Abstract
Hemobilia represents an uncommon cause of gastrointestinal bleeding that can present both diagnostic and therapeutic challenges. The evaluation of hemobilia typically involves cross-sectional imaging and endoscopic retrograde cholangiopancreatography (ERCP). There is limited data regarding the diagnostic utility of endoscopic ultrasound (EUS) in the evaluation of hemobilia. We present a case of a hepatic artery pseudoaneurysm as the etiology of hemobilia that was detected via EUS only. We conclude that EUS can serve as an important diagnostic tool in the evaluation of obscure hemobilia, especially in cases where imaging, ERCP, and percutaneous transhepatic cholangiography have been unsuccessful or inconclusive.
Introduction
Hemobilia is a rare cause of upper gastrointestinal (GI) bleeding resulting from an abnormal communication between a biliary duct and a blood vessel. The most common etiologies of hemobilia include trauma and iatrogenic injury following hepatic or biliary tract instrumentation. Choledocholithiasis, malignancy, and vascular malformations are less frequently cited causes of hemobilia.1-4 Notably, hemobilia from vascular lesions has a propensity to cause massive GI bleeding. Endoscopic retrograde cholangiopancreatography (ERCP) typically plays a central role in the diagnosis and treatment of hemobilia, whereas endoscopic ultrasound (EUS) has not been routinely employed.
Case Report
An 80-year-old female presented to an outside hospital with abdominal pain and mild transaminitis. A right upper quadrant ultrasound (US) raised concern for a mass in the gallbladder. A cholescintigraphy scan revealed biliary obstruction. Magnetic resonance cholangiopancreatography was unremarkable. Laparoscopic cholecystectomy was notable for a necrotic gallbladder with a large volume of blood and blood clots. Pathology was negative for neoplasm. The patient developed postoperative jaundice, and her hepatic panel remained abnormal. She was discharged to home on postoperative day 2 but was readmitted the following day after an episode of hematemesis. Labs on readmission showed hemoglobin 5.9 g/dL, alkaline phosphatase 160 U/L, aspartate aminotransferase 165 U/L, alanine aminotransferase 160 U/L, and total bilirubin 4.2 mg/dL. Esophagogastroduodenoscopy (EGD) revealed multiple erosions in the stomach but no other significant findings. Computed tomography (CT) of the abdomen/pelvis noted only a 5-cm hematoma inferior to the gallbladder fossa. ERCP was attempted but the bile duct could not be cannulated secondary to extrinsic compression from the hematoma and bleeding from the papilla.
The patient was transferred to our institution, and ERCP was attempted again but cannulation was unsuccessful due to a large protuberant mass-like ampulla. Biopsies of the ampulla were unremarkable. A pancreas protocol CT scan noted biliary and pancreatic duct dilation to the level of the ampulla. A percutaneous transhepatic cholangiography (PTC) was then placed and brushings from the biliary tract and ampulla were obtained, but these also came back unremarkable. The patient continued to have bloody output from the PTC with repeated transfusion requirements. CT angiography and venography demonstrated unremarkable vascular structures and no evidence of active bleeding. On EUS with a linear echoendoscope, hyperechoic material consistent with clots was visualized in the common bile duct, the cystic duct stump, and the common hepatic duct, surrounding the PTC tube. The ampulla/major papilla was bulging and distorted though without a definite mass. Notably, there was arterial vascular flow within the common hepatic duct near the crossover of the hepatic artery (Video 1, Figure 1). No shadowing stones were seen.
Figure 1.
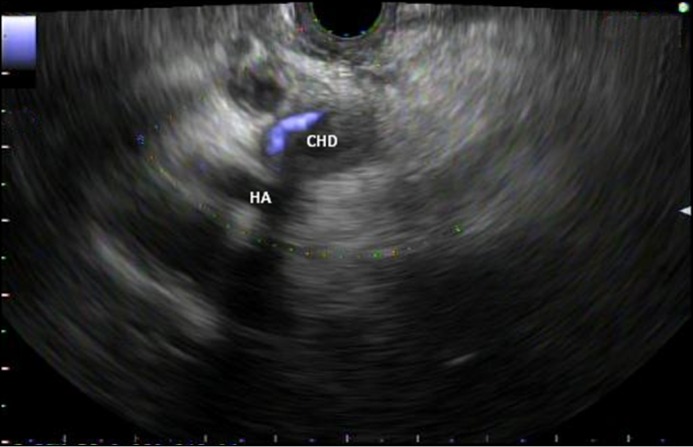
Endoscopic ultrasound image demonstrating arterial vascular flow within the common hepatic duct (CHD) near the crossover of the hepatic artery (HA).
Video 1.
Endoscopic ultrasound demonstrating suspected arterial vascular flow within the common hepatic duct near the crossover of the hepatic artery.
At this point ERCP with interventional radiology rendezvous was performed to exclude stone disease as a cause of hemobilia. Biliary sphincterotomy, biliary brushings, and repeat biopsy of the ampulla were performed, and pathology again was unremarkable. No choledocholithiasis was seen. Sludge and old blood clots were extracted by balloon catheter. Arteriograms of the celiac axis, superior mesenteric artery, left hepatic artery, and gastroduodenal artery revealed no identifiable source of hemobilia. A Tc-99m red blood cell scan revealed evidence of active GI bleeding in the second part of the duodenum. A repeat angiogram identified a pseudoaneurysm with extravasation of contrast associated with a branch of the right hepatic artery and segment VI of the liver (Figure 2). This was treated with coil embolization with resultant resolution of persistent hemobilia (Figure 3).
Figure 2.
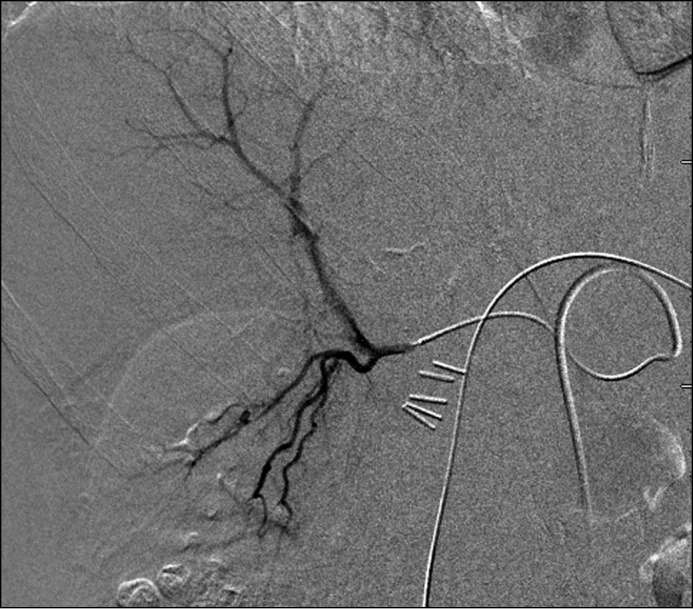
Angiogram pre-embolization noting a pseudoaneurysm and extravasation of contrast associated with a branch of the right hepatic artery and segment VI of the liver.
Figure 3.
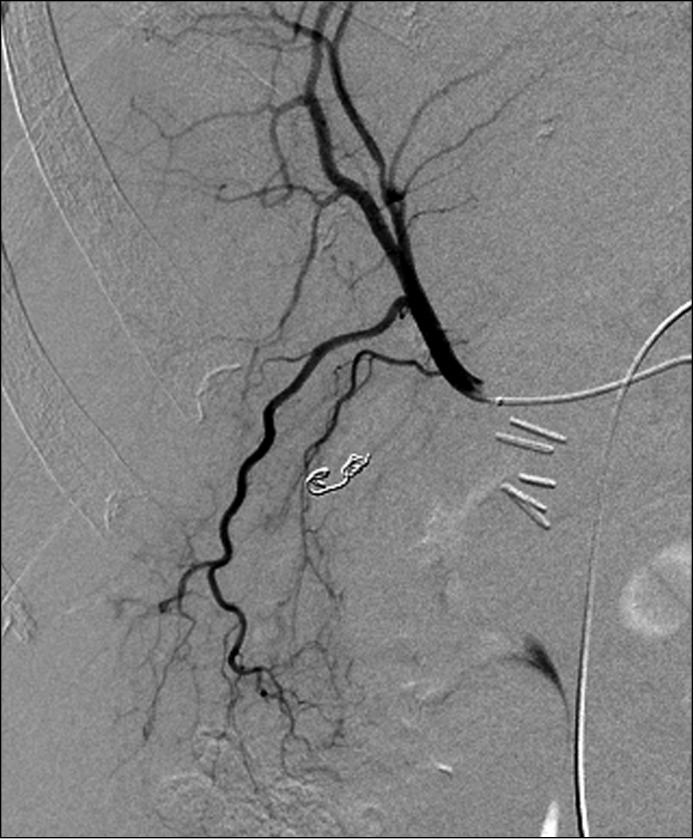
Angiogram following coil embolization of right hepatic artery pseudoaneurysm.
On follow-up the patient did not have subsequent recurrence of her hemobilia. She was discharged home and had a follow-up ERCP due to the previous biliary dilatation and ampullary mass. No evidence of an ampullary mass was seen, and repeat ampullary and intraductal biopsies were unremarkable. A balloon sweep of the biliary tree was performed, and her prior biliary sphincterotomy was extended. Her PTC was removed at that time without subsequent complications or recurrence of bleeding.
Discussion
ERCP has played a central diagnostic and therapeutic role in the evaluation of hemobilia, with some case series suggesting a diagnostic yield close to 100% in patients with severe bleeding.1-4 EUS has not played a significant role in the evaluation of hemobilia.5-6 Hepatic artery pseudoaneurysms in the majority of cases result from iatrogenic or accidental trauma, but causes can also include tumors, stones, and inflammatory and other vascular disorders.4,7-8 While our patient developed bleeding following cholecystectomy, the presence of blood and clots within the gallbladder at the time of resection is consistent with a preceding vascular abnormality. In this patient, we speculate that prior inflammation of the gallbladder may have led to the development of the pseudoaneurysm; intrabiliary bleeding may then have precipitated her presentation with abdominal pain, biliary obstruction, and a gallbladder full of blood. Given the abnormal appearance of the ampulla in this case, we remained concerned about an underlying malignancy, though repeated biopsies and brushings were unremarkable.
Treatment options for hepatic artery pseudoaneurysms include embolization of the aneurysm or common hepatic artery, endovascular stenting, or surgical repair.9 Angiographic coil embolization via interventional radiology has become the treatment of choice and was successful in our patient. This case adds to the existing literature to suggest a role for EUS in the evaluation of hemobilia, especially in cases where other testing modalities such as cross-sectional imaging, ERCP, and even angiography have been unsuccessful or unrevealing.10-11 As illustrated in this case, EUS can serve as an important diagnostic tool in the evaluation of obscure hemobilia.
Disclosures
Author contributions: M. Konerman prepared the manuscript and is the article guarantor, C. Piraka edited the manuscript and video, and Z. Zhang edited the manuscript and images.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1. Green MH, Duell RM, Johnson CD, et al.. Haemobilia. Br J Surg. 2001; 88:773–86. [DOI] [PubMed] [Google Scholar]
- 2. Chin MW, Enns R. Hemobilia. Curr Gastroenterol Rep. 2010; 12:121–9. [DOI] [PubMed] [Google Scholar]
- 3. Kim KH, Kin TN. Etiology, clinical features, and endoscopic management of hemobilia: A retrospective analysis of 37 cases. Korean J Gastroenterol. 2012; 59:296–302. [DOI] [PubMed] [Google Scholar]
- 4. Bloechle C, Izbicki JR, Rashed MY, et al.. Hemobilia: Presentation, diagnosis, and management. Am J Gastroenterol. 1994; 89:1537–40. [PubMed] [Google Scholar]
- 5. Cattan P, Cuillerier E, Cellier C, et al.. Hemobilia caused by a pseudoaneurysm of the hepatic artery diagnosed by EUS. Gastrointest Endosc. 1999; 49:252–5. [DOI] [PubMed] [Google Scholar]
- 6. Trakarnsanga A, Sriprayoon T, Akaraviputh T, et al.. Massive hemobilia from a ruptured hepatic artery aneurysm detected by endoscopic ultrasound (EUS) and successfully treated. Endoscopy. 2010; 42:340–1. [DOI] [PubMed] [Google Scholar]
- 7. Abbas MA, Fowl RJ, Stone WM, et al.. Hepatic artery aneurysm: Factors that predict complications. J Vasc Surg. 2003; 38:41–5. [DOI] [PubMed] [Google Scholar]
- 8. Messina LM, Shanley CJ. Visceral artery aneurysms. Surg Clin North Am. 1997; 77:425–42. [DOI] [PubMed] [Google Scholar]
- 9. Dougherty MJ, Gloviczki P, Cherry KJ, et al.. Hepatic artery aneurysms: Evaluation and current management. Int Angiol. 1993; 12:178–84. [PubMed] [Google Scholar]
- 10. Seicean A. Endoscopic ultrasound in the diagnosis and treatment of upper digestive bleeding: A useful tool. J Gastrointestin Liver Dis. 2013; 22(4):465–9. [PubMed] [Google Scholar]
- 11. Srinivasan I, Tang SJ, Vilmann AS, et al.. Hepatic applications of endoscopic ultrasound: Current status and future directions. World J Gastroenterol. 2015; 21(44):12544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]


