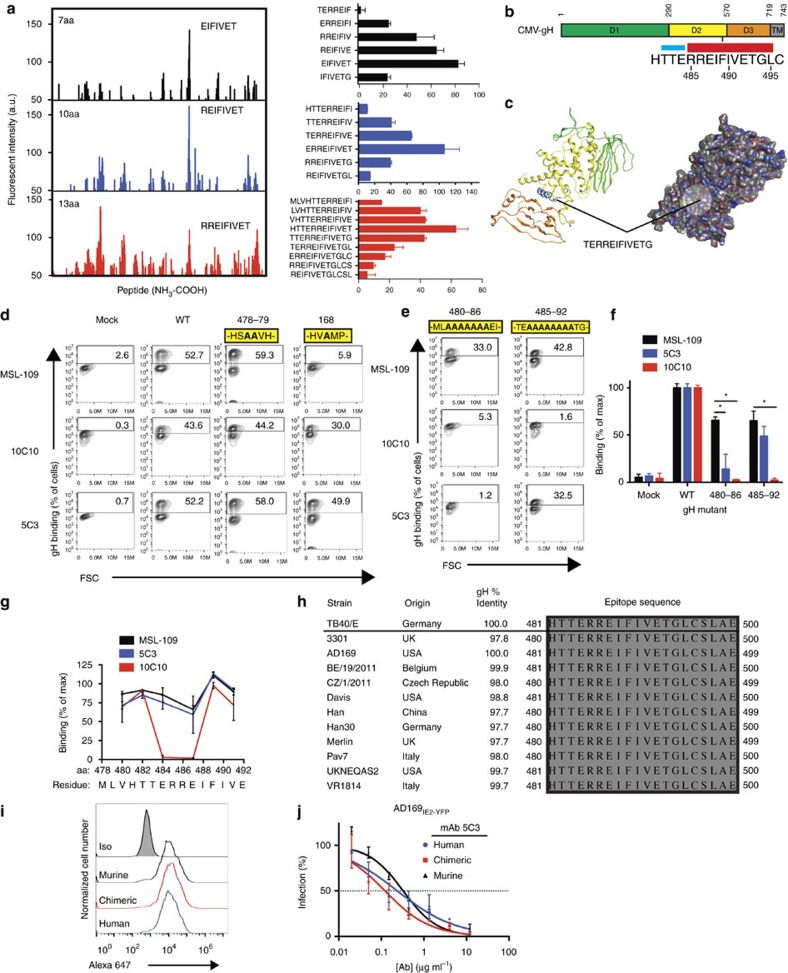
Truncation analysis revealed that 5C3 recognized an epitope between residues 295–717 and 10C10 binds to residues 132–717 (Supplementary Fig. 7). To further define the epitope of the mAbs, we utilized a cyclic peptide microarray platform (PEPperCHIP) consisting of overlapping cyclic peptides of 7, 10 and 13aa derived from gH (Fig. 8a). Remarkably, 5C3 bound consistently to a peptide (RREIFIVET) corresponding to domain 2 of the gH (aa. 485–493) protein44 (Fig. 8b). A 3D model of CMV-gH based on the crystal structure of HSV-1 gH (ref. 45) predicted that aa481–493 lies within an alpha helix-rich domain on the surface of CMV-gH (Fig. 8c).
Figure 8. Characterization of the α-gH mAb epitopes.
(a) Spot intensities were quantified following incubation of 5C3 followed by anti-mouse Dylight680 mAb with overlapping conformational gH peptide libraries of 7 amino acids (aa; black), 10aa (blue) and 13aa (red; left panel). Reactivity of 5C3 with overlapping peptide series near the region of peak binding are shown (right panel). Data represents mean intensity from duplicate samples. (b) The region of 5C3 reactivity is located between aa's 481–495 within domain 2 of gH. The region includes a β-sheet (blue) and a 10aa-long alphahelical region (red). (c) Structural modeling of CMV-gH based on the crystal structure of HSV-1 indicates the location of the putative epitope (blue; left). Predicted surface interactions indicate that the 5C3-reactive alpha helical region is exposed on the surface of gH (circle; right). (d) 293 cells transfected with gL and a transfection control, wild-type gH, gH mutant 478–79 or gH mutant 168 were stained with MSL-109 (top row), 10C10 (middle row) or 5C3 (bottom row). Percentage of cells positive for gH are indicated. (e) 293 cells transfected with gL and gH mutant 480–86 or 485–92 were stained with MSL-109 (top row), 10C10 (middle row) or 5C3 (bottom row). Percentage of cells positive for gH are indicated. (f) The data from Fig. 6e was quantified and normalized compared with WT binding. (g) Binding of MSL-109 (black), 5C3 (blue) and 10C10 (red) to 293 cells transfected with gH containing 2aa alanine substitutions along the length of the epitope region was measured. Percentage of gH-positive cells compared with WT-transfected cells was calculated and plotted. (h) The epitope region from 12 geographically distinct CMV strains were aligned to the TB40/E sequence. (i) U373gH/gL cells were exposed to murine, chimeric and humanized 5C3 mAbs and analyzed by flow cytometry with cells exposed to an isotype control (grey peak). (j) Murine, chimeric and humanized 5C3 mAbs were pre-incubated with AD169IE2-YFP at various concentrations before exposure to MRC5 fibroblasts. Infection levels were measured and compared with an isotype control. Data in d–g, i, j represents duplicates from three experiments. S.d. is depicted in all relevant figures. *P<0.05 (Student's two-tailed t test).
To confirm the identity of the 5C3 epitope and determine if 10C10 also targeted this domain, a flow cytometry-based binding assay was established using gH alanine mutants. Assay conditions were initially established using HEK-293 cells expressing wild-type-gH, gH478-79AA (a mutant substituting two alanines upstream of the putative epitope region (aa478–79), and gHW168A (an alanine point mutant that disrupts α-gH mAb MSL-109 binding46 and used as a control; Fig. 8d and Supplementary Table 4). 10C10, 5C3 and MSL-109 recognized wild-type gH and gH478-79AA; whereas, MSL-109 as expected, weakly bound to gHW168A (Fig. 8d). Interestingly, the slight decrease in 10C10 and 5C3 binding to gHW168A demonstrates the sensitivity of these antibodies to conformational changes of gH.
Next, two mutants with alanine substitutions spanning the putative epitope region were generated for binding analysis (Fig. 8e–g and Supplementary Table 4). Binding of MSL-109 to both constructs validated the intact conformation of gH (Fig. 8e). Remarkably, 10C10 and 5C3 were unable to bind to the gH480-A-486 mutant; yet 5C3 binding was restored for the gH485-A-492 mutant and not for 10C10 (Fig. 8e,f). For higher-resolution mapping of the epitope, 10C10 or 5C3 binding to additional alanine mutants (gH480AA481, gH482AA483, gH484AA485, gH487AA488, gH489AA490 and gH491AA492) was performed (Fig. 8g and Supplementary Table 4). While MSL-109 and 5C3 bound to all of the mutants, 10C10 binding was completely abrogated on disruption of amino acids 484–487. These data demonstrate that the epitopes of 5C3 and 10C10 are contained within residues 481-HTTERREIFI-490. This region is highly conserved (100% identity) among diverse CMV strains (Fig. 8h) defining the mAbs' broad specificity.
Notably, a recent study implicated gH residues 485–493 and 671–675 as critical for binding of the anti-gH mAb 14-4b (ref. 47). To investigate the requirement of residues 485–493 for binding by 14-4b, the gH alanine mutants were evaluated for recognition by this mAb (Supplementary Fig. 8). Strikingly, aa's 487–488 were more critical for interaction with 14-4b than residues 484–485 based on the abrogation of antibody binding. Interestingly, the 14-4b binding to gH mutants was more similar to the binding profile of 10C10, yet the neutralization data was in line with the IC50 values of 5C3 (Supplementary Fig. 8). Further, competition studies using 10C10 and 5C3 conjugated with a fluorescent probe (Alexa-647) and non-labelled 14-4b for binding to U373gH/gL cells demonstrated that 14-4b does not compete with 10C10647 binding and only limits 5C3647 binding at higher 14-4b concentrations. These findings suggest that the three anti-gH antibodies differ in their exact epitope, yet likely target a proximal region within Domain 2 of gH (Fig. 8b). Collectively, these data confirm the existence of an ‘antigenic hotspot' within the central alpha helix region of gH capable of eliciting mAbs with potent and broad-spectrum neutralizing capacity.
Humanized mAb 5C3 maintains specificity and potency
To demonstrate the therapeutic potential of murine neutralizing CMV mAbs, the sequence of mAb 5C3 was engineered to create a chimeric mAb consisting of the murine Fv domains fused to human constant domains, as well as a humanized antibody. Flow cytometry staining indicated that the re-engineered mAbs bind U373gH/gL cells to the same extent as murine 5C3 (Fig. 8i). AD169IE2-YFP-neutralization assays conducted in MRC5 fibroblasts demonstrated nearly identical IC50 values for all three of the mAb variants (Fig. 8j). Together these data demonstrate that chimeric and humanized variants of mAb 5C3 do not display loss in specificity or neutralizing potency and exhibit the feasibility of functional murine hybridoma screening for the development of novel therapeutic mAbs.
Discussion
We utilized a high-throughput infection platform based on fluorescent reporter viruses to identify broadly neutralizing CMV mAbs targeting the gH envelope protein. The neutralizing mAbs were effective at blocking diverse strains of virus and different cell types by binding to the trimer and PCs (Fig. 5 and Supplementary Table 3). Thus, targeting a functionally relevant and conserved region of the gH protein is an attractive strategy for generating broad-spectrum biologics.
CMV-gH likely contributes to viral entry primarily through activation of the fusion event, rather than serving a receptor-binding role48. Given that 10C10 and 5C3 block infection at a post-attachment step (Fig. 6), the mAbs may function by interrupting a fusion-triggering signal to gB following viral attachment. Alternatively, gH may constitute part of the fusion complex and thus the mAbs directly interfere with the fusion event. Interestingly, the α-gB mAbs (Fig. 3) neutralized only AD169 and not the TB40/E strain; despite the mAbs' ability to bind to gB derived from TB40/E (Fig. 3d). These results suggest that alternative viral envelope proteins in TB40/E may compensate for mAb-mediated inhibition of gB to allow for CMV entry. Together, these data indicate that assessing neutralization by laboratory-adapted viral strains is insufficient for determining the utility of a therapeutic mAb.
The coevolution of CMV with its human host over millions of years has enabled it to evade immune clearance49,50. Besides well-characterized strategies to evade T cell and NK cell activation, CMV may limit the generation of neutralizing antibodies to essential domains of its envelope50. Thus, generating antibodies in mice immunized with purified virions will allow for the identification of neutralizing mAbs targeting functionally critical regions of envelope proteins that are obscured in the human host. Supplementation of serum from pregnant women with mAb 5C3 demonstrated enhanced CMV-neutralizing ability regardless of serostatus (Supplementary Fig. 9). Neutralization of both fibroblast and epithelial cell infection by Cytogam was also augmented by the addition of 5C3 (Supplementary Fig. 9) indicating that human antibodies to the 10C10/5C3 epitope region is minimally represented in human serum. This underscores the usefulness of murine-generated antibodies in developing antiviral biologics.
The 20aa region of gH Domain 2 surrounding the 10C10 and 5C3 epitope is 100% identical among geographically distinct CMV strains (Fig. 8h), despite sequence variation of gH within those strains51,52. Interestingly, mAb 14-4b binding to gH requires the wild-type residues 481–488 and 671–675 (ref. 47) suggesting a potential overlap with the 5C3 and 10C10 epitope. Analysis of neutralization efficacy (IC50 values), epitope mapping using gH mutants, and competition studies (Supplementary Fig. 8) demonstrate that the mAbs' epitopes possess different binding requirements and do not overlap. Collectively, these findings support the existence of a highly immunogenic region of the gH protein susceptible to antibody-mediated neutralization using murine-derived mAbs.
Additionally, the MSL-109 epitope53 has been identified to bind to Domain 2 of gH, yet MSL-109 binding to gH is dependent on essential residues in Domain 1. Generation of MSL-109 escape mutants identified residues W168, P171 or D446 as important residues for gH binding and neutralization46. Consistent with the model that 5C3 and 10C10 do not share an epitope with MSL-109, 5C3 and 10C10 strongly bind to W168A (Fig. 8d). Further, the epitope of MSL-109 may include multiple regions including aa 380–397, 403–423 and 442–446 (refs 45, 53) that does not include the 5C3 and 10C10 epitope of residues 485–493. The inability of alanine pair substitutions across aa480–486 to abrogate 5C3 binding indicates that a significant sequence disruption within this region may be required to abrogate 5C3 binding. This implies that CMV may not easily escape 5C3 neutralization, as a considerable sequence alteration may be necessary in order for virus quasi-species to evade antibody-mediated inhibition. This may be an especially important characteristic for a neutralizing anti-gH mAb given that escape mutants to MSL-109 have been observed in vitro46.
Advancements in antibody engineering permit the facile conversion of murine mAbs into human biologics54. In fact, chimeric and humanized variants of mAb 5C3 revealed no change in specificity or neutralizing potency of the mAbs (Fig. 8i,j). Further, the comparison of mAb 5C3 with the human-derived α-gH mAb, MSL-109 in neutralization assays in both fibroblasts and epithelial cells revealed nearly identical IC50 values (Supplementary Fig. 10) further demonstrating the value of murine-derived neutralizing mAbs. Human mAbs directed to gH or PC have been identified following a labour-intensive isolation and neutralization strategy31, however these exhibit limited efficacy in neutralization of fibroblast cells. The α-gH mAbs identified to date bind to diverse regions of gH (Supplementary Fig. 11) and their mechanism(s) are not well-characterized46,53. Assessment of the clinical efficacy of MSL-109 did not demonstrate any benefit55,56,57,58 possibly due to suboptimal dosing, viral escape or epitope variability among strains46,59. Also, its function may rely on disruption of gH/gL complexes that may be compensated for by alternative envelope complexes46. Thus, targeting a functional domain with an essential role in viral fusion would provide a more effective means of blocking infection. Alternatively, a cocktail of α-gB, -gH and -PC mAbs may be the most effective strategy to block infection while preventing the generation of resistant strains60,61,62,63.
In conclusion, we developed a high-throughput platform to identify a panel of potent CMV-neutralizing α-gH mAbs that recognize a highly conserved domain exposed in both the gH/gL/gO complex and the PC. These novel mAbs can be advanced for use in immunotherapeutics, in delineating CMV entry pathways, and for identification of critical protein domains to be targeted by vaccination. Moreover, the platform described here represents a rapid and effective framework for creating potent and broadly specific CMV biologics. Identification of the 10C10 and 5C3 epitopes points to the existence of conserved and highly immunogenic regions of the CMV-gH protein and provides valuable insight for the development of future antiviral immunotherapeutics.
Methods
Cell lines, antibodies and viruses
Human U373-MG astrocytoma cells (gift from Dr Hidde Ploegh, Whitehead Institute, MIT) and MRC5 lung fibroblasts (ATCC #CCL-171) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal bovine serum (FBS). THP-1 cells (ATCC #TIB0202) were cultured in Roswell Park Memorial Institute (RPMI) supplemented with 10% FBS. Gp2–293 cells (Takara #631505) were employed to generate retroviruses and were cultured in identical conditions. Human retinal epithelial ARPE-19 cells (ATCC #CRL-2302) were cultured in DMEM and F-12 (1:1) with 10% FBS. All cell lines were tested for mycoplasma contamination. Culture media was supplemented with 1 mM HEPES, 100 U ml−1 penicillin, and 100 g ml−1 streptomycin at 37 °C in a humidified atmosphere (5% CO2). The monoclonal anti-gH antibodies (clone 14-4b (ref. 64) and AP86 (ref. 65)), and the anti-gB antibody 27–156, kind gifts from William J. Britt, were purified from hybridoma culture supernatant. Polyclonal anti-gL immunoglobulins were raised in rabbits by inoculation with a peptide derived from the CMV TB40/E gL sequence (aa. 265–278, PAHSRYGPQAVDAR). Monoclonal antibody W6/32 (anti-MHCI; gift from Dr Hidde Ploegh, Whitehead Institute, MIT), which recognizes properly folded MHC class I molecules was purified from hybridoma culture supernatant. Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased (Chemicon, Billeriza, MA). CMV virus was propagated in MRC5 cells and virus from infected-cell supernatant and cell lysate (pooled) following sonication was purified by density gradient centrifugation by spinning at 20,000 r.p.m. at room temperature for 1.5 h over a 20% sorbitol cushion35.
Infectious virus yield of AD169IE2-YFP (a gift from Dr Leor Weinberger (Gladstone Institute UCSF) was assayed on MRC5 fibroblasts by median tissue culture infectious dose (TCID50). All other strains were titered by plaque assay.
Production of murine hybridomas
Five mice were immunized with AD169 (100 μg) followed by boost of 50 μg of virus preparations. Following the analysis of CMV neutralization using sera from the mice, two of the mice with highest neutralization titre were euthanized and their spleens were utilized to generate B-cell hybridomas using a standard protocol. The individual B-cell clones were selected from soft agar with a robotic Hamilton/Stem Cell Technology ClonaCell Easy Pick instrument, allowing direct screening of individual clones to identify those that neutralize a CMV infection.
High-throughput neutralization assay
AD169IE2-YFP was pre-incubated with hybridoma supernatant (25 μl of supernatant, 25 μl of DMEM for total inoculum volume of 50 μl; MOI: 3) and incubated for 2 h at 4 °C. Development of the neutralization assay by varying incubation time and temperature did not limit virus infection on the incubation of virus at 4 °C (Supplementary Fig. 12A). The incubation of virus with supernatant for 2 h at 4 °C was selected due to the logistics of analysing a large number of hybridoma clones. The inoculum was then added to MRC5 cells (10,000 cells per well) in a 96-well plate. After 2 h the virus/antibody inoculum was removed and replaced with 100 μl DMEM. The plate was then read in an Acumen eX3 laser-scanning fluorescence microplate cytometer to measure YFP fluorescence levels at 16 hpi. Serum samples were diluted with DMEM before virus incubation and then pre-incubated with AD169IE2-YFP as described above. Further, neutralization assays in which AD169IE2-YFP was pre-incubated with 5C3 for different times and temperatures demonstrated equivalent inhibition of infection (Supplementary Fig. 12) demonstrating the versatility of HTN assay. Experiments involving TB40/EFlag-YFP (TB40/E-Bac clone (gift from Dr Christian Sinzger (University of Ulm, Germany) was used to generate TB40/EFlag-YFP) were conducted as described above and analyzed by fluorescent cytometer at 72 hpi. Following initial screen, all samples were tested in technical replicates of 3 to avoid confounding data from outliers.
Immunostain assay
Infected cells were fixed with 4% paraformaldehyde at 16 hpi for 15 min. at 4 °C. Cells were permeablized with 0.1% Triton X-100 and stained with mAb 810-X (1:250) (Millipore), before analysis by fluorescent cytometer.
Plaque reduction assay
MRC5 cells were seeded in duplicate with DMEM at a density of 5 × 104 cells per well in a 24-well plate (BD-Falcon, Franklin Lakes, NJ). The next day cells were infected with virus that was pre-incubated with a range of mAb concentrations (MOI 0.002). Following infection (2 h at 37 °C), cells were washed 2x with DMEM and wells were refilled with 3% DMEM containing DMSO supplemented with 10 μg ml−1 Cytogam (CSL Behring, King of Prussia, PA). At 7–10 dpi, cells were fixed in 4% paraformaldehyde (20 min at 4 °C), and stained with Giemsa (Harleco, EMD Millipore; 1 h at 37 °C). Wells were washed with dH2O 5 × and plaques were blind counted using phase contrast microscopy.
THP-1 neutralization assay
THP-1 cells (250,000 cells per well) were infected with TB40/EFLAG-YFP that was pre-incubated with 0.5 μg ml−1 mAb (MOI 3). Following infection (37 °C, 2 h) inoculum was removed and cells were washed 1 × with RPMI. Cells were cultured for 72 h and then analyzed for YFP fluorescence by flow cytometry on an Intellicyt HTFC flow cytometer.
Transwell assays
MRC5 cells (10,000 per well) were seeded on permeable inserts. The next day cells were infected with TB40/E (MOI: 1). Following inoculation (2 h at 37 °C), cells were washed 2 × and wells were filled with fresh media. At 2 dpi the well insert was transferred to a receiver plate containing either MRC5 cells or ARPE-19 cells (10,000 cells per well) that were seeded the previous day. Wells were then filled with media supplemented with various concentrations of mAb, such that both layers of cells were exposed to the media, and the permeable insert layer did not directly contact the bottom receiver layer. At 7 dpi receiver layer cells were fixed and analyzed by immunostain.
ARPE-19 cell–cell dissemination assay
ARPE-19 cells were seeded in a 96-well plate (10,000 cells per well) and the following day infected with TB40/E (MOI: 0.1). At 48 hpi, cell supernatant was replaced with media containing various concentrations of α-gH mAb, Cytogam or an isotype control. At 10 dpi, cells were fixed and analyzed by immunostain.
Monocyte-fibroblast coculture experiments
Monocytes were infected with TB40/E (MOI: 3) in RPMI with 1% FBS for 1 h at 37 °C with shaking every 15 min and then washed twice with 1 × PBS before culturing in complete RPMI containing 500 U ml−1 granulocyte–macrophage colony-stimulating factor. Following three days of subculture, TB40/E-infected monocytes were transferred to fibroblasts monolayers containing equal number of cells and a range of mAb concentrations. At 6 dpi, monolayers were analyzed by immunostain and quantified using a fluorescent cytometer.
Generation of stable U373 cell lines
gH, gL, gO and UL128 cDNA from the CMV TB40/E sequence was cloned into the pLGPW, pLNCX and pLHCX vectors (Clontech) and stably introduced into U373 astrocytoma cells by retroviral transduction. The gO nucleotide sequence was optimized for expression in human cells.
Flow cytometry analysis
Flow cytometry analysis was performed as previously described66. Briefly, cells were incubated with 1%BSA/PBS, and then antibody (2 μg ml−1) followed by secondary staining with Alexa-647 goat anti-mouse antibody (1:500) (ThermoFisher Scientific). Fluorescent data was collected on an Intellicyt HTFC flow cytometer. For analysis of permeabilized cells, cells were fixed with BD Biosciences Cytofix/Cytoperm solution (20 min. at 4 °C) and maintained in 0.1% saponin throughout the staining procedure. The data were quantified using Flow Jo software (Tree Star, Inc).
Cell lysis and immunoprecipitation
Total cell lysates from U373 transgenic cell lines or infected MRC5 cells were subjected to SDS–PAGE followed by immunoblot analysis using the anti-gH mAb AP86 (1:5 hybridoma supernatant), polyclonal anti-gL IgG (1:1,000), and an anti-GAPDH (1:10,000; Chemicon, Billeriza, MA). Uncropped immunoblot and autoradiograph films are displayed in Supplementary Fig. 13.
Metabolic labelling analysis
MRC5 fibroblasts were infected and pulsed at 72 hpi with 35S-methionine for 6 h at 37 °C. Cells were lysed in NP40 and immunoprecipitated using neutralizing gB mAbs 2F4, 5A6, 7H7, 8H2 or gH mAbs 10C10 and 5C3. Anti-GFP antibody was used as an isotype control. Following incubation of immune complexes with protein A agarose beads (RepliGen), samples were treated with 1 × SDS sample buffer and resolved using SDS–PAGE. For experiments involving N-glycanase F treatment, following immunoprecipitation the protein A beads were split into 2 fractions and one fraction was incubated with 500 units of PNGase F (New England Biolabs) for 2 h before addition of sample buffer and resolution by SDS–PAGE.
Pre/post-attachment neutralization assay
MRC5 cells (10,000 cells per well) were seeded overnight and the following day incubated at 4 °C, 30 min. AD169IE2-YFP virus prep (MOI: 3) was chilled to 4 °C and exposed to the cells for 30 min. followed by addition of pre-chilled mAbs (50 μg ml−1 final concentration) and a final 30 min. incubation step at 4 °C. For pre-incubation with mAbs, virus was pre-incubated with mab at 4 °C, 2 h before exposure to cells. Cells were transferred to 37 °C for 2 h, followed by a 2 × DMEM wash. Fluorescence levels were measured at 16 hpi by fluorescent cytometer.
Analysis of truncation mutants
gH mutants were cloned into pCDNA 3.1 and transfected with lipofectamine. Cells were harvested 2 days post transfection and analyzed by Immunoprecipitation followed by resolution with SDS–PAGE and immunoblot with a monoclonal anti-HA antibody (12CA5; 1 μg ml−1).
Virion binding assay
TB40/E cell-free virus prep was blocked with normal rabbit serum at 1:500, (4 °C, 1 h.). A range of mAb concentrations was then added and incubated at 4 °C, 2 h. Virus/antibody mixture was then ultracentrifuged at 24,000 r.p.m. in a SW55ti rotor for 90 min over a 20% sorbitol cushion. Supernatant was removed and the pelleted virus/antibody was resuspended with 1 × sample buffer and resolved by SDS–PAGE followed by detection with an anti-mouse HRP antibody. Immunoblot using Cytogam permitted the visualization of virus levels.
Mass spectrometry proteomic analysis
mAb 10C10 (10 μg) was incubated with lysates from TB40/E-infected (MOI: 3) MRC5 cells (10 million) at 72 hpi. The precipitates were directly subjected to quantitative analysis of the protein contents at Bioproximity, Inc (Chantilly, VA) using global proteome profiling by LC-MS/MS utilizing Orbitrap mass spectrometry. The protein intensity was calculated as the sum of the peptide-associated features that map to a protein sequence. Protein intensities were adjusted (1) by normalization based on total intensity within a LC-MS/MS run and then across all considered runs, and (2) by background subtraction. Multiple quantitation methods were supported as MS1 peak area, MS2 intensity and spectral counting-derivatives. Proteins were required to have one or more unique peptides across the analyzed samples with low E-value scores. The mascot generic format files were searched using the most recent human and CMV sequence libraries obtained from UniProt. All searches were performed on Amazon Web Services-based cluster compute instances using the Proteome Cluster interface.
Generation of gH peptide library
The C- and N-terminus of gH was elongated by neutral GSGSGSG linkers to avoid truncated peptides. The elongated sequence was translated into 7, 10 and 13 amino acid peptides with peptide-peptide overlaps of 6, 9 and 12 amino acids. After peptide synthesis, all peptides were cyclized via a thioether linkage between a C-terminal cysteine side chain thiol group and an appropriately modified N-terminus. The resulting cyclic peptide microarrays contained 1,815 different cyclic peptides printed in duplicate (3,630 peptides spots) and were complemented on right and left of the array by additional HA (YPYDVPDYAG) and Flag (DYKDDDDKGG) control peptides (60 spots each control).
Analysis of epitope reactivity of the gH microarray
Incubation of peptide microarray libraries was carried out with mAb 5C3 at concentrations of 10 μg ml−1, 100 μg ml−1 and 500 μg ml−1 in incubation buffer followed by staining with the secondary goat anti-mouse IgG (H+L) DyLight680 antibody and read-out at a scanning intensity of 7 (red). Additional HA peptides on right and left of the peptide arrays were finally stained as internal quality control to confirm the assay quality and the peptide microarray integrity (scanning intensities: 7/7, red/green). Quantification of spot intensities and peptide annotation were based on the 16-bit grey scale tiff files at scanning intensities of 7 that exhibit a higher dynamic range than the 24-bit colorized tiff files; microarray image analysis was done with PepSlide Analyzer. An intensity map based on averaged foreground intensities was generated.
Serum supplementation experiments
Human serum (1:1,000) was supplemented with a range of mAb concentration and pre-incubated with TB40/E (MOI: 3; 4 °C, 2 h). Following incubation, virus/serum/mAb mixtures were added to MRC5 or ARPE-19 cells (10,000 cells per well). Following infection (37 °C, 2 h), inoculum was removed and cells were washed 2 × . Infection levels were analyzed at 16 hpi by anti-IE immunostain and fluorescent cytometer.
mAb binding competition experiment
mAbs 10C10 and 5C3 were conjugated to Alexa Fluor 647 using a labelling kit (ThermoFisher). U373gH/gL cells were blocked with 1% BSA/PBS and then simultaneously exposed to a fixed concentration of Alexa647-conjugated antibody, and a range of concentrations of non-labelled antibody. Fluorescent data was collected on an Intellicyt HTFC flow cytometer. The data were quantified by using Flow Jo software.
Chimerization/humanization of 5C3
Sequencing and chimerization for 5C3 variable heavy and kappa chains were obtained by an adaptation of methods previously published for single mouse cells67. Briefly, RNA was extracted from the 5C3 hybridoma using Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA), followed by first stand cDNA synthesis using random hexamers and poly-T primers (Integrated DNA Technologies, Caralville, IA) and SuperScript 3 reverse transcriptase (ThermoFisher Scientific, NY, NY). PCR with FastStart Taq DNA polymerase (Roche) was performed according to factory supplier using degenerate broad primers specific for 5′ UTR ends of heavy and kappa mouse V genes and specific IgG constant and kappa constant 3′ primers67. Purified PCR product was then submitted for Sanger sequencing in both directions using same primers as PCR (GeneWiz, South Plainfield, NJ). Sequence results were blasted against the IMGT mouse databank of germ-line genes using V-Quest (http://imgt.org) and specific 5′ and 3′ primers were built to the exact 5′ start of the V gene and the exact 3′ end of the J gene. For 5C3, sequence blasted as a mouse VH1–61/JH3 for heavy and mouse VK 3–12/JK2 for kappa. Cloning PCRs using specific cloning primers based on germ-line gene information were performed from cDNA and cloned into pFuseSS human IgG1 and human kappa constant containing vectors (InvivoGen, San Diego, CA). Clones were transfected into human Expi293 cells and chimerized antibody was purified from supernatant using protein A columns on an Akta FPLC protein purification system (GE Healthcare Life Sciences, Pittsburgh, PA).
Mouse 5C3 (parental) was humanized by germ-line framework shuffling following methodologies in ref. 68 with each mouse framework region (FW1–4) for the heavy and kappa chain independently replaced with a human acceptor framework region of highest homology. In brief, the process of humanizing started with prediction of 5C3 Fv structure using the ROSIE online server for antibody structure (http://rosie.rosettacommons.org/antibody)69,70. Using a Kabat numbering scheme, framework residues for heavy and light chain were blasted independently using NCBI IgBlast (http://www.ncbi.nlm.nih.gov/igblast/). When disagreements between parental and human residues were encountered, residues were changed to human unless the residue was identified as a key residue or in a structurally relevant position. In this case, residues were evaluated individually and in context of structure model, and parental sequence was maintained (backmutation) if a residue was hypothesized to greatly compromise structural integrity and/or binding activity of antibody. The human acceptor framework sequences containing all identified backmutations for each framework were then grafted between mouse CDR sequences in silico using Geneious DNA software (Biomatters Limited, Newark, NJ) and were synthesized as gBlocks (Integrated DNA Technologies) containing same 5′/3′ restriction sites as for chimerization. Constructs were cloned into same human constant gene containing vectors, transfected and purified from supernatant as for chimerization.
Statistical analysis
Student's unpaired, two-tailed t tests were performed using GraphPad Prism (La Jolla, CA). Asterisks represent statistical significance of P<0.05 or less. For calculation of half maximal inhibitory concentration (IC50), 4 parameter non-linear regression analysis was performed using GraphPad Prism. Standard deviation is depicted in all relevant figures.
Data availability
All data are available from the authors upon request.
Additional information
How to cite this article: Gardner, T. J. et al. Functional screening for anti-CMV biologics identifies a broadly neutralizing epitope of an essential envelope protein. Nat. Commun. 7, 13627 doi: 10.1038/ncomms13627 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures 1 - 13 and Supplementary Tables 1 - 4
Acknowledgments
This work was supported in part by the NIH grants AI101820 and AI112318. T.J.G. was a pre-doctoral trainee supported in part by an American Heart Association pre-doctoral fellowship. T.J.G. and T.M.S. are in part supported by the pre-and post-doctoral USPHS Institutional Research Training Award T32-AI07647.
Footnotes
Author contributions T.J.G. contributed significantly to the acquisition, analysis and interpretation of the data, study design and drafting of the manuscript. K.R.S., J.A.D., T.M.S., V.M.N. and T.K. contributed significantly to the acquisition of data and critical review of the manuscript. T.M.M. and D.T. contributed significantly to the interpretation of data, study design, drafting and critical review of the manuscript.
References
- Reinke P., Prosch S., Kern F. & Volk H. D. Mechanisms of human cytomegalovirus (HCMV) (re)activation and its impact on organ transplant patients. Transpl. Infect. Dis. 1, 157–164 (1999). [DOI] [PubMed] [Google Scholar]
- Khan N. The immunological burden of human cytomegalovirus infection. Arch. Immunol. Ther. Exp. (Warsz) 55, 299–308 (2007). [DOI] [PubMed] [Google Scholar]
- Damato E. G. & Winnen C. W. Cytomegalovirus infection: perinatal implications. J. Obstet. Gynecol. Neonatal Nurs. 31, 86–92 (2002). [DOI] [PubMed] [Google Scholar]
- Connolly S. A., Jackson J. O., Jardetzky T. S. & Longnecker R. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 9, 369–381 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke H. G. & Heldwein E. E. Crystal structure of the human cytomegalovirus glycoprotein B. PLoS Pathog. 11, e1005227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler N. et al. Structural basis for the recognition of human cytomegalovirus glycoprotein B by a neutralizing human antibody. PLoS Pathog. 10, e1004377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanarsdall A. L., Ryckman B. J., Chase M. C. & Johnson D. C. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J. Virol. 82, 11837–11850 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T., Nepomuceno R. R. & Nowlin D. M. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191, 387–395 (1992). [DOI] [PubMed] [Google Scholar]
- Ryckman B. J., Jarvis M. A., Drummond D. D., Nelson J. A. & Johnson D. C. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J. Virol. 80, 710–722 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille P. T., Knoche A. J., Nelson J. A., Jarvis M. A. & Johnson D. C. A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells. J. Virol. 84, 2585–2596 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X. J. et al. UL74 of human cytomegalovirus contributes to virus release by promoting secondary envelopment of virions. J. Virol. 82, 2802–2812 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Lanchy J. M. & Ryckman B. J. Human Cytomegalovirus gH/gL/gO promotes the fusion step of entry into all cell types, whereas gH/gL/UL128-131 broadens virus tropism through a distinct mechanism. J. Virol. 89, 8999–9009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. & Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl Acad. Sci. USA 102, 18153–18158 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn G. et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 78, 10023–10033 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G. et al. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J. Gen. Virol. 86, 275–284 (2005). [DOI] [PubMed] [Google Scholar]
- Chou S., Marousek G., Li S. & Weinberg A. Contrasting drug resistance phenotypes resulting from cytomegalovirus DNA polymerase mutations at the same exonuclease locus. J. Clin. Virol. 43, 107–109 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed E. C., Bowden R. A., Dandliker P. S., Lilleby K. E. & Meyers J. D. Treatment of cytomegalovirus pneumonia with ganciclovir and intravenous cytomegalovirus immunoglobulin in patients with bone marrow transplants. Ann. Intern. Med. 109, 783–788 (1988). [DOI] [PubMed] [Google Scholar]
- Snydman D. R. et al. A further analysis of primary cytomegalovirus disease prevention in renal transplant recipients with a cytomegalovirus immune globulin: interim comparison of a randomized and an open-label trial. Transplant. Proc. 20, 24–30 (1988). [PubMed] [Google Scholar]
- Snydman D. R. et al. Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N. Engl. J. Med. 317, 1049–1054 (1987). [DOI] [PubMed] [Google Scholar]
- Japanese Congenital Cytomegalovirus Infection Immunoglobulin Fetal Therapy Study Group. A trial of immunoglobulin fetal therapy for symptomatic congenital cytomegalovirus infection. J. Reprod. Immunol. 95, 73–79 (2012). [DOI] [PubMed] [Google Scholar]
- Visentin S. et al. Early primary cytomegalovirus infection in pregnancy: maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clin. Infect. Dis. 55, 497–503 (2012). [DOI] [PubMed] [Google Scholar]
- Nigro G. et al. Immunoglobulin therapy of fetal cytomegalovirus infection occurring in the first half of pregnancy--a case-control study of the outcome in children. J. Infect. Dis. 205, 215–227 (2012). [DOI] [PubMed] [Google Scholar]
- Buxmann H. et al. Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: a retrospective analysis. J. Perinat. Med. 40, 439–446 (2012). [DOI] [PubMed] [Google Scholar]
- Revello M. G. et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N. Engl. J. Med. 370, 1316–1326 (2014). [DOI] [PubMed] [Google Scholar]
- Ohlin M. & Soderberg-Naucler C. Human antibody technology and the development of antibodies against cytomegalovirus. Mol. Immunol. 67, 153–170 (2015). [DOI] [PubMed] [Google Scholar]
- Potzsch S. et al. B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog. 7, e1002172 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlin M., Sundqvist V. A., Mach M., Wahren B. & Borrebaeck C. A. Fine specificity of the human immune response to the major neutralization epitopes expressed on cytomegalovirus gp58/116 (gB), as determined with human monoclonal antibodies. J. Virol. 67, 703–710 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Vugler L., Butfiloski E. J. & Stephens E. B. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J. Virol. 64, 1079–1085 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Meza B. P., Adler S. P. & McVoy M. A. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine 26, 5760–5766 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanova A. et al. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc. Natl Acad. Sci. USA 111, 17965–17970 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macagno A. et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J. Virol. 84, 1005–1013 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed D. C. et al. Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine. Proc. Natl Acad. Sci. USA 110, E4997–E5005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genini E. et al. Serum antibody response to the gH/gL/pUL128-131 five-protein complex of human cytomegalovirus (HCMV) in primary and reactivated HCMV infections. J. Clin. Virol. 52, 113–118 (2011). [DOI] [PubMed] [Google Scholar]
- Fouts A. E., Chan P., Stephan J. P., Vandlen R. & Feierbach B. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J. Virol. 86, 7444–7447 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner T. J. et al. Development of a high-throughput assay to measure the neutralization capability of anti-cytomegalovirus antibodies. Clin. Vaccine Immunol. 20, 540–550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner T. J. et al. Development of a high-content screen for the identification of inhibitors directed against the early steps of the cytomegalovirus infectious cycle. Antiviral Res. 113, 49–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J. & Auger D. Synthesis and processing of the envelope gp55-116 complex of human cytomegalovirus. J. Virol. 58, 185–191 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter P. et al. Two novel spliced genes in human cytomegalovirus. J. Gen. Virol. 84, 1117–1122 (2003). [DOI] [PubMed] [Google Scholar]
- Wang D. & Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J. Virol. 79, 10330–10338 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. et al. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl Acad. Sci. USA 100, 14976–14981 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega V. M. et al. Human cytomegalovirus US28 facilitates cell-to-cell viral dissemination. Viruses 6, 1202–1218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampe K., Briem-Richter A., Fischer L., Nashan B. & Ganschow R. The value of immunoprophylaxis for cytomegalovirus infection with intravenous immunoglobulin in pediatric liver transplant recipients receiving a low-dose immunosupressive regimen. Pediatr. Transplant. 14, 67–71 (2010). [DOI] [PubMed] [Google Scholar]
- Huber M. T. & Compton T. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72, 8191–8197 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdary T. K. et al. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat. Struct. Mol. Biol. 17, 882–888 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C. et al. Structural and biochemical studies of HCMV gH/gL/gO and Pentamer reveal mutually exclusive cell entry complexes. Proc. Natl Acad. Sci. USA 112, 1767–1772 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts A. E. et al. Mechanism for neutralizing activity by the anti-CMV gH/gL monoclonal antibody MSL-109. Proc. Natl Acad. Sci. USA 111, 8209–8214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E. P., Lanchy J. M., Ellerbeck E. E. & Ryckman B. J. Scanning mutagenesis of human cytomegalovirus glycoprotein gH/gL. J. Virol. 90, 2294–2305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Yu Q., Wechsler A. & Ryckman B. J. Comparative analysis of gO isoforms reveals that strains of human cytomegalovirus differ in the ratio of gH/gL/gO and gH/gL/UL128-131 in the virion envelope. J. Virol. 87, 9680–9690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega V., Redmann V., Gardner T. & Tortorella D. Diverse immune evasion strategies by human cytomegalovirus. Immunol. Res. 54, 140–151 (2012). [DOI] [PubMed] [Google Scholar]
- Gardner T. J. & Tortorella D. Virion glycoprotein-mediated immune evasion by human cytomegalovirus: a sticky virus makes a slick getaway. Microbiol. Mol. Biol. Rev. 80, 663–677 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzette N., Bhattacharjee B., Jensen J. D., Gibson L. & Kowalik T. F. Extensive genome-wide variability of human cytomegalovirus in congenitally infected infants. PLoS Pathog. 7, e1001344 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzer I., Guelly C., Trajanoski S. & Puchhammer-Stockl E. Deep sequencing reveals highly complex dynamics of human cytomegalovirus genotypes in transplant patients over time. J. Virol. 84, 7195–7203 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C. et al. Antigenic characterization of the HCMV gH/gL/gO and pentamer cell entry complexes reveals binding sites for potently neutralizing human antibodies. PLoS Pathog. 11, e1005230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta L. G. Molecular engineering and design of therapeutic antibodies. Curr. Opin. Immunol. 20, 460–470 (2008). [DOI] [PubMed] [Google Scholar]
- Borucki M. J. et al. A phase II, double-masked, randomized, placebo-controlled evaluation of a human monoclonal anti-Cytomegalovirus antibody (MSL-109) in combination with standard therapy versus standard therapy alone in the treatment of AIDS patients with Cytomegalovirus retinitis. Antiviral Res. 64, 103–111 (2004). [DOI] [PubMed] [Google Scholar]
- MSL-109 adjuvant therapy for cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome: the Monoclonal Antibody Cytomegalovirus Retinitis Trial. The Studies of Ocular Complications of AIDS Research Group. AIDS Clinical Trials Group. Arch Ophthalmol 115, 1528–1536 (1997). [PubMed] [Google Scholar]
- Gilpin A. M., Holbrook J. T., Jabs D. A. & Meinert C. L. (Studies of Ocular Complications of AIDS Research Group). Data and safety monitoring board deliberations resulting in the early termination of the Monoclonal Antibody Cytomegalovirus Retinitis Trial. Control Clin. Trials 24, 92–98 (2003). [DOI] [PubMed] [Google Scholar]
- Boeckh M. et al. Randomized, placebo-controlled, double-blind study of a cytomegalovirus-specific monoclonal antibody (MSL-109) for prevention of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 7, 343–351 (2001). [DOI] [PubMed] [Google Scholar]
- Manley K. et al. Human cytomegalovirus escapes a naturally occurring neutralizing antibody by incorporating it into assembling virions. Cell Host Microbe. 10, 197–209 (2011). [DOI] [PubMed] [Google Scholar]
- Lantto J. et al. Capturing the natural diversity of the human antibody response against vaccinia virus. J. Virol. 85, 1820–1833 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X. et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514, 47–53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregenholt S., Jensen A., Lantto J., Hyldig S. & Haurum J. S. Recombinant human polyclonal antibodies: a new class of therapeutic antibodies against viral infections. Curr. Pharm. Des. 12, 2007–2015 (2006). [DOI] [PubMed] [Google Scholar]
- Bregenholt S. & Haurum J. Pathogen-specific recombinant human polyclonal antibodies: biodefence applications. Expert Opin. Biol. Ther. 4, 387–396 (2004). [DOI] [PubMed] [Google Scholar]
- Simpson J. A. et al. Neutralizing monoclonal antibodies that distinguish three antigenic sites on human cytomegalovirus glycoprotein H have conformationally distinct binding sites. J. Virol. 67, 489–496 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban M., Britt W. & Mach M. The dominant linear neutralizing antibody-binding site of glycoprotein gp86 of human cytomegalovirus is strain specific. J. Virol. 66, 1303–1311 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega V. M. et al. Human cytomegalovirus US3 modulates destruction of MHC class I molecules. Mol. Immunol. 51, 245–253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T., Busse C. E. & Wardemann H. Cloning and expression of murine Ig genes from single B cells. J. Immunol. Methods 350, 183–193 (2009). [DOI] [PubMed] [Google Scholar]
- Kuramochi T., Igawa T., Tsunoda H. & Hattori K. Humanization and simultaneous optimization of monoclonal antibody. Methods Mol. Biol. 1060, 123–137 (2014). [DOI] [PubMed] [Google Scholar]
- Lyskov S. et al. Serverification of molecular modeling applications: the Rosetta Online Server that Includes Everyone (ROSIE). PLoS ONE 8, e63906 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasubramanian A., Sircar A., Chaudhury S. & Gray J. J. Toward high-resolution homology modeling of antibody Fv regions and application to antibody-antigen docking. Proteins 74, 497–514 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1 - 13 and Supplementary Tables 1 - 4
Data Availability Statement
All data are available from the authors upon request.