Abstract
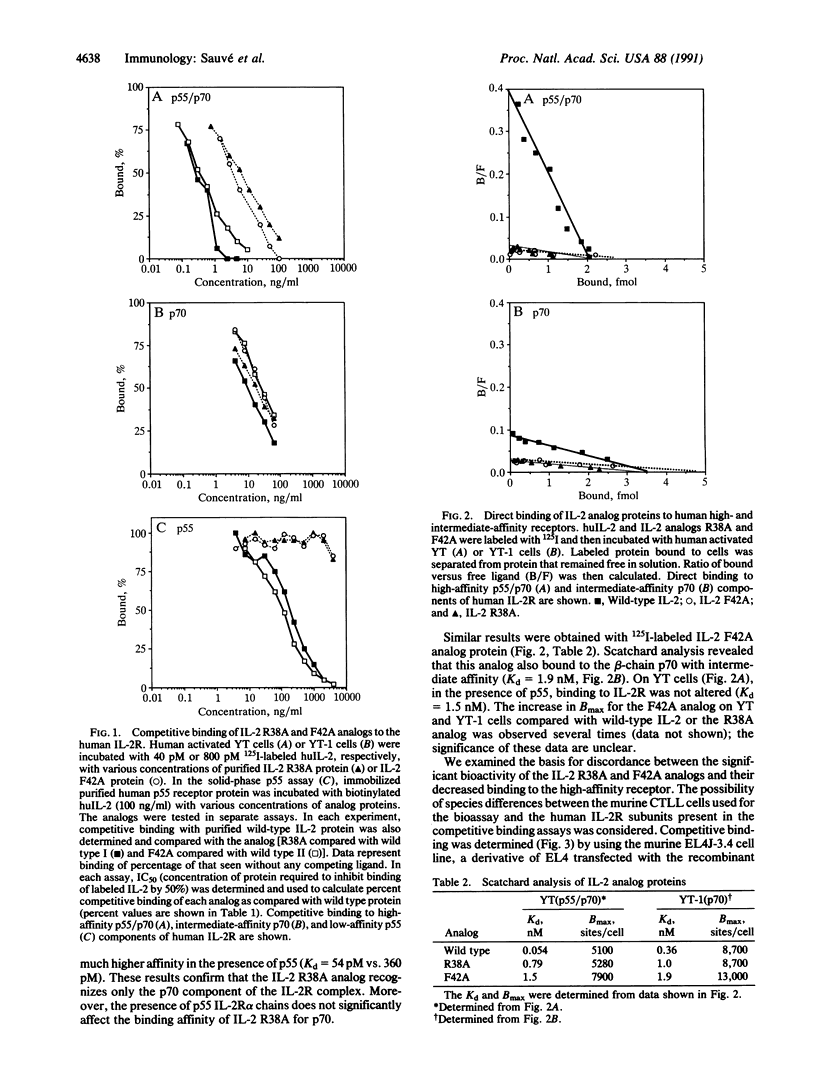
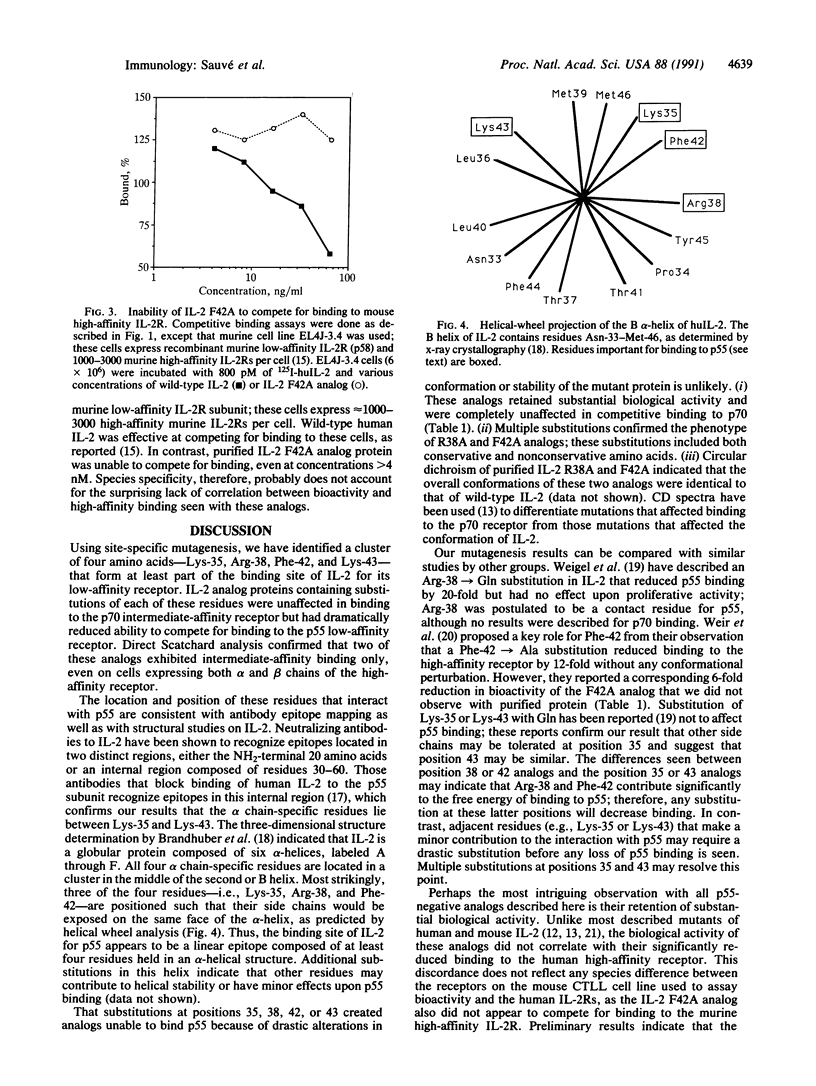
Human interleukin 2 (IL-2) analogs with defined amino acid substitutions were used to identify specific residues that interact with the 55-kDa subunit (p55) or alpha chain of the human IL-2 receptor. Analog proteins containing specific substitutions for Lys-35, Arg-38, Phe-42, or Lys-43 were inactive in competitive binding assays for p55. All of these analogs retained substantial competitive binding to the intermediate-affinity p70 subunit (beta chain) of the receptor complex. The analogs varied in ability to interact with the high-affinity p55/p70 receptor. Despite the lack of binding to p55, all analogs exhibited significant biological activity, as assayed on the murine CTLL cell line. The dissociation constants of Arg-38 and Phe-42 analogs for p70 were consistent with intermediate-affinity binding; the Kd values were not significantly affected by the presence of p55 in binding to the high-affinity IL-2 receptor complex. These results confirm the importance of the B alpha-helix in IL-2 as the locus for p55-receptor binding and support a revised model of IL-2-IL-2 receptor interaction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandhuber B. J., Boone T., Kenney W. C., McKay D. B. Three-dimensional structure of interleukin-2. Science. 1987 Dec 18;238(4834):1707–1709. doi: 10.1126/science.3500515. [DOI] [PubMed] [Google Scholar]
- Collins L., Tsien W. H., Seals C., Hakimi J., Weber D., Bailon P., Hoskings J., Greene W. C., Toome V., Ju G. Identification of specific residues of human interleukin 2 that affect binding to the 70-kDa subunit (p70) of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7709–7713. doi: 10.1073/pnas.85.20.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D., Cerretti D. P., Larsen A., Park L., March C., Dower S., Gillis S., Urdal D. Cloning, sequence and expression of human interleukin-2 receptor. Nature. 1984 Dec 20;312(5996):768–771. doi: 10.1038/312768a0. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Herrmann T., Diamantstein T. The human intermediate-affinity interleukin 2 receptor consists of two distinct, partially homologous glycoproteins. Eur J Immunol. 1988 Jul;18(7):1051–1057. doi: 10.1002/eji.1830180713. [DOI] [PubMed] [Google Scholar]
- Ju G., Collins L., Kaffka K. L., Tsien W. H., Chizzonite R., Crowl R., Bhatt R., Kilian P. L. Structure-function analysis of human interleukin-2. Identification of amino acid residues required for biological activity. J Biol Chem. 1987 Apr 25;262(12):5723–5731. [PubMed] [Google Scholar]
- Kondo S., Kinoshita M., Shimizu A., Saito Y., Konishi M., Sabe H., Honjo T. Expression and functional characterization of artificial mutants of interleukin-2 receptor. Nature. 1987 May 7;327(6117):64–67. doi: 10.1038/327064a0. [DOI] [PubMed] [Google Scholar]
- Kuo L. M., Robb R. J. Structure-function relationships for the IL 2-receptor system. I. Localization of a receptor binding site on IL 2. J Immunol. 1986 Sep 1;137(5):1538–1543. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Yokota T., Kastelein R., Zurawski S. M., Arai N., Takebe Y. Species-specificity of T cell stimulating activities of IL 2 and BSF-1 (IL 4): comparison of normal and recombinant, mouse and human IL 2 and BSF-1 (IL 4). J Immunol. 1987 Mar 15;138(6):1813–1816. [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Internalization of interleukin 2 is mediated by the beta chain of the high-affinity interleukin 2 receptor. J Exp Med. 1987 Apr 1;165(4):1201–1206. doi: 10.1084/jem.165.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Sabe H., Suzuki N., Kondo S., Ogura T., Shimizu A., Honjo T. A larger number of L chains (Tac) enhance the association rate of interleukin 2 to the high affinity site of the interleukin 2 receptor. J Exp Med. 1988 Nov 1;168(5):1563–1572. doi: 10.1084/jem.168.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragovi H., Malek T. R. The murine interleukin 2 receptor. Irreversible cross-linking of radiolabeled interleukin 2 to high affinity interleukin 2 receptors reveals a noncovalently associated subunit. J Immunol. 1987 Sep 15;139(6):1918–1926. [PubMed] [Google Scholar]
- Siegel J. P., Sharon M., Smith P. L., Leonard W. J. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987 Oct 2;238(4823):75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Tsudo M., Goldman C. K., Bongiovanni K. F., Chan W. C., Winton E. F., Yagita M., Grimm E. A., Waldmann T. A. The p75 peptide is the receptor for interleukin 2 expressed on large granular lymphocytes and is responsible for the interleukin 2 activation of these cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5394–5398. doi: 10.1073/pnas.84.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- Wang H. M., Smith K. A. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987 Oct 1;166(4):1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel U., Meyer M., Sebald W. Mutant proteins of human interleukin 2. Renaturation yield, proliferative activity and receptor binding. Eur J Biochem. 1989 Mar 15;180(2):295–300. doi: 10.1111/j.1432-1033.1989.tb14647.x. [DOI] [PubMed] [Google Scholar]
- Weir M. P., Chaplin M. A., Wallace D. M., Dykes C. W., Hobden A. N. Structure-activity relationships of recombinant human interleukin 2. Biochemistry. 1988 Sep 6;27(18):6883–6892. doi: 10.1021/bi00418a034. [DOI] [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski S. M., Zurawski G. Mouse interleukin-2 structure-function studies: substitutions in the first alpha-helix can specifically inactivate p70 receptor binding and mutations in the fifth alpha-helix can specifically inactivate p55 receptor binding. EMBO J. 1989 Sep;8(9):2583–2590. doi: 10.1002/j.1460-2075.1989.tb08397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



