Abstract
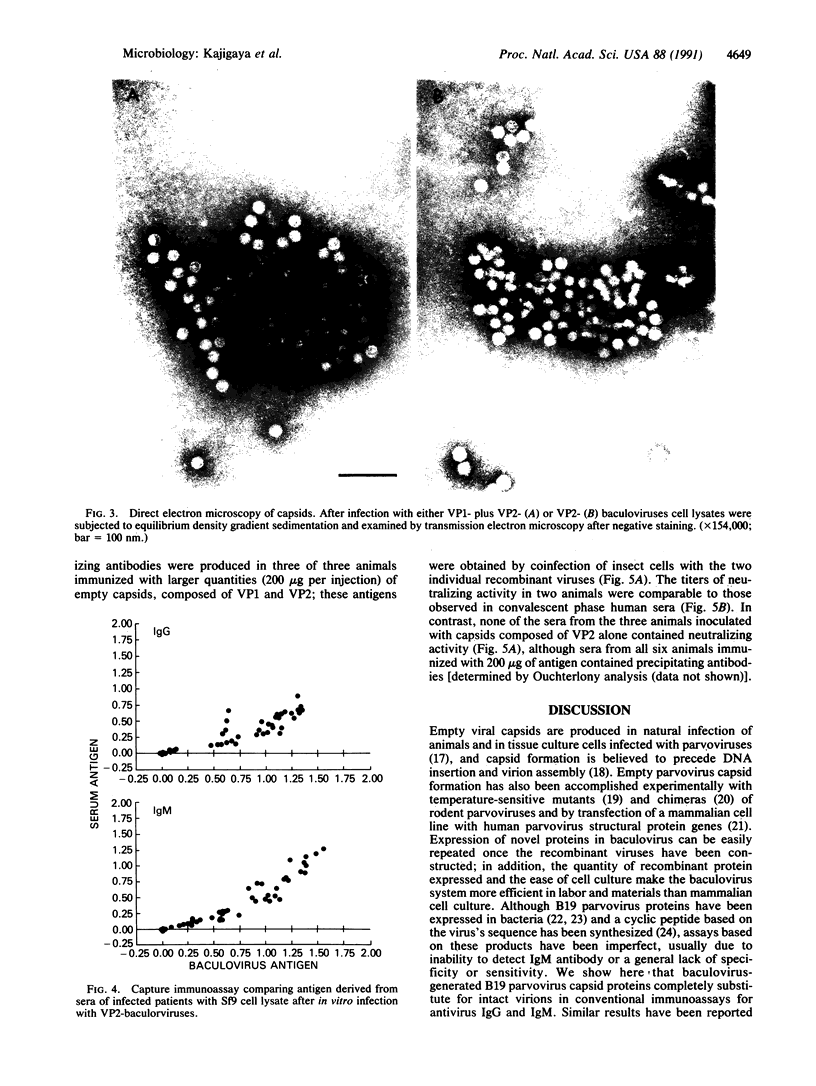
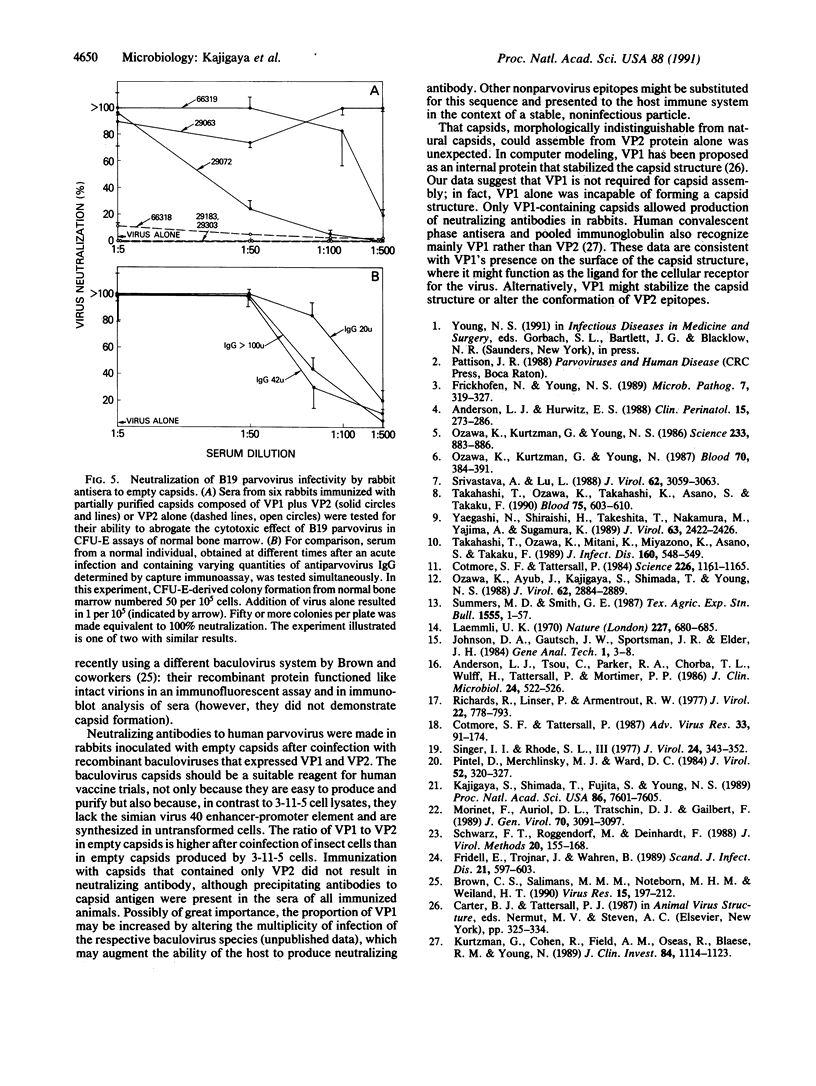
B19 parvovirus is pathogenic in humans, causing fifth disease, transient aplastic crisis, some cases of hydrops fetalis, and acquired pure red cell aplasia. Efforts to develop serologic assays and vaccine development have been hampered by the virus's extreme tropism for human bone marrow and the absence of a convenient culture system. We constructed recombinants containing either the major (VP2) or minor (VP1) structural proteins of B19 in a baculovirus-based plasmid, from which the polyhedrin gene had been deleted; these recombinant plasmids were used to generate recombinant infectious baculovirus. Subsequent infection of insect cells in vitro resulted in high-level expression of either B19VP1 or VP2. Parvovirus capsids were obtained by self-assembly in cell cultures coinfected with either VP1- and VP2-containing baculoviruses or, surprisingly, VP2-containing baculoviruses alone. Empty B19 capsids composed of VP1 and VP2 could replace serum virus as a source of antigen in a conventional immunoassay for detection of either IgG or IgM antiparvovirus antibodies in human serum. Immunization of rabbits with capsids composed of VP1 and VP2 resulted in production of antisera that recognized serum parvovirus on immunoblot and neutralized parvovirus infectivity for human erythroid progenitor cells. Baculovirus-derived parvovirus antigen can substitute for scarce viral antigen in immunoassays and should be suitable as a human vaccine.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. J., Hurwitz E. S. Human parvovirus B19 and pregnancy. Clin Perinatol. 1988 Jun;15(2):273–286. [PubMed] [Google Scholar]
- Anderson L. J., Tsou C., Parker R. A., Chorba T. L., Wulff H., Tattersall P., Mortimer P. P. Detection of antibodies and antigens of human parvovirus B19 by enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Oct;24(4):522–526. doi: 10.1128/jcm.24.4.522-526.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. S., Salimans M. M., Noteborn M. H., Weiland H. T. Antigenic parvovirus B19 coat proteins VP1 and VP2 produced in large quantities in a baculovirus expression system. Virus Res. 1990 Mar;15(3):197–211. doi: 10.1016/0168-1702(90)90028-a. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Characterization and molecular cloning of a human parvovirus genome. Science. 1984 Dec 7;226(4679):1161–1165. doi: 10.1126/science.6095448. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Frickhofen N., Young N. S. Persistent parvovirus B19 infections in humans. Microb Pathog. 1989 Nov;7(5):319–327. doi: 10.1016/0882-4010(89)90035-1. [DOI] [PubMed] [Google Scholar]
- Fridell E., Trojnar J., Wahren B. A new peptide for human parvovirus B19 antibody detection. Scand J Infect Dis. 1989;21(6):597–603. doi: 10.3109/00365548909021686. [DOI] [PubMed] [Google Scholar]
- Kajigaya S., Shimada T., Fujita S., Young N. S. A genetically engineered cell line that produces empty capsids of B19 (human) parvovirus. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7601–7605. doi: 10.1073/pnas.86.19.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman G. J., Cohen B. J., Field A. M., Oseas R., Blaese R. M., Young N. S. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J Clin Invest. 1989 Oct;84(4):1114–1123. doi: 10.1172/JCI114274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morinet F., D'Auriol L., Tratschin J. D., Galibert F. Expression of the human parvovirus B19 protein fused to protein A in Escherichia coli: recognition by IgM and IgG antibodies in human sera. J Gen Virol. 1989 Nov;70(Pt 11):3091–3097. doi: 10.1099/0022-1317-70-11-3091. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Kajigaya S., Shimada T., Young N. The gene encoding the nonstructural protein of B19 (human) parvovirus may be lethal in transfected cells. J Virol. 1988 Aug;62(8):2884–2889. doi: 10.1128/jvi.62.8.2884-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Productive infection by B19 parvovirus of human erythroid bone marrow cells in vitro. Blood. 1987 Aug;70(2):384–391. [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Replication of the B19 parvovirus in human bone marrow cell cultures. Science. 1986 Aug 22;233(4766):883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- Pintel D., Merchlinsky M. J., Ward D. C. Expression of minute virus of mice structural proteins in murine cell lines transformed by bovine papillomavirus-minute virus of mice plasmid chimera. J Virol. 1984 Nov;52(2):320–327. doi: 10.1128/jvi.52.2.320-327.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R., Linser P., Armentrout R. W. Kinetics of assembly of a parvovirus, minute virus of mice, in synchronized rat brain cells. J Virol. 1977 Jun;22(3):778–793. doi: 10.1128/jvi.22.3.778-793.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T. F., Roggendorf M., Deinhardt F. Human parvovirus B19: ELISA and immunoblot assays. J Virol Methods. 1988 Jun;20(2):155–168. doi: 10.1016/0166-0934(88)90149-8. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Ultrastructural studies of H-1 parvovirus replication. IV. Crystal development and structure with the temperature-sensitive mutant ts1. J Virol. 1977 Oct;24(1):343–352. doi: 10.1128/jvi.24.1.343-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Lu L. Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal human bone marrow. J Virol. 1988 Aug;62(8):3059–3063. doi: 10.1128/jvi.62.8.3059-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Ozawa K., Mitani K., Miyazono K., Asano S., Takaku F. B19 parvovirus replicates in erythroid leukemic cells in vitro. J Infect Dis. 1989 Sep;160(3):548–549. doi: 10.1093/infdis/160.3.548. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Ozawa K., Takahashi K., Asano S., Takaku F. Susceptibility of human erythropoietic cells to B19 parvovirus in vitro increases with differentiation. Blood. 1990 Feb 1;75(3):603–610. [PubMed] [Google Scholar]
- Yaegashi N., Shiraishi H., Takeshita T., Nakamura M., Yajima A., Sugamura K. Propagation of human parvovirus B19 in primary culture of erythroid lineage cells derived from fetal liver. J Virol. 1989 Jun;63(6):2422–2426. doi: 10.1128/jvi.63.6.2422-2426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]