Abstract
Ginseng occupies a prominent position in the list of best-selling natural products worldwide. Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolius) show different properties and medicinal applications in pharmacology, even though the main active constituents of them are both thought to be ginsenosides. Metabolomics is a promising method to profile entire endogenous metabolites and monitor their fluctuations related to exogenous stimulus. Herein, an untargeted metabolomics approach was applied to study the overall urine metabolic differences between Asian ginseng and American ginseng in mice. Metabolomics analyses were performed using gas chromatography-mass spectrometry (GC-MS) together with multivariate statistical data analysis. A total of 21 metabolites related to D-glutamine and D-glutamate metabolism, glutathione metabolism, TCA cycle and glyoxylate and dicarboxylate metabolism, differed significantly under the Asian ginseng treatment; 34 metabolites mainly associated with glyoxylate and dicarboxylate metabolism, TCA cycle and taurine and hypotaurine metabolism, were significantly altered after American ginseng treatment. Urinary metabolomics reveal that Asian ginseng and American ginseng can benefit organism physiological and biological functions via regulating multiple metabolic pathways. The important pathways identified from Asian ginseng and American ginseng can also help to explore new therapeutic effects or action targets so as to broad application of these two ginsengs.
Asian ginseng, the dried root and rhizome of Panax ginseng C. A. Meyer, is known to reinforce “qi”, invigorating spleen for benefiting lung and tranquilizing the mind and promoting the intelligence. American ginseng is derived from the dried root of Panax quinquefolium L., with the property of cool and mainly used to tonify “qi” and nourish “yin”, which means removing “heat” and promoting the production of body fluids1. Ginseng saponins are usually considered to be the major bioactive components in these two ginseng species, however, there are somewhat different in their effects. Asian ginseng has a high ratio of Rg1: Rb1 thought to have stimulatory effects on central nervous system (CNS) while American ginseng has a lower ratio of Rg1: Rb1 but can calm the CNS2,3. The previous literatures evidenced that Asian ginseng and American ginseng exert lowering and neutral effects on blood pressure, respectively4,5,6,7. PARK et al. concluded that Panax ginseng did not change body temperature but a high dose of Panax quinquefolius may reduce mice night-time body temperature and pyrogen-related factors8. Besides, there are evidences to support that both Asian ginseng and American ginseng can improve human cognitive function9,10. In addition, several controlled clinical trials demonstrated ginseng’s therapeutic potential for glycemic control whereas Asian ginseng yields greater fast plasma insulin reductions relative to American ginseng11,12,13. Furthermore, both Asian ginseng and American ginseng have anti-cancer effects by modulating signaling pathways associated with inflammation, oxidative stress, angiogenesis, metastasis, and stem/progenitor-like properties of cancer cells14.
Over the past decades, most of the pharmacology researches on Asian ginseng and American ginseng are independent conducted, few is carried out simultaneously. With the character of complex composition, ginseng usually acts on multireceptor systems or non-target impacts such as intestinal microbiota15. Thus, we need seek for integrative approach to deepen pharmacology mechanism. As an important part of systems biology, metabolomics characterized by holistic perspective which is consistent with the integral thinking of traditional Chinese medicine (TCM) and the comprehensive actions of TCM has been applied to pharmaceutical industry, drug toxicity and clinical diagnosis16,17,18. Modern pharmacological tools provide a favorable opportunity to accelerate research on mechanism of action of herbs, especially a systems-biology-based pharmacological study of TCM19. In the period of systems biology, metabolomics abridge TCM with molecular pharmacology to an in-depth understanding of molecular mechanism and signal transduction pathway20.
In recent metabolomics study, most of which have focused on chemical markers and quality control between Asian ginseng and American ginseng21,22,23,24. This work provides new insight into different metabolome disturbance in urine along with a four-week administration of Asian ginseng and American ginseng coupling the GC-MS platform which presents unique strengths of high sensitivity, reproducibility and available spectral libraries25. Because urine contains most of the body’s metabolic end products revealing the state of the organism and its noninvasive feature, urinary metabolic profiling has been a favorable technique and has been adopted in this protocol26.
Both Asian ginseng and American ginseng are recognized as tonic herbs and used as dietary supplement worldwide due to its regulation and promotion on body. In our study, we target ginsengs in their nourishing functions and aim to further guide for ginseng daily use in different physical crowd. Also, this study also offers new insight into TCM properties and their traditional application.
Results
Metabolic changes in response to Asian ginseng and American ginseng treated mice
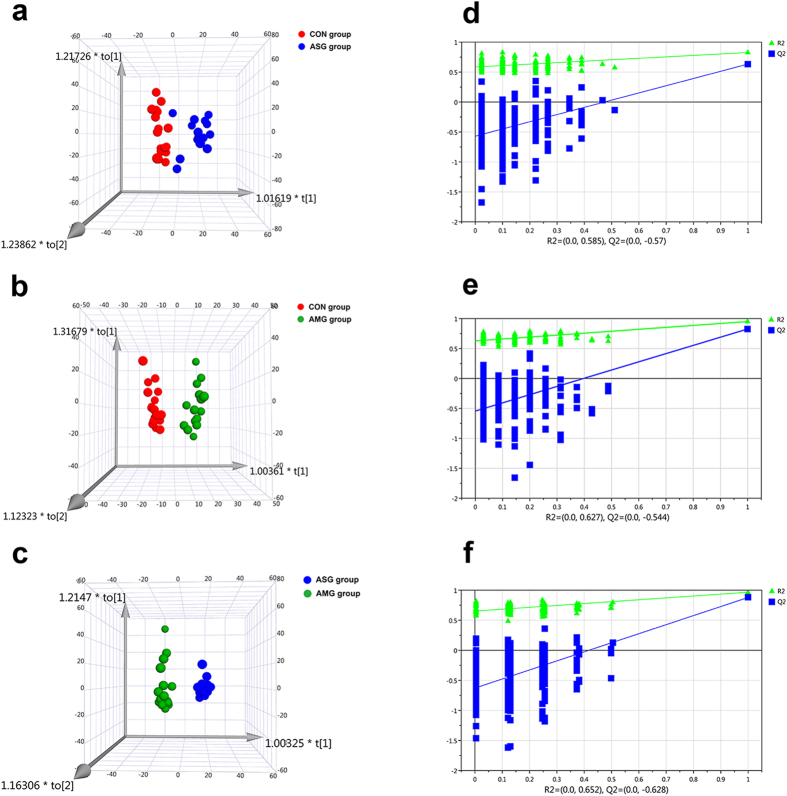
GC-MS was a powerful tool for metabolomics research and it extracted a total of 1797 metabolite peaks in this study. The representative total ion current (TIC) chromatograms obtained from Control group (CON group), Asian ginseng group (ASG group) and American ginseng group (AMG group) were shown in Fig. 1. Although subtle differences between control and treatment groups could be observed from chromatograms, it was still inadequate to clarify definite differentiation exists among control, Asian ginseng and American ginseng administration group. Therefore, intergroup differences were represented by constructing orthogonal partial least squares discriminant analysis (OPLS-DA) model in Fig. 2. It is obvious that clear separation was observed between CON group and ASG group (R2Y (cum) = 0.826, Q2 (cum) = 0.633, Fig. 2a) as well as CON group and AMG group (R2Y (cum) = 0.951, Q2 (cum) = 0.827, Fig. 2b), which indicated urinary metabolic pattern has changed in response to Asian ginseng and American ginseng treated mice. Moreover, a well-separated tendency from Asian ginseng and American ginseng individuals was similarly displayed, suggesting that a different metabolic phenotype existed between Asian ginseng and American ginseng (Fig. 2c). The parameters R2Y (cum) and Q2 (cum) value of 0.966 and 0.879 were considered to be a good fitness and predictability of the constructed OPLS-DA model, respectively (Fig. 2c). Metabolomics usually covered megavariate dataset which meant that the total number of variables is much larger than the number of observations, may leading a chance correlation26,27. To validate the model and avoid overfitting, permutation test (n = 200) was necessary and presented. As shown in Fig. 2d–f, the GC-MS analytical platform has excellent performance in stability and repeatability, and could be exploited in subsequently metabolomics research.
Figure 1. Typical total ion current (TIC) chromatograms of the three groups obtained from GC-MS analysis.
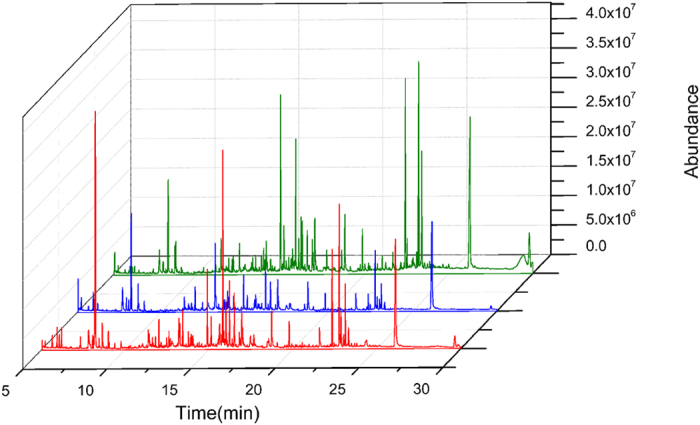
Red represents CON group, blue represents ASG group, green represents AMG group.
Figure 2. OPLS-DA score plots for discriminating the urine metabolome from control group and treatment group and corresponding permutation test obtained from GC-MS.
(a) CON vs ASG group, (b) CON vs AMG group, (c) ASG vs AMG group. Chance permutation at 200 times was used for the discrimination between (d) CON vs ASG, (e) CON vs AMG, and (f) ASG vs AMG.
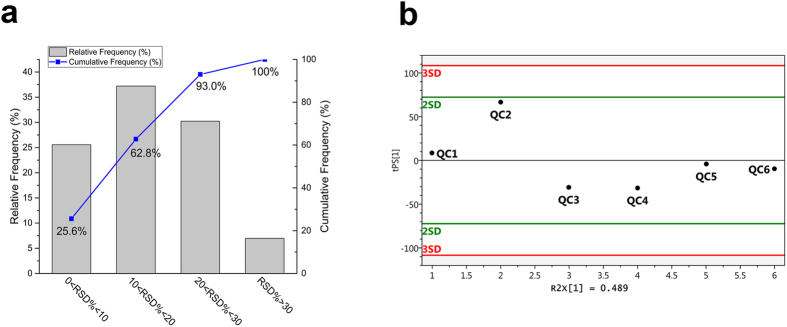
The reproducibility of analysis was assessed by using QC samples. The relative standard deviations (RSD%) were calculated differential metabolites concentrations in the QC samples. As is shown in Fig. 3, 93% of differential metabolites have a RSD% less than 30% and QC samples behaved stable for the duration of the run. Based on these results, the high quality data was sufficient to warrant further statistical analysis of the results to detect biomarkers28.
Figure 3. Analytical variation determined by quality control (QC) samples performance.
(a) Distribution of RSD% for differential metabolites’ peak area in 6 QC samples, (b) PCA first component score plot for QC samples.
Identification of Asian ginseng-responsive and American ginseng-responsive differential metabolites
Differential metabolites contributing to the separation were identified using variable importance in the projection (VIP) value and p value. In general, a threshold of VIP >1 was considered as the relevant metabolites for interpreting the discrimination and Wilcoxon-Mann U test p value set to 0.05 (p < 0.05) was believed to a significant difference. Based on above strategy, a total of 21 and 34 discriminating metabolites resulting from Asian ginseng and American ginseng treatment groups were respectively selected (Tables 1 and 2). The significant changed metabolites were distributed as organic acids, amino acids and sugar alcohols, and 12 common metabolites were altered after Asian ginseng and American ginseng exposure.
Table 1. Differential metabolites in response to Asian ginseng treatment.
| No. | RT (min) | Compound | Formula | VIP valuea | p valueb | FCc | Trend |
|---|---|---|---|---|---|---|---|
| 1 | 21.70 | Glycerol 1-palmitate* | C19H38O4 | 1.646 | 1.11E-04 | 0.631 | Down |
| 2 | 6.42 | Tartaric acid | C4H6O6 | 1.607 | 5.03E-06 | 4.130 | Up |
| 3 | 11.49 | Pyroglutamic acid* | C5H7NO3 | 1.604 | 3.33E-05 | 2.945 | Up |
| 4 | 18.78 | Stearic acid* | C18H36O2 | 1.537 | 1.34E-04 | 4.558 | Up |
| 5 | 17.76 | Uric acid* | C5H4N4O3 | 1.475 | 3.86E-04 | 40.648 | Up |
| 6 | 5.15 | L-Lactic acid* | C3H6O3 | 1.471 | 6.66E-06 | 0.352 | Down |
| 7 | 14.10 | Aconitic acid* | C6H6O6 | 1.370 | 9.18E-05 | 2.499 | Up |
| 8 | 8.24 | Glycerol | C3H8O3 | 1.336 | 2.37E-03 | 2.815 | Up |
| 9 | 13.70 | 5-Hydroxyindole | C8H7NO | 1.305 | 1.72E-05 | 2.672 | Up |
| 10 | 21.03 | Ribothymidine* | C10H14N2O6 | 1.285 | 1.56E-03 | 2.426 | Up |
| 11 | 14.01 | Putrescine* | C4H12N2 | 1.256 | 1.37E-05 | 10.298 | Up |
| 12 | 17.70 | Myo-Inositol* | C6H12O6 | 1.221 | 1.61E-04 | 2.433 | Up |
| 13 | 11.43 | Erythritol* | C4H10O4 | 1.221 | 1.47E-02 | 0.684 | Down |
| 14 | 8.69 | Glycine* | C2H5NO2 | 1.191 | 2.75E-04 | 29.617 | Up |
| 15 | 15.19 | D-Pinitol | C7H14O6 | 1.166 | 1.00E-03 | 4.144 | Up |
| 16 | 8.71 | Succinic acid | C4H6O4 | 1.141 | 7.47E-03 | 4.862 | Up |
| 17 | 12.80 | 4-Hydroxyphenylacetic acid* | C8H8O3 | 1.113 | 2.37E-03 | 1.608 | Up |
| 18 | 17.00 | Palmitic acid* | C16H32O2 | 1.047 | 1.82E-02 | 1.973 | Up |
| 19 | 12.65 | L-Glutamic acid* | C5H9NO4 | 1.047 | 3.86E-04 | 20.301 | Up |
| 20 | 14.961 | Citric acid* | C6H8O7 | 1.031 | 5.19E-03 | 3.083 | Up |
| 21 | 16.54 | Gluconic acid | C6H12O7 | 1.015 | 8.40E-03 | 1.855 | Up |
aVariable importance in the projection (VIP) was obtained from OPLS-DA with a threshold of 1.0.
bp value was calculated from Wilcoxon-Mann U test.
cFold change (FC) was calculated from the arithmetic mean values between ASG and CON group. The metabolites marked with “*” were structurally identified by reference standards.
Table 2. Differential metabolites in response to American ginseng treatment.
| No. | RT (min) | Compound | Formula | VIP valuea | p valueb | FCc | Trend |
|---|---|---|---|---|---|---|---|
| 1 | 17.00 | Palmitic acid* | C16H32O2 | 1.633 | 8.82E-10 | 0.687 | Down |
| 2 | 5.13 | L-Lactic acid* | C3H6O3 | 1.541 | 2.43E-05 | 0.682 | Down |
| 3 | 9.05 | Glyceric acid | C3H6O4 | 1.480 | 2.30E-05 | 0.335 | Down |
| 4 | 19.83 | Pseudouridine | C9H12N2O6 | 1.349 | 4.03E-04 | 21.931 | Up |
| 5 | 21.03 | Ribothymidine* | C10H14N2O6 | 1.320 | 3.82E-03 | 2.893 | Up |
| 6 | 23.07 | Glycerol 1-octadecanoate* | C21H42O4 | 1.298 | 1.03E-05 | 2.176 | Up |
| 7 | 23.18 | Cellobiose* | C12H22O11 | 1.296 | 1.34E-04 | 2.898 | Up |
| 8 | 13.90 | Tricarballylic acid | C6H8O6 | 1.289 | 8.34E-03 | 8.748 | Up |
| 9 | 22.38 | Sucrose* | C12H22O11 | 1.288 | 8.34E-03 | 2.854 | Up |
| 10 | 13.41 | 3-Hydroxyadipic acid | C6H10O5 | 1.286 | 4.67E-04 | 3.432 | Up |
| 11 | 21.74 | 1,3-Dipalmitin | C35H68O5 | 1.275 | 4.67E-04 | 1.979 | Up |
| 12 | 13.70 | 5-Hydroxyindole | C8H7NO | 1.264 | 2.18E-04 | 2.043 | Up |
| 13 | 23.37 | Maltose* | C12H22O11 | 1.262 | 5.39E-03 | 4.248 | Up |
| 14 | 12.05 | Threonic acid | C4H8O5 | 1.260 | 2.16E-03 | 0.530 | Down |
| 15 | 16.27 | Sorbitol* | C6H14O6 | 1.250 | 4.87E-02 | 0.691 | Down |
| 16 | 16.31 | Galactitol* | C6H14O6 | 1.246 | 4.13E-02 | 0.693 | Down |
| 17 | 15.18 | D-Pinitol | C7H14O6 | 1.181 | 4.82E-03 | 3.231 | Up |
| 18 | 16.55 | Gluconic acid | C6H12O7 | 1.176 | 4.13E-02 | 0.688 | Down |
| 19 | 13.26 | Taurine* | C2H7NO3S | 1.170 | 2.24E-02 | 0.789 | Down |
| 20 | 16.66 | Pantothenic acid* | C9H17NO5 | 1.162 | 1.36E-02 | 0.661 | Down |
| 21 | 16.25 | Mannitol* | C6H14O6 | 1.159 | 5.39E-03 | 3.116 | Up |
| 22 | 19.13 | Xanthurenic acid* | C10H7NO4 | 1.156 | 5.70E-03 | 7.300 | Up |
| 23 | 21.66 | Glycerol 1-palmitate* | C19H38O4 | 1.156 | 2.98E-04 | 1.467 | Up |
| 24 | 12.87 | 4-Hydroxyphenylacetic acid* | C8H8O3 | 1.155 | 6.03E-03 | 0.437 | Down |
| 25 | 12.15 | Glutaric acid | C5H8O4 | 1.125 | 3.40E-03 | 0.349 | Down |
| 26 | 14.98 | Citric acid* | C6H8O7 | 1.120 | 4.28E-04 | 4.327 | Up |
| 27 | 14.10 | Aconitic acid* | C6H6O6 | 1.093 | 2.48E-03 | 2.233 | Up |
| 28 | 11.53 | Pyroglutamic acid* | C5H7NO3 | 1.087 | 2.45E-02 | 1.372 | Up |
| 29 | 7.34 | Ketoleucine* | C6H10O3 | 1.081 | 4.07E-02 | 0.643 | Down |
| 30 | 20.21 | 1-Myristoyl-glycerol | C17H34O4 | 1.076 | 3.02E-03 | 1.207 | Up |
| 31 | 18.79 | Stearic acid* | C18H36O2 | 1.066 | 2.24E-02 | 1.076 | Up |
| 32 | 23.15 | Lactose* | C12H22O11 | 1.061 | 7.20E-04 | 1.641 | Up |
| 33 | 10.01 | Methionine* | C5H11NO2S | 1.049 | 2.45E-02 | 0.366 | Down |
| 34 | 17.67 | N-Acetyl-D-glucosamine | C8H15NO6 | 1.009 | 1.39E-02 | 4.973 | Up |
aVariable importance in the projection (VIP) was obtained from OPLS-DA with a threshold of 1.0.
bp value was calculated from Wilcoxon-Mann U test.
cFold change (FC) was calculated from the arithmetic mean values between AMG and CON group. The metabolites marked with “*” were structurally identified by reference standards.
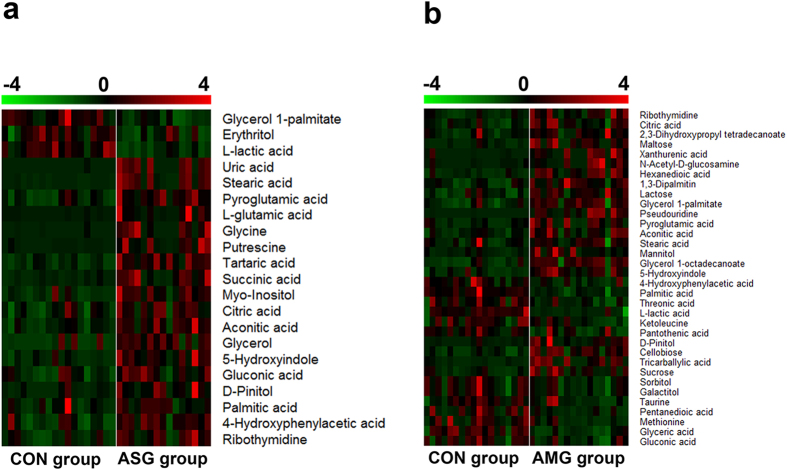
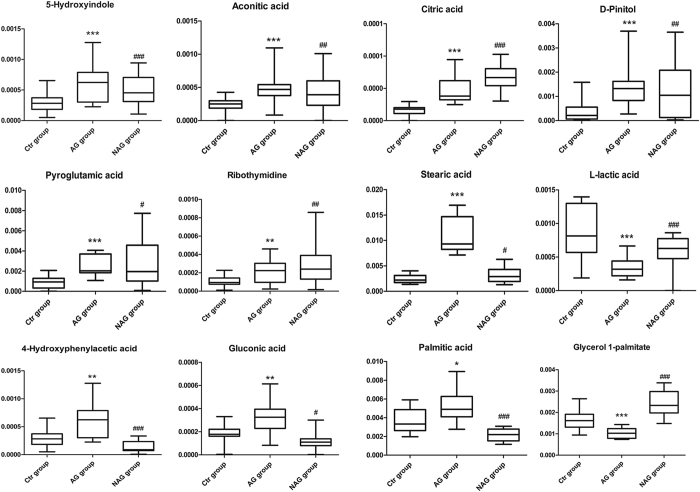
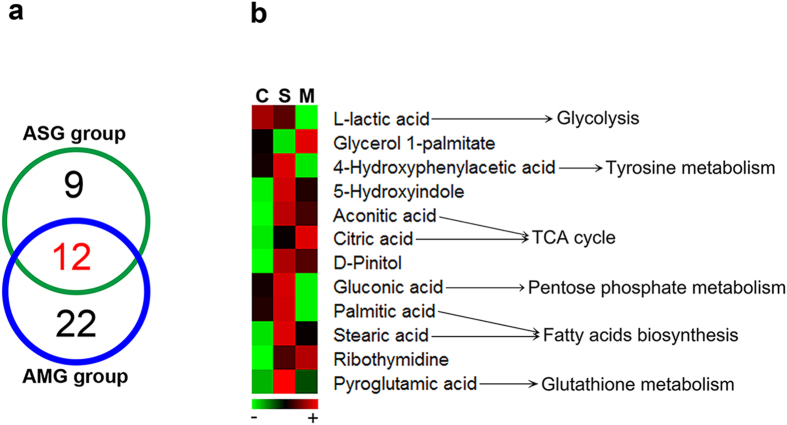
To intuitively inspect the tendency of the variation of the metabolite concentrations between non-treated control group and treatment groups, heat maps were produced according to the relative quantities of each marker. As shown in Fig. 4, compared to CON group, the content of 18 metabolites increased and 3 metabolites decreased in ASG group, while 21 metabolites enhanced and 13 metabolites reduced in AMG group. Among 12 common altered metabolites, the excretion of lactic acid was both declined under the two treatments, whereas coordinated expanded in the content of 7 metabolites including pyroglutamic acid, 5-hydroxyindole, aconitic acid, citric acid, D-pinitol, stearic acid and ribothymidine. In addition, 4-hydroxyphenylacetic acid, gluconic acid and palmitic acid displayed a converse trend that enlarged in response to Asian ginseng and fell in response to American ginseng while glycerol 1-palmitate had an opposite tendency (Fig. 5).
Figure 4. Heatmaps visulization of the differential metabolites responding to Asian ginseng and American ginseng.
(a) ASG group compared to CON group, (b) AMG group compared to CON group. The colors from green to red indicates the increasing expression of metabolites.
Figure 5. Box plots showing the levels of 12 common differential metabolites in the CON group, ASG group and AMG group.
*p < 0.05, **p < 0.01, ***p < 0.001 for ASG group vs CON group; #p < 0.05, ##p < 0.01, ###p < 0.001 for AMG group vs CON group.
Metabolic pathway analysis of Asian ginseng and American ginseng
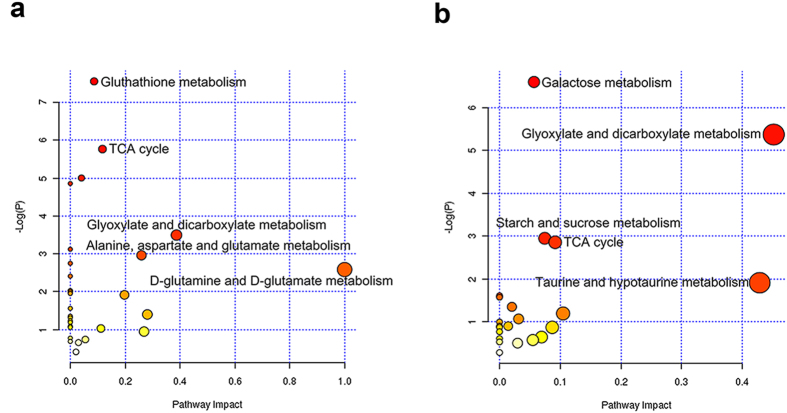
Metabolic pathway analysis was further studied on the basis of the important metabolites listed in Tables 1 and 2. MetaboAnalyst (www. metaboanalyst. ca) is a web-based server supporting pathway analysis which integrated enrichment analysis and pathway topology analysis to reveal the significant relevant pathways influenced by Asian ginseng and American ginseng administration, respectively. Figure 6a showed that elevated D-glutamine and D-glutamate metabolism was thought to be involved in the most relevant pathway influenced by Asian ginseng with the impact value of 1.0. In addition, another four pathways including glutathione metabolism, TCA cycle, glyoxylate and dicarboxylate metabolism as well as alanine, aspartate and glutamate metabolism were promoted responding to Asian ginseng intervention compared with control group. Meanwhile, it can be seen that American ginseng administration upregulated two pathways related to glyoxylate and dicarboxylate metabolism pathway and TCA cycle whereas down-regulated three pathways such as taurine and hypotaurine metabolism pathway, galactose metabolism pathway as well as starch and sucrose metabolism pathway which filtered out to the important metabolic pathways (Fig. 6b). A metabolic network was constructed by searching the KEGG pathway database (Fig. 7).
Figure 6. Pathway mapping based on the differential metabolites.
(a) ASG group versus CON group, (b) AMG group versus CON group.
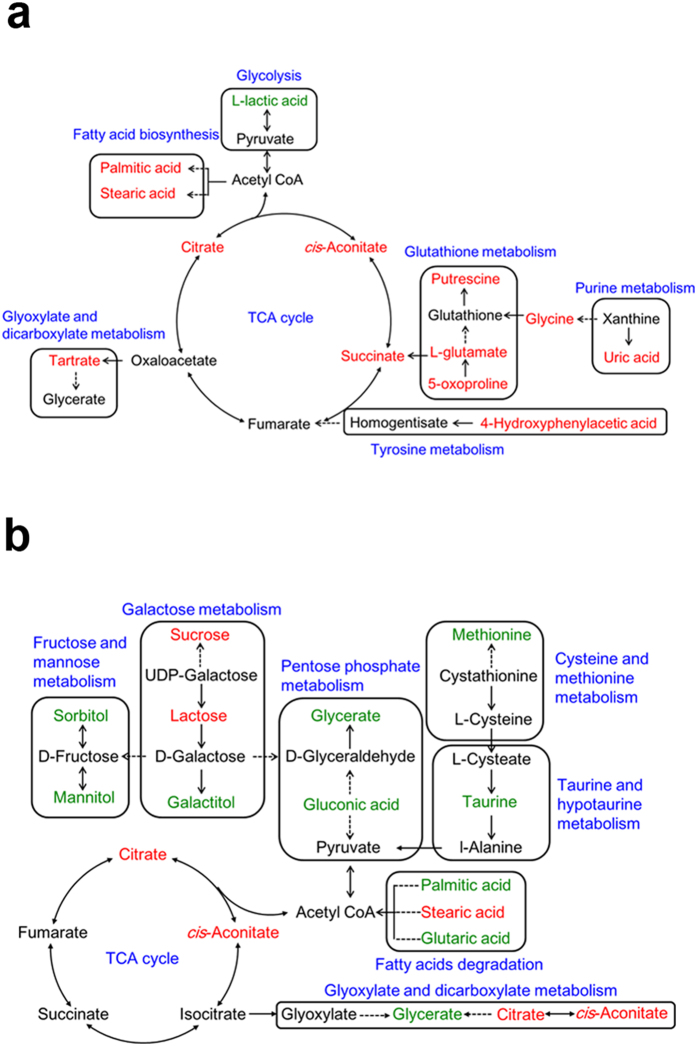
Figure 7.
Metabolic pathway networks of significant different metabolites in response to Asian ginseng (a) and American ginseng (b). The level of metabolites compared to that of control group is marked in red (up-regulated) and green (down-regulated), respectively. The black color represents the undetected metabolites. The words in blue besides the pane indicate the related pathways.
Discussion
In this study, we describe urinary metabolic footprints change responding to Asian ginseng and American ginseng intragastric administration using GC-MS techniques combined with multivariate analysis. Urine is a complex matrix mixing with lots of end products of metabolites (including pharmacy) such as fatty acids, steroids, amino acids and esters. However, some of compounds cannot be detected by GC-MS owing to its nature of non-volatile and polar. Chemical derivatization serves functions of reducing the polarity, increasing the thermal stability and volatility of the analytes25. Resultantly, we adopt a two-step derivatization procedure in which the residue is subjected to oximation using methoxyamine hydrochloride followed by trimethylsilyl derivatization using N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS).
L-glutamic acid is believed to be a predominant position in 21 altered metabolites in response to Asian ginseng, the level of which elevated 20 times compared with that of control group. Furthermore, glutamate-centered metabolism (D-glutamine and D-glutamate metabolism, glutathione metabolism, alanine, aspartate and glutamate metabolism) was considered to be the most relevant pathways influenced by Asian ginseng. As the principal excitatory neurotransmitter in brain, glutamic acid plays a crucial role in functions of learning and memory29. It can be suggested that Asian ginseng improve cognitive performance via modulate glutamate release in agreement with the previous study30. All Asian ginseng-regulated metabolites involved in glutathione metabolism were increased which shows protection on tissues from toxic molecules produced by oxidative stress31. These include L-glutamic acid (20.3 fold enrichment), glycine (29.6 fold enrichment), pyroglutamic acid (2.9 fold enrichment) and putrescine (10.3 fold enrichment).
A panel of altered saccharide metabolites including sorbitol, galactitol, sucrose, lactose and maltose which is responsible for galactose metabolism as well as starch and sucrose metabolism pathway presents a strong link to glycometabolism in AMG group. A relative low level of monosaccharides such as sorbitol and galactitol as well as a high level of disaccharides including sucrose, lactose and maltose points out that a biotransformation from monosaccharide to disaccharide has been enhanced. These results agree with the hypoglycemic activity of American ginseng published previously13. In addition, decreasing content of taurine in AMG group indicates the disturbance of taurine and hypotaurine metabolism. Taurine is a sulfur amino acid that is highly abundant in excitable tissues, especially in brain and heart. It has been proven that the protective effects of taurine appear to involved antioxidant, osmoregulatory and ion regulatory activities32. An inconspicuous descending tendency found in our results implies that American ginseng is likely to enhance taurine utilization followed by reduction of excretion under physiological conditions.
Moreover, 12 common differential metabolites from ASG and AMG group perform a closed connection to energy expenditure (Fig. 8). The decreased level of lactic acid produced from aerobic glycolysis (Warburg effect) is attributed to anticancer action of ginseng33,34. These observations are similar with the data showed here regarding low urinary lactic acid in ASG and AMG group. D-pinitol, an active antidiabetic principle isolated from natural herbs35, was observed at a high level in Asian ginseng and American ginseng group, which might lead to the improved glycaemic control. In epidemiologic and clinical studies, stearic acid was found to be associated with lowered LDL cholesterol in comparison with other saturated fatty acids36. A significant enrichment in stearic acid was found in Asian ginseng treatment group, implying Asian ginseng probably attenuate cardiovascular disease risk resulted from elevated content of stearic acid. Different trends in gluconic acid lead to the variation level of NADPH which is generated from pentose phosphate pathway and NADPH is employed to fatty acids biosynthesis, which corresponds with the level of palmitic acid influenced by Asian ginseng and American ginseng.
Figure 8. A panel of 12 common differential metabolites from ASG and AMG group and their related pathways.
(a) Venn diagram showing the number of metabolites exhibiting a significant difference from ASG and AMG group, (b) Heatmaps of 12 common differential metabolites contructed from average normalized peak areas of three groups and their related metabolic pathways. C: CON group, S: ASG group, M: AMG group.
Intriguingly, both Asian ginseng and American ginseng elevate the level of aconitic acid and citric acid which belongs to glyoxylate and dicarboxylate metabolism pathway as well as TCA cycle highly associated with energy metabolism. Furthermore, it is well-established that Asian ginseng has been widely used for replenishing qi, so as to American ginseng. Such restoration of potency may partially attribute to the improvement of energy metabolism consistent with the experiment conducted in deficiency of vital energy rat37. 4-hydroxyphenylacetic acid responsible for tyrosine metabolism is enrichment in ASG group, however, appears a lower strength in AMG group. The previous literature has showed that spleen-deficiency patients are characterized by disorder of tyrosine metabolism38, and it is general knowledge that both Asian ginseng and American ginseng have been used for invigorating the spleen, but present diversified ability. In the attributive channel theory of Chinese herbal medicine, both Asian ginseng and American ginseng are attributable to the kidney. The improvement in renal function may due to increased urinary content of pyroglutamic acid identified from ASG and AMG group. Also, metabolomics study has uncovered that a clear decrease in pyroglutamic acid in urine of chronic kidney disease (CKD) patients and a higher level of pyroglutamic acid is significantly related to lower risk of incident CKD39,40.
In summary, our present work do a research on comparison of physiological function between Asian ginseng and American ginseng with a systematic metabolomics method. Clearly, a distinct metabolic pattern between Asian ginseng and American ginseng is discovered by OPLS-DA with a panel of urinary characteristic metabolites. The important pathway identified from Asian ginseng and American ginseng may be useful for further mechanism analysis and can also help to explore new therapeutic effects or action targets so as to broad application of Asian ginseng and American ginseng. It is notable that several fatty acids and amino acids which are primary metabolites in Asian ginseng and American ginseng41,42,43 were highlighted as the differential metabolites in response to the treatments of two ginsengs, the effect of exogenous metabolites should take into consideration in future study. However, there still are some limitations on this study. First, a normal animal model instead of disease model adopted in this paper might not be in the direction of target action mechanism. Second, all metabolites are only detected by a single technique of GC-MS. Thus, further analysis combined with LC-MS should be considerable.
Materials and Methods
Chemicals
Chromatographic grade methanol was from Hanbang Tech Co. (Jiangsu, China). Urease (Type III), methoxyamine hydrochloride, N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) and all standard compounds were purchased from Sigma-Aldrich. Ultrapure water was prepared using a Milli-Q purification system. Six-year-old Asian ginseng (Panax ginseng) was provided by Infinitus (China) Company Ltd. American ginseng (Panax quinquefolius) was obtained from BaiXin Pharmacy (Nanjing, China). Asian Ginseng and American ginseng were pulverized through a 100-mesh sieve and suspended in ultrapure water for animal experiments.
Animal protocols
All procedures were conducted in accordance with Administrative Measures of Experimental Animals in Jiangsu Province, and the experimental protocols were approved by the Animal Ethics Committee of China Pharmaceutical University. Fifty-four male ICR mice (18–22 g) were purchased from the Experimental Animal Center of Yangzhou University (Yangzhou, China) and housed under controlled environmental conditions (23–27 °C and 12 h light/dark cycle) with free access to standard diet and water. After one week adaptive feeding, the mice were randomly divided into three groups: CON group (n = 18), ASG group (n = 18) and AMG group (n = 18). The treatment groups were intragastrically administrated with Asian ginseng solution (2 g/kg) and American ginseng solution (2 g/kg) once a day, respectively. And the control group was dosed with equivalent volumes of ultrapure water. 24 h urine was collected from all mice in individual metabolic cages after 30 days administration. All urine samples were immediately centrifuged at 3000 rpm for 10 min and the supernatants were stored at −80 °C until analysis.
Sample preparation and analysis
Urine samples were prepared following previous published protocol with minor modification26. An aliquot of 50 μl urine sample was mixed with 120 μl urease (10 mg/ml, 37 °C, 60 min) to remove high content of urea. 400 μl methanol was used to precipitate protein followed by vortex mixing for 5 min before centrifuged at 10000 g for 10 min at 4 °C. A 300 μl aliquot of supernatant was transferred to a clean Eppendorf tube and was dried under a gentle stream of nitrogen gas. The residue was derivatized by addition of 40 μl methoxyamine hydrochloride (15 mg/ml in pyridine) at 60 °C for 2 h. To each sample add 60 μl BSTFA (1% TMCS) and heat the mixture at 70 °C (1200 rpm) for 60 min. Allow the derivative to cool and centrifuge at 13000 rpm for 10 min prior to GC-MS analysis. Quality control (QC) samples were prepared by pooling aliquots of all the urine samples and were processed with the same procedure as that for the experiment samples.
Each 2 μl aliquot of derivatized sample was injected in 30:1 split ratio into an Agilent 7890B/5977A gas chromatography-mass spectrometry (GC-MS) equipped with HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm, Agilent J & W Scientific, USA). Helium was used as the carrier gas with a constant flow rate of 1 ml/min. The temperature program was as follows: the initial temperature was 80 °C hold for 2 min, elevated to 300 °C at a rate of 10 °C/min and was maintained for 6 min. The temperature of the injector, transfer line and ion source was set to 250, 280 and 230 °C, respectively. The mass range (50–600 m/z) in a full-scan mode for electron impact ionization (70 eV) was applied. The solvent delay time was set to 5 min.
Data processing and statistical analysis
Raw GC-MS data were exported to mzData format by MassHunter Workstation Software (Version B.06.00, Agilent Technologies) and subsequently sent to XCMS package under the R Project. The pretreatment process included novel nonlinear retention time alignment, baseline filtration, peak identification, matching and integration. The resulting data matrix which consists of variables, sample code and peak area was further processed using Microsoft Excel 2010. Total chromatographic area normalization was applied to reduce the deviation originates from discrepant urine concentration between each sample. Finally, the normalized dataset was imported into SIMCA 14 (Umetrics, Sweden) for multivariate statistically analysis and SPSS (Version 19) for univariate data analysis.
To verify separation trends between different groups, supervised orthogonal partial least squares discriminant analysis (OPLS-DA) was used. On the basis of variable importance in the projection (VIP) values greater than 1.0 obtained from OPLS-DA model and p values less than 0.05 acquired from Wilcoxon-Mann U test, a set of discriminating metabolites were determined. All metabolites were identified by comparing mass fragments with the standard mass spectra on the commercial database NIST with a similarity of more than 70%.
Additional Information
How to cite this article: Yang, L. et al. Distinct urine metabolome after Asian ginseng and American ginseng intervention based on GC-MS metabolomics approach. Sci. Rep. 6, 39045; doi: 10.1038/srep39045 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was partly supported by the Jiangsu Province Science Fund for Distinguished Young Scholars (BK20130025).
Footnotes
The authors declare no competing financial interests.
Author Contributions L.Q. and Q.L.W. conceived and designed the research; Y.L. and Z.W.S. carried out the animal experiments; Y.L. drafted the manuscript and analyzed the data; Y.Q.T., G.Y.Z. and M.C.W. conducted the sample preparation; F.Y. revised the manuscript. All authors approved the final version of the manuscript.
References
- Commission C. P. Pharmacopoeia of the People’ Republic of China. Vol. 1 8–9 and 131–132 (Chinese medical science and technology press, 2015). [Google Scholar]
- Chen C. F., Chiou W. F. & Zhang J. T. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin 29, 1103–1108 (2008). [DOI] [PubMed] [Google Scholar]
- Qi L. W., Wang C. Z. & Yuan C. S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry 72, 689–699 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha T. W., Kim M., Kim M., Chae J. S. & Lee J. H. Blood pressure-lowering effect of Korean red ginseng associated with decreased circulating Lp-PLA activity and lysophosphatidylcholines and increased dihydrobiopterin level in prehypertensive subjects. Hypertens Res 39, 449–456 (2016). [DOI] [PubMed] [Google Scholar]
- Han K. H. et al. Effect of red ginseng on blood pressure in patients with essential hypertension and white coat hypertension. Am J Chin Med 26, 199–209 (1998). [DOI] [PubMed] [Google Scholar]
- Stavro P. M., Woo M., Heim T. F., Leiter L. A. & Vuksan V. North American ginseng exerts a neutral effect on blood pressure in individuals with hypertension. Hypertension 46, 406–411 (2005). [DOI] [PubMed] [Google Scholar]
- Stavro P. M. et al. Long-term intake of North American ginseng has no effect on 24-hour blood pressure and renal function. Hypertension 47, 791–796 (2006). [DOI] [PubMed] [Google Scholar]
- Park E. Y. et al. Efficacy comparison of Korean ginseng and American ginseng on body temperature and metabolic parameters. Am J Chin Med 42, 173–187 (2014). [DOI] [PubMed] [Google Scholar]
- Scholey A. et al. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: an acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology 212, 345–356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. O. & Scholey A. B. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Be 75, 687–700 (2003). [DOI] [PubMed] [Google Scholar]
- Shishtar E. et al. The effect of ginseng (the genus panax) on glycemic control: a systematic review and meta-analysis of randomized controlled clinical trials. PLoS One 9, e107391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S. W. et al. Effect of Panax ginseng supplementation on biomarkers of glucose tolerance, antioxidant status and oxidative stress in type 2 diabetic subjects: results of a placebo-controlled human intervention trial. Diabetes Obes Metab 10, 1125–1127 (2008). [DOI] [PubMed] [Google Scholar]
- Vuksan V. et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med 160, 1009–1013 (2000). [DOI] [PubMed] [Google Scholar]
- Wong A. S., Che C. M. & Leung K. W. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep 32, 256–272 (2015). [DOI] [PubMed] [Google Scholar]
- Zhou S. S. et al. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci Rep 6, 22474 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. G., Reily M. D. & Baker J. D. Metabonomics in pharmaceutical discovery and development. J Proteome Res 6, 526–539 (2007). [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Connelly J., Lindon J. C. & Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discovery 1, 153–161 (2002). [DOI] [PubMed] [Google Scholar]
- Claudino W. M. et al. Metabolomics: available results, current research projects in breast cancer, and future applications. J Clin Oncol 25, 2840–2846 (2007). [DOI] [PubMed] [Google Scholar]
- Liu X., Wu W. Y., Jiang B. H., Yang M. & Guo D. A. Pharmacological tools for the development of traditional Chinese medicine. Trends Pharmacol Sci 34, 620–628 (2013). [DOI] [PubMed] [Google Scholar]
- Wang M. et al. Metabolomics in the context of systems biology: bridging traditional Chinese medicine and molecular pharmacology. Phytother Res 19, 173–182 (2005). [DOI] [PubMed] [Google Scholar]
- Zhao H., Xu J., Ghebrezadik H. & Hylands P. J. Metabolomic quality control of commercial Asian ginseng, and cultivated and wild American ginseng using (1)H NMR and multi-step PCA. J Pharm Biomed Anal 114, 113–120 (2015). [DOI] [PubMed] [Google Scholar]
- Pace R., Martinelli E. M., Sardone N. & Combarieu Eric D. E. Metabolomic evaluation of ginsenosides distribution in Panax genus (Panax ginseng and Panax quinquefolius) using multivariate statistical analysis. Fitoterapia 101, 80–91 (2015). [DOI] [PubMed] [Google Scholar]
- Chen Y. et al. Chemical differentiation and quality evaluation of commercial Asian and American ginsengs based on a UHPLC-QTOF/MS/MS metabolomics approach. Phytochem Anal 26, 145–160 (2015). [DOI] [PubMed] [Google Scholar]
- Park H. W. et al. Metabolomic approach for discrimination of processed ginseng genus (Panax ginseng and Panax quinquefolius) using UPLC-QTOF MS. J Ginseng Res 38, 59–65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasikanti K. K., Ho P. C. & Chan E. C. Gas chromatography/mass spectrometry in metabolic profiling of biological fluids. J Chromatogr B: Anal Technol Biomed Life Sci 871, 202–211 (2008). [DOI] [PubMed] [Google Scholar]
- Chan E. C., Pasikanti K. K. & Nicholson J. K. Global urinary metabolic profiling procedures using gas chromatography-mass spectrometry. Nat Protoc 6, 1483–1499 (2011). [DOI] [PubMed] [Google Scholar]
- Trygg J., Holmes E. & Lundstedt T. Chemometrics in metabonomics. J Proteome Res 6, 469–479 (2007). [DOI] [PubMed] [Google Scholar]
- Gika H. G., Theodoridis G. A., Wingate J. E. & Wilson I. D. Within-day reproducibility of an HPLC-MS-based method for metabonomic analysis: application to human urine. J Proteome Res 6, 3291–3303 (2007). [DOI] [PubMed] [Google Scholar]
- McEntee W. J. & Crook T. H. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology 111, 391–401 (1993). [DOI] [PubMed] [Google Scholar]
- Naval M. V., Gomez-Serranillos M. P., Carretero M. E. & De Arce C. Value of high-performance liquid chromatographic analysis of amino acids in the determination of Panax ginseng radix extract effect in cultured neurons. J Chromatogr A 1121, 242–247 (2006). [DOI] [PubMed] [Google Scholar]
- Liu H., Wang H., Shenvi S., Hagen T. M. & Liu R. M. Glutathione metabolism during aging and in Alzheimer disease. Ann NY Acad Sci 1019, 346–349 (2004). [DOI] [PubMed] [Google Scholar]
- Huxtable R. J. Physiological actions of taurine. Physiol Rev 72, 101–163 (1992). [DOI] [PubMed] [Google Scholar]
- Vander Heiden M. G., Cantley L. C. & Thompson C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. et al. Ginsenoside 20(S)Rg3 inhibits the Warburg effect through STAT3 pathways in ovarian cancer cells. Int J Oncol 46, 775–781 (2015). [DOI] [PubMed] [Google Scholar]
- Bates S. H., Jones R. B. & Bailey C. J. Insulin-like effect of pinitol. Br J Pharmacol 130, 1944–1948 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J. E., Zhang J. & Kris-Etherton P. M. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr 91, 46–63 (2010). [DOI] [PubMed] [Google Scholar]
- Lin H. et al. Uinary metabonomic study of Panax ginseng in deficiency of vital energy rat using ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Ethnopharmacol (2016). [DOI] [PubMed] [Google Scholar]
- Zhang A. et al. Exploratory urinary metabolic biomarkers and pathways using UPLC-Q-TOF-HDMS coupled with pattern recognition approach. Analyst 137, 4200–4208 (2012). [DOI] [PubMed] [Google Scholar]
- Posada-Ayala M. et al. Identification of a urine metabolomic signature in patients with advanced-stage chronic kidney disease. Kidney Int 85, 103–111 (2014). [DOI] [PubMed] [Google Scholar]
- Yu B. et al. Serum metabolomic profiling and incident CKD among African Americans. Clin J Am Soc Nephrol 9, 1410–1417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y. H., Ikegami F. & Lambein F. Neuroactive and other free amino acids in seed and young plants of Panax ginseng. Phytochemistry 62, 1087–1091 (2003). [DOI] [PubMed] [Google Scholar]
- Wan J. Y. et al. Integrated evaluation of malonyl ginsenosides, amino acids and polysaccharides in fresh and processed ginseng. J Pharm Biomed Anal 107, 89–97 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang X. J. et al. Fatty acid variability in three medicinal herbs of Panax species. Chem Cent J 7, 12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]