Abstract
We recently reported the use of a gene-trapping approach to isolate cell clones in which a reporter gene had integrated into genes modulated by T-cell activation. We have now tested a panel of clones from that report and identified the one that responds to a variety of G-protein coupled receptors (GPCR). The β-lactamase tagged EGR-3 Jurkat cell was used to dissect specific GPCR signaling in vivo. Three GPCRs were studied, including the chemokine receptor CXCR4 (Gi-coupled) that was endogenously expressed, the platelet activation factor (PAF) receptor (Gq-coupled), and β2 adrenergic receptor (Gs-coupled) that was both stably transfected. Agonists for each receptor activated transcription of the β-lactamase tagged EGR-3 gene. Induction of EGR-3 through CXCR4 was blocked by pertussis toxin and PD58059, a specific inhibitor of MEK (MAPK/ERK kinase). Neither of these inhibitors blocked isoproterenol or PAF-mediated activation of EGR-3. Conversely, β2- and PAF-mediated EGR-3 activation was blocked by the p38, specific inhibitor SB580. In addition, both β2- and PAF-mediated EGR-3 activation could be synergistically activated by CXCR4 activation. This combined result indicates that EGR-3 can be activated through distinct signal transduction pathways by different GPCRs and that signals can be integrated and amplified to efficiently tune the level of activation.
Key words: functional genomics, GPCR signaling, CXCR4, EGR-3, MAPK
Introduction
A variety of extracellular stimuli can target transmembrane molecules to activate intracellular signal transduction. These signals are then transduced through the cell by signaling cascades to regulate cellular processes such as differentiation, proliferation and apoptosis 1., 2.. One specific intracellular event is the activation of mitogen-activated protein kinases (MAPKs; ref. 3) which are divided into three major classes: MAPK/ERK, JNK/SAPK, and p38 MAPK (4). These three classes of MAPKs are likely to work independently and synergistically to regulate cellular processes. MAPK/ERK is commonly stimulated by growth factors and cytokines, and phosphorylates various target proteins to transmit signals and regulate cellular events. JNK/SAPK is activated by stresses such as osmotic shock and ultraviolet radiation, and leads to phosphorylation of C-Jun, a component of AP-1 (5), which subsequently activates transcription. The third class including p38 MAPK was identified as the target of lipopolysaccharide (LPS) treatment (6), but targets of p38 MAPK are currently unknown.
Recently, a number of receptors that couple to heterotrimeric G-proteins (guanine nucleotide-binding regulatory proteins) have been shown to stimulate MAPKs 7., 8. through differential coupling of their various G-protein subunits to specific MAPKs (9). It is therefore possible that MAPK activation by GPCRs (G-protein coupled receptors) can be proceed by multiple signal transduction pathways, leading to the activation of specific downstream genes and the desired biological response. Since the identification of genes that are regulated by specific stimuli is of fundamental importance in understanding cellular processes, much effort has been made to develop an effective approach to identify these genes and their mechanism of activation. In MAPK signaling, cross talk between pathways occurs in many cases, making the study of specific functions of certain genes a difficult task.
In our previous paper (10), we described a functional genomic assay to isolate cell clones and identify genes responsive to a specific signal transduction pathway. Here we report the use of one clone (JTIC-3) generated with this approach to study specific gene regulation activated by GPCR signaling. We show how these three GPCRs, which are coupled to one of the three major G-protein α-subunits Gq, Gs, or Gi, induce activation of a common transcription factor through MAPK signaling pathways.
Results and Discussion
GPCRs modulate transcription of β-lactamase tagged EGR-3
Gene trapping is a powerful approach for the study of gene regulation and function. In our previous report, we combined flow cytometry and gene trapping in live cells to isolate cell clones induced by a specific stimuli (T-cell activation). The signal transduction of the cell clones isolated by this method was shown to be dependent on various second messenger cascades including Ca2+ and PKC (protein kinase C). Specifically, all clones were isolated for their activation by PHA (phytohemagglutinin), but individual clones showed selective activation by PMA (phorbol myristate acetate), thapsigargin, or PMA+thapsigargin. A panel of these clones with robust signal, low background and varied dependence for stimuli was selected to test for activation by selected GPCRs. One specific clone (JTIC-3) responded to a variety of GPCRs at different levels. This clone, JTIC-3, has the β-lactamase reporter integrated into and functionally tagged on the EGR-3 gene 11., 12.. EGR-3 and related proteins are a class of Zinc finger transcription factors that recognize the same consensus DNA sequence. The transcription activity of EGR-3 is highly concentrated in the CNS (central nervous system; ref. 13). It was previously showed that EGR-3 was activated by T-cell receptor activation. In addition, several reports from experiments in mice have shown that mouse analogs egr-1, egr-2, egr-3, and egr-4 signal transduction pathways are connected with some GPCRs 14., 15..
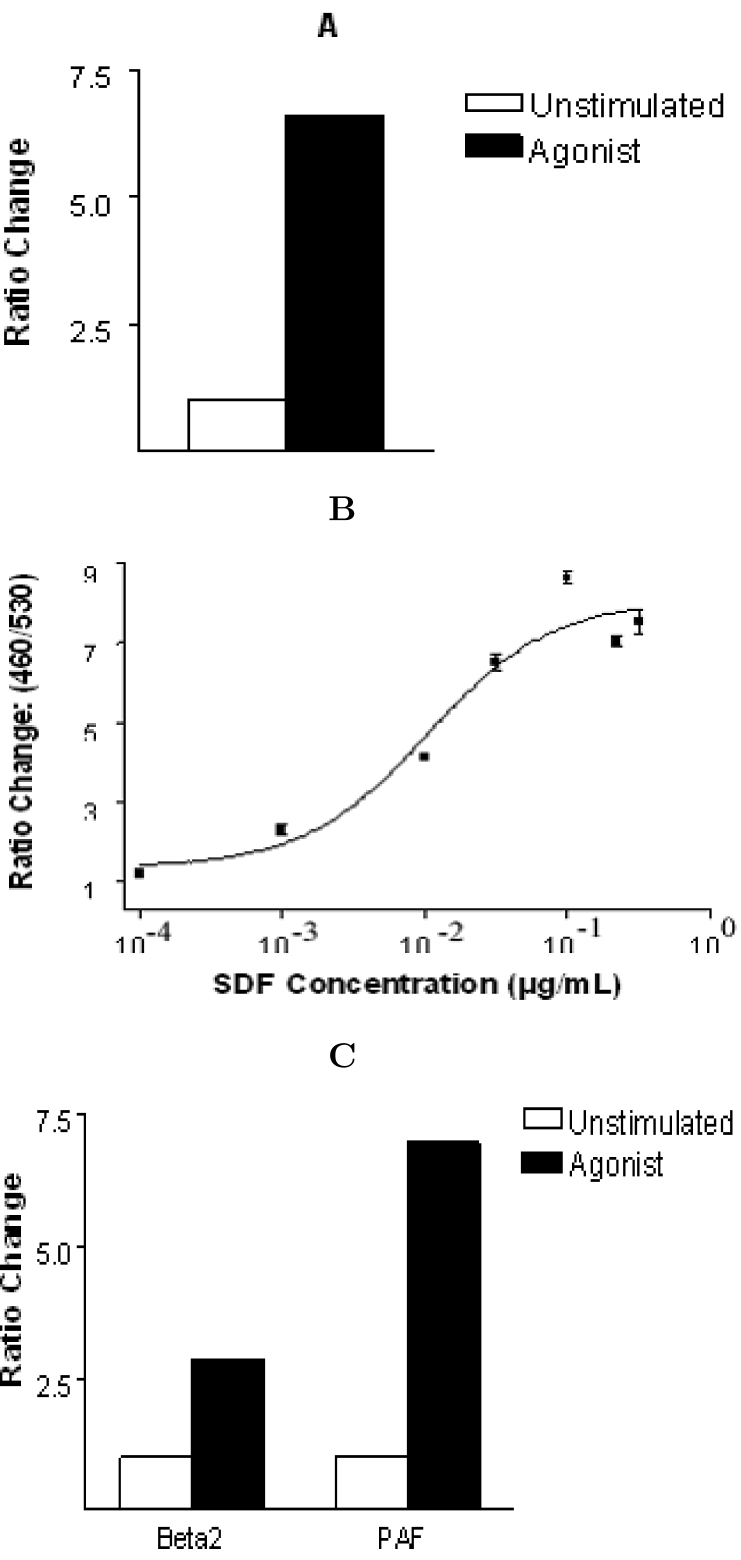
Recently, CXCR4 was identified as a native receptor for chemokine peptide stromal cell-derived factor 1 (SDF-1; ref. 16., 17.). This receptor is expressed endogenously in Jurkat cells and has recently been shown to function as a co-receptor for lymphocyte-tropic HIV-1 required for cellular infection (18). When human SDF-1 was tested on Jurkat cells or on a CHO (Chinese hamster ovary) cell clone stably expressing CXCR4, a transient rise of cytosolic free Ca2+ was observed. When we tested the activation of JTIC-3 by different GPCRs, CXCR4 was one of the receptors to activate EGR-3 when stimulated with SDFα peptide, an active fragment of SDF-1 (Figure 1A). The activation of the EGR-3 gene was in a dose-dependent manner with an EC50 of ~10 nM (Figure 1B). This result indicated that besides T-cell stimulation shown in prior reports, SDF-1 could also induce transcription of EGR-3. Interestingly, SDF-1 has been shown to inhibit infection by HIV-1 and this inhibition is believed to occur through SDF-1 competition with viruses for binding to CXCR4 16., 17.. Because of this, the JTIC-3 cell line may be useful to identify inhibitors or analogs of SDF-1 that can inhibit HIV-1 infection.
Fig. 1.
β-lactamase reporter gene activation by GPCRs expressed endogenously or exogenously in genomic clone JTIC-3. T cell endogenous CXC-4 receptor, or stably transfected PAF receptor, β2 receptor in JTIC-3 was stimulated with (solid bar) or without (open bar) their respective agonist, with 0.1 μg/mL SDFα (A), 1 μΜ PAF or 10 μM isoproterenol (C). All the cells were plated in 96-well plates at 100,000 cells per well. After 6 h incubation with agonist at 37°C, the cells were loaded with 1 μΜ CCF2/AM at room temperature for 1 h and then read on a Cytofluor 4000 fluorescence plate reader, with excitation 390 nm, emission 460 (blue) and 530 (green). Ratio changes of 460 vs 530 were used to determine β-lactamase activity. Bars represent means ± S.D. for triplicate samples. EC50 value for SDFα dose response (B) was determined using PrismTM software.
For assessing the dependence of EGR-3 induction by GPCRs that are not natively expressed in Jurkat cells, we generated stable transfect cell lines derived from JTIC-3 expressing an exogenous receptor. JTIC-3 was transfected with the PAFR (platelet activation factor receptor) and β2R so that one member of each of the three classes of GPCRs (Gi, Gs, and Gq) was represented. Individual cell clones responding to PAF and β2 receptor agonist isoproterenol were isolated using flow cytometry followed by testing for activation by their respective agonist. The results showed that β2 receptor activated the β-lactamase tagged EGR-3 less than CXCR4 or PAFR (Figure 1C). To confirm the specific involvement of β2 receptor, we used two potent β2 receptor antagonists, ICI-158 and proprenolol, to perform inhibition assays. Both antagonists totally blocked the isoproterenol mediated β-lactamase expression (data not shown). This result indicated that the isoproterenol induced activation of EGR-3 is specifically mediated by β2 receptor. Taken together, the above results demonstrate that all three classes of GPCRs (Gi, Gq, and Gs) can activate the transcription of EGR-3.
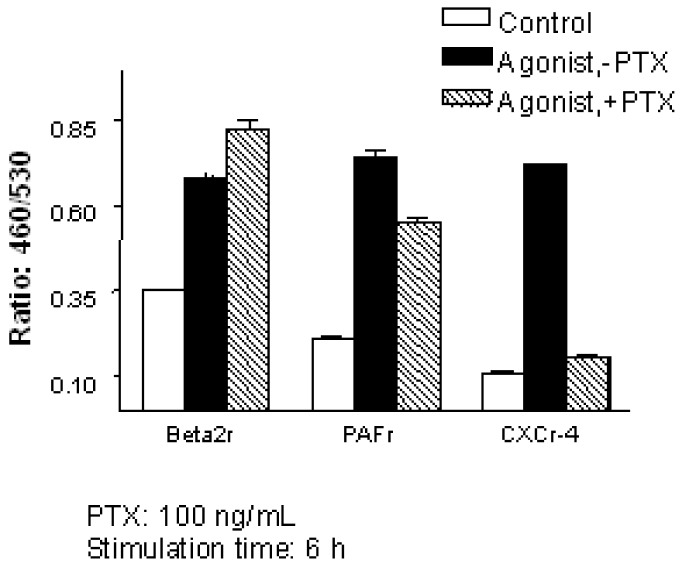
To investigate which receptor-linked effector might be responsible for EGR-3 activation, we also examined the effects of various inhibitors on the GPCR-linked activation. PTX totally blocked the EGR-3 activation induced by SDF-1 (Figure 2), but had no effect on the EGR-3 activation induced by PAF or isoproterenol. PTX (pertussis toxin) is known to induce the ADP-ribosylation of Gi/Go subunits of the G-protein complex, thus preventing the dissociation of Gαi/o and β/γ subunits (1). This result indicated that as expected, the PTX-sensitive Gαi/o was involved in the EGR-3 activation mediated by SDF-1.
Fig. 2.
Effect of PTX on different GPCR mediated signal transduction. Individual cell line was pre-incubated in the presence or absence of 100 ng/mL PTX overnight. The cells were then stimulated with their respective agonist (1 μΜ PAF, 10 μΜ isoproterenol, 0.1 μg/mL SDFα). Cell stimulation and CCF-2 dye loading condition are the same as described in Figure 1.
It has been reported that Gi and Gs coupled receptors activate MAPK pathway through Gβγ. To confirm the role of Gα in MAPK signaling, we transfected the constitutively activated Gαq, Gαs, and Gαi mutants into our reporter cell line transiently. The Gαq mutant activated EGR-3 reporter gene expression while Gαs and Gαi mutants failed to show any effect (data not shown). This result, together with the result from PTX assay, suggested that GβΥ subunits may be responsible for the Gs and Gi coupled receptor activation of EGR-3.
GPCRs activate EGR-3 expression through various MAPK pathways
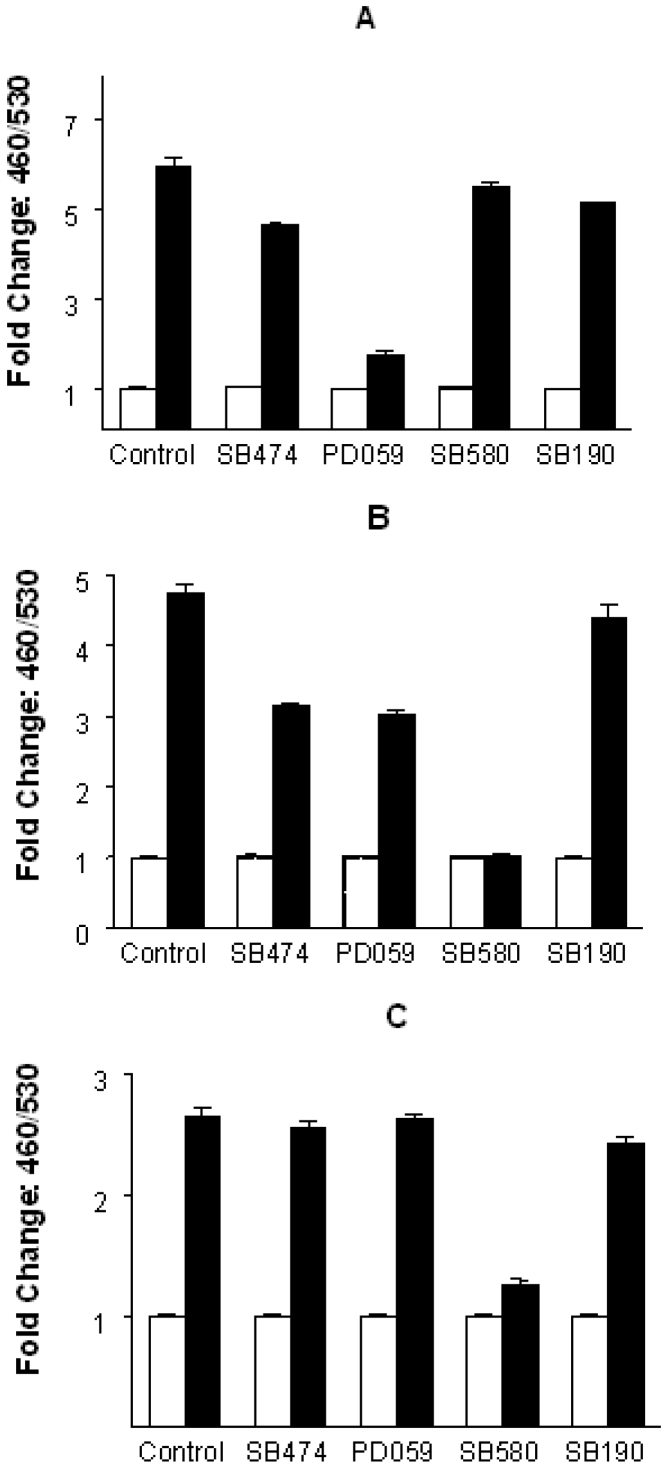
MAPKs are activated in response to diverse extracellular signals. They then phosphorylate a variety of intracellular substrates including other kinases and transcription factors, which in turn modulate the expression of target genes implicated in cell growth 4., 19.. It has recently become apparent that GPCRs can induce intracellular signal transduction through MAPK pathways 9., 20.. It is therefore quite logical to investigate the involvement of MAPKs in EGR-3 activation. For this analysis we used the JTIC-3 clone and its subclones that had been transfected with the PAF or β2 receptor. In Figure 3, we show the inhibition profiles of different GPCR-mediated transcription activity by different MAPK inhibitors. Compound PD58059, which is specific for MEK (MAPK/ERK kinase), blocked SDFα-induced β-lactamase induction (Figure 3A), but failed to block β2- and PAF-receptor induced β-lactamase induction. Conversely, the p38 specific inhibitor SB580 blocked β2- and PAF-receptor mediated activation of EGR-3 (Figure 3B and 3C), but failed to act on SDF induced activation. These results indicate that all the three GPCRs activate EGR-3 transcription through MAPK pathways, but the CXCR4 pathway is distinct from the pathway used by PAF and β2 receptors. When some GPCRs were found to activate p38 MAPK, one question that remained to be answered was whether all GPCRs elicit their signals to p38 (21). Our results indicate that GPCR signals could go through diversified MAPK pathways, but may result in regulating the same cellular target. The significance of the diversity of MAPK activation by different GPCRs should be further investigated.
Fig. 3.
Inhibition of GPCR-MAPK mediated signal transduction. Individual cell line was pre-incubated in the presence of different MAPK inhibitor including SB202190, SB202358, PD98059 or negative inhibitor control SB202474 at 37°C overnight (see Materials and Methods). The cells were then stimulated with their respective agonist (1 μM PAF, 10 μM isoproterenol, 0.1 μg/mL SDF). Cell stimulation and CCF-2 dye loading condition are the same as described in Figure 1.
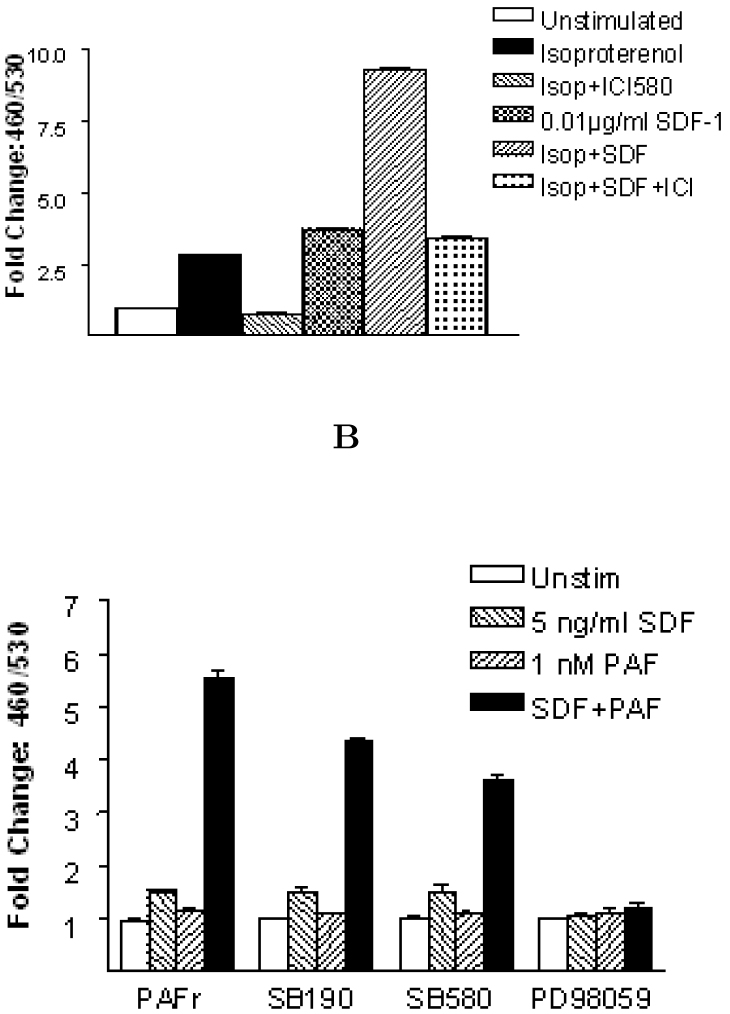
Synergistic effects in MAPK cascades
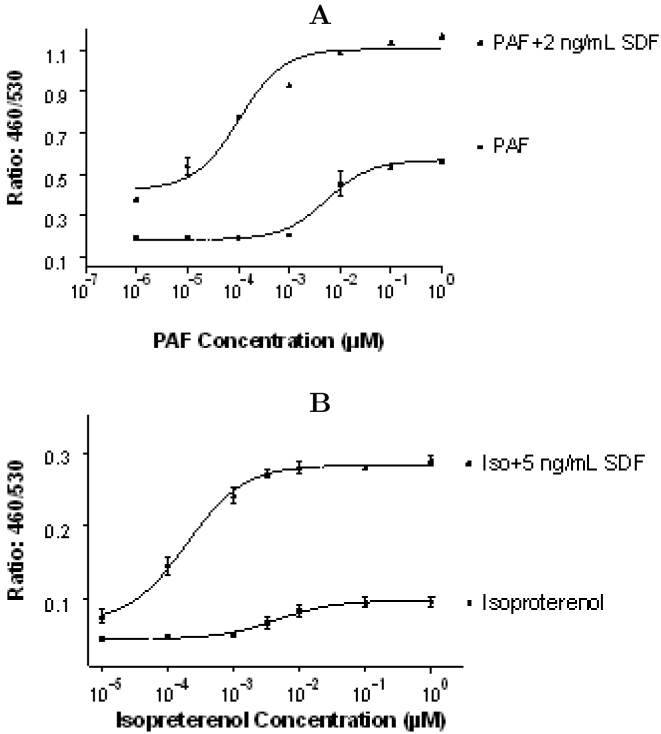
Even though different MAPK subgroups are activated through distinct, sometime partially overlapping cascades, there are cases when MAPKs can respond synergistically to different upstream signals, resulting in the integration of effects from distinct stimuli 22., 23.. To test possible synergistic effects among GPCRs on MAPK signaling pathways, we co-stimulated the JTIC-3/PAFR, JTIC-3/β2R clones with sub-threshold concentration of SDFα and various concentration of PAF or isoproterenol respectively. In the presence of minimum amounts of SDFα, both isoproterenol and PAF treatment resulted in a significant synergistic activation of EGR-3 (Figure 4A and 4B). Treatment of JTIC-3/β2R clone with β2 receptor antagonist ICI-158 inhibited the synergistic effect (Figure 5A). On the other hand, treatment of JTIC-3/PAFR clone with MAPK inhibitor PD98059 (specific to CXCR4-induced MAPK signaling) totally abolished the synergistic effect of SDFα and PAF, and the effects were also partially blocked by MAPK inhibitor SB203580 (specific to PAFR-induced MAPK signaling; Figure 5B). This experiment clearly indicated that the synergy of different GPCRs in cellular signaling activities and the activation of EGR-3 were transmitted from integration of multiple MAPK pathways. Synergistic activation of MAPKs by different stimuli has been reported for various physiological functions such as regulating intracellular signaling and correlating the cell growth (23). The significance of synergistic effects applied by multiple GPCRs has not been clearly studied.
Fig. 4.
Synergistic effect of GPCR-MAPK mediated signal transduction. A. Stimulation of cells with different concentration of PAF in the presence or absence of minimum amount of SDF peptide. B. Stimulation of cells with different concentration of isoproterenol in the presence or absence of minimum amount of SDF peptide. Cell stimulation and CCF-2 dye loading condition are the same as described in Figure 1.
Fig. 5.
A. β2 receptor specific response and synergistic effect of β2R and CXCR4 can be blocked by β2 receptor antagonist ICI-158. The cells were pre-incubated with ICI-158 for 1 h before agonist stimulation. B. Inhibition of synergistic effect of PAFR and CXCR4 by specific MAPK inhibitor PD98059. Cell stimulation and CCF-2 dye loading condition are the same as described in Figure 1.
Using our gene-trapping technique, we have established that EGR-3 is a target of MAPK activation. The illustration demonstrates the relationship between different GPCRs/MAPKs to enhance our current understanding of how cells are able to respond coordinately to diverse extra-cellular signals. Before these findings, very few downstream targets for p38 MAPK had been identified. One of the p38 MAPK isoforms, p38b, which has 74% sequence homology to p38, displays activity toward activating transcription factor 2 (ATF2; ref 24), while p38 shows little effect on this transcription factor. Whether the EGR-3 activation was due to p38 or p38b remains to be seen. The p38 protein is found to be a major tyrosine-phosphorylated protein following LPS treatment (25). It has been suggested that the p38 MAPK pathway may be involved in the inhibition of cell growth and the promotion of cell death (26). Involvement of GPCRs in the p38 regulation may provide a new insight into the function of both p38 and GPCRs.
Materials and Methods
Materials
SDF-1α was from R&D Systems (Minneapolis, USA). Isoproterenol, PAF, MAP Kinase Inhibitor Set, Phorbol-12-myristate-13-acetate were from Calbiochem (San Diego, USA). PTX and β2 receptor antagonist ICI-158 were from RBI (Natick, USA). Phytohemagglutinin-M, RPMI 1640 media, heat inactivated FBS, were from Gibco/BRL (Gaithersburg, USA). Human β2 receptor expression plasmid (pβ2r) was from Dr. M. Simon. Human PAFR expression plasmid (pPAFR) was provided by Dr. R. Yeh.
Genomic clone JTIC-3
The method to generate clone JTIC-3 in which endogenous EGR-3 was functionally tagged with a β-lactamase reporter is as described by Whitney et al. (10). Briefly, a cell library was created using a gene-trapping approach to have exogenous gene (promoterless β-lactamase reporter gene) spliced into an endogenous gene and generate a fusion RNA when the gene trap vector has properly inserted into a host intron. The plasmid vector GAS-1 was introduced into Jurkat cells by electroporation and subsequent antibiotic selection. The entire Jurkat cell pool, with at least one million independent cell clones, was then treated with PHA. Flow cytometry was used to isolate cell clones in which β-lactamase had inserted into a PHA inducible gene. Individual cell clones were then expanded and characterized, and reporter genes were identified.
Cell culture, transfection, and clone selection
Jurkat cells were maintained in RPMI medium supplemented with 10% fetal bovine serum. JTIC-3 cells were transfected with pPAFr and pβ2r via electroporation. The transfected cells were then transferred into normal culture medium and two days later, replaced with a medium containing 150 μg/mL zeocin. After about a two-week selection, a total of 5-10×6 cells were stimulated with 10 μM isoproterenol or 1 μM PAF at 37°C for 6 h and were then loaded with 1 μM CCF2/AM at room temperature for 1 h. Flow cytometry was conducted using a Becton Dickinson FACS VantageTM with an argon laser producing 351-364 nm multi-line UV excitation. Fluorescence emission was detected via 460/50 (blue) and 535/40 (green) emission filters. Individual blue cells were identified and single cells were dispensed into 96-well micro-titer plates using CloneCyt on the FACS VantageTM. Clones were subsequently screened, selected, and expanded.
β-lactamase reporter gene assay
Cells with the β-lactamase reporter gene and endogenous or exogenous receptor were plated into 96-well plates at 100,000 cells per well. CXCR4 receptor was stimulated with 0.5 μg/mL SDFα; PAF receptor was stimulated with 1 μM PAF; β2 receptor was stimulated with 1 μM isoproteronol. Each receptor was stimulated at 37°C for 6 h. The cells were loaded for 1 h with 1 μM CCF-2/AM and were read on a Cytofluor plate reader. β-lactamase enzyme levels were determined by comparing the fluorescence emission ratio changes of 460 nm vs 530 nm for unstimulated and stimulated cells.
Inhibition of β-lactamase reporter gene expression by MAPK inhibitors
Cells were pre-incubated in the presence or absence of 10 μM SB202190, 20 μM SB203580, 10 μM SB202474 or 10 μM PD58019 in culture medium overnight, and were then spun down and re-suspended in serum-free medium containing the same amount of inhibitor. Reporter gene assay for stimulated or unstimulated cells is the same as described above.
Effects of PTX
Cells were pre-incubated in the presence or absence of 100 μg/mL PTX overnight, and then were spun down by centrifugation and were re-suspended in assay medium with or without PTX. Reporter gene assay for stimulated or unstimulated cells is the same as described above.
References
- 1.Casey P.J., Gilman A.G. G protein involvement in receptor-effector coupling. J. Biol. Chem. 1988;263:2577–2580. [PubMed] [Google Scholar]
- 2.Loetscher M. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J. Biol. Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- 3.Blumer K.J. Mammalian mitogen-activated protein kinase kinase kinase (MEKK) can function in a yeast mitogen-activated protein kinase pathway downstream of protein kinase C. Proc. Natl. Acad. Sci. USA. 1994;91:4925–4929. doi: 10.1073/pnas.91.11.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su B., Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 5.Davis R.J. MAPKs: new JNK expands the group. Trends Biochem. Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 6.Rouse. J. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 7.Koch W.J. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J. Biol. Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 8.Crespo P. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 9.Faure M. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J. Biol. Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- 10.Whitney M. A genome-wide functional assay of signal transduction in living mammalian cells. Nat. Biotechnol. 1998;16:1329–1333. doi: 10.1038/4302. [DOI] [PubMed] [Google Scholar]
- 11.Mages H.W. Expression of PILOT, a putative transcription factor, requires two signals and is cyclosporin A sensitive in T cells. Int. Immunol. 1993;5:63–70. doi: 10.1093/intimm/5.1.63. [DOI] [PubMed] [Google Scholar]
- 12.Patwardhan S. EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene. 1991;6:917–928. [PubMed] [Google Scholar]
- 13.Beckmann A.M. Differential expression of Egr-1-like DNA-binding activities in the naive rat brain and after excitatory stimulation. J. Neurochem. 1997;69:2227–2237. doi: 10.1046/j.1471-4159.1997.69062227.x. [DOI] [PubMed] [Google Scholar]
- 14.Bouaboula M. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem. J. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von der Kammer H. Muscarinic acetylcholine receptors activate expression of the EGR gene family of transcription factors. J. Biol. Chem. 1998;273:14538–14544. doi: 10.1074/jbc.273.23.14538. [DOI] [PubMed] [Google Scholar]
- 16.Oberlin E. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 17.Bleul C.C. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J. Exp. Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Y. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 19.Waskiewicz A.J., Cooper J.A. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr. Opin. Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 20.Hawes B.E. Distinct pathways of Gi- and Gq-mediated mitogen-activated protein kinase activation. J. Biol. Chem. 1995;270:17148–17153. doi: 10.1074/jbc.270.29.17148. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi J. Activation of p38 mitogen-activated protein kinase by signaling through G protein-coupled receptors. Involvement of Gbetagamma and Galphaq/11 subunits. J. Biol. Chem. 1997;272:27771–27777. doi: 10.1074/jbc.272.44.27771. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Smithgall T.E. Fibroblast transformation by Fps/Fes tyrosine kinases requires Ras, Rac, and Cdc42 and induces extracellular signal-regulated and c-Jun N-terminal kinase activation. J. Biol. Chem. 1998;273:13828–13834. doi: 10.1074/jbc.273.22.13828. [DOI] [PubMed] [Google Scholar]
- 23.Sui X. Synergistic activation of MAP kinase (ERK1/2) by erythropoietin and stem cell factor is essential for expanded erythropoiesis. Blood. 1998;92:1142–1149. [PubMed] [Google Scholar]
- 24.Jiang Y. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta) J. Biol. Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 25.Han J. Ulevitch RJ, Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J. Biol. Chem. 1993;268:25009–25014. [PubMed] [Google Scholar]
- 26.Kyriakis J.M., Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]