Abstract
Nutrients may affect the invasiveness of alien plants and the invasibility of native plant communities. We performed a greenhouse experiment to investigate the interactive effect of invasion by a clonal herb Hydrocotyle vulgaris and nutrient enrichment on biomass and evenness of native plant communities. We established three types of plant communities (H. vulgaris alone, native plant communities without or with H. vulgaris) under low and high levels of nutrients. Native communities consisted of eight native, terrestrial species of three functional groups, i.e. four grasses, two legumes, and two forbs. Invasion of H. vulgaris had no effect on biomass of the native community, the functional groups, or the individual species. High nutrients increased biomass of grasses, but reduced evenness of the community. High nutrients also decreased the competitive effect, and the relative dominance index of H. vulgaris. Therefore, high nutrients reduced the competitive ability of H. vulgaris and enhanced the resistance of the native community to invasion. The results provide a basis for management strategies to control the invasion and spread of H. vulgaris by manipulating resource availability to support native communities.
Biological invasion has become a serious ecological problem1. Invasion of plant species can decrease native biodiversity1,2 and alter community structure3, biogeochemical cycles4 and ecosystem services1. In the past few decades, the number of introduced invasive plant species has increased dramatically5, and many of them are clonal plants6,7,8. Nutrient enrichment as a result of anthropogenic landscape modifications has also become widely recognized as a serious threat to biodiversity maintenance and ecosystem functioning9,10. Although many studies have investigated responses of invasive plants and native plant communities to nutrient enrichment11,12, impacts of nutrient enrichment on the interaction between invasive plants and native plant communities remains an unresolved issue in invasion biology13,14,15.
The invasibility of native communities depends on biotic factors16,17. For example, native plants can create spatial heterogeneity in soil nutrients18 or act as physical obstacles, blocking the spread of the belowground rhizomes and tubers of invasive clonal plants19,20. The invasibility of native communities also depends on the functional similarity between the invasive species and the dominant species of the native communities21. Native species with ecological characteristics that are similar to those of invasive species tend to resist invasions more strongly than those exhibit distinct characteristics because they have a higher niche overlap and thus a higher demand for the same resources22,23.
Abiotic factors, such as resource enrichment, can confer invasive species with advantages over native species24. Nutrients are an important resource and may therefore affect the invasibility of native plant communities and the invasiveness of exotic species25,26. Many studies have shown that the invasion success of exotic plants can be enhanced by enrichment of nutrients that are limiting27. For example, nitrogen addition enhanced richness and abundance of invasive annual herbs10,11, promoted the spread of introduced plants into terrestrial habitats12, but increased the resistance of resident communities to the invasion by Bromus tectorum28. Thus, different habitat-dependent pathways mediate interactions between invasion and nutrient enrichment to drive community change29.
Nutrients are correlated with primary production of ecosystems at large scales because nitrogen and phosphorus are limiting in most freshwater, marine, and terrestrial ecosystems26,30. Post-industrial anthropogenic activities have amplified nitrogen and phosphorus cycles by 100% and 400%, respectively30, and rates of nutrient deposition have increased dramatically31. Higher rates of atmospheric nutrient deposition can enhance plant invasion32. Native species have different nutrient-acquisition strategies and are key functional components of vegetation. Changes in functional group representation can disrupt key ecosystem function such as productivity. Nutrient enrichment may favor invasive species over native species and thus change the structure and function of communities. However, the complex associations among nutrient enrichment, plant species invasion, and specific invasion patterns are unclear33,34.
We conducted a greenhouse experiment to test whether high nutrient availability would promote the invasion of a clonal exotic plant species Hydrocotyle vulgaris into a terrestrial plant community. We simulated the invasion of H. vulgaris into a community consisting of eight native terrestrial plant species of three functional groups (grasses, legumes, and forbs) under two levels of nutrient availability. Specifically, we addressed four questions. (1) Does nutrient availability affect the invasiveness of H. vulgaris? (2) Does increasing nutrient availability decrease the invasibility of the native communities? (3) Does nutrient availability affect performance of the functional groups of the plant community? (4) Does the interaction between the invasive plant species and the native community rely on the nutrient availability?
Results
The growth of H. vulgaris
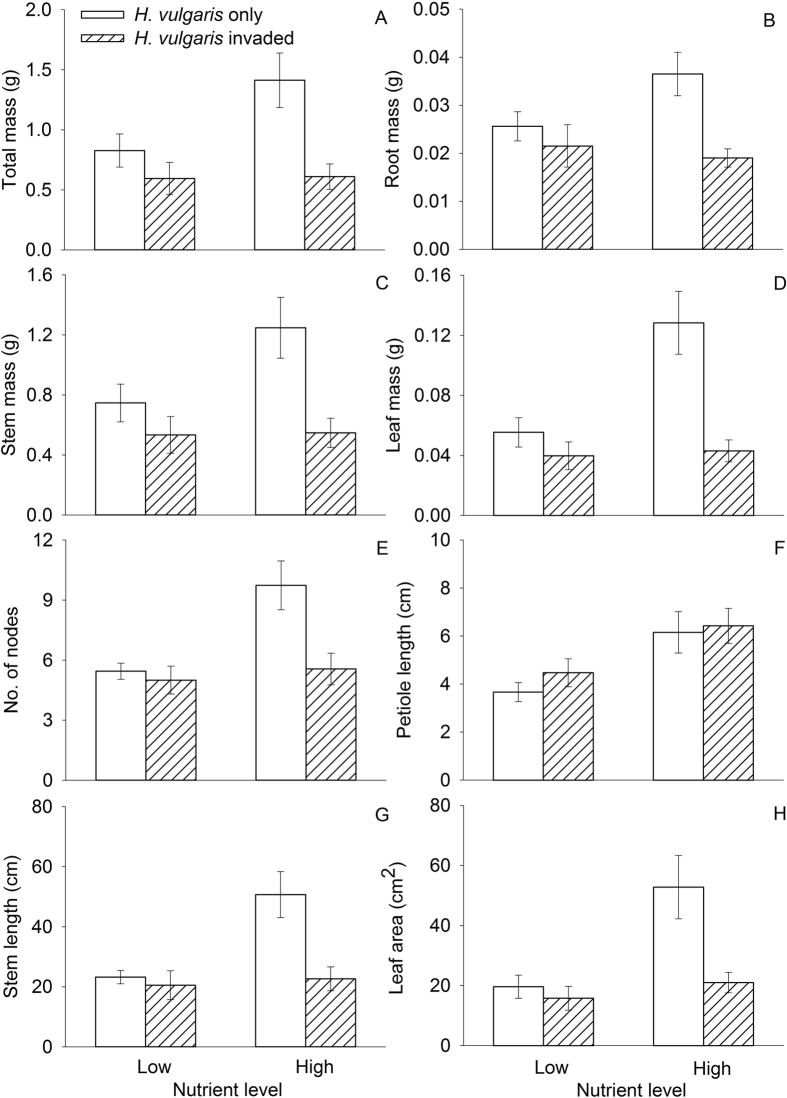
The presence of the native plant community significantly decreased in all traits of H. vulgaris measured, except stem mass and petiole length. Nutrient enrichment also increased all traits, except total mass and root mass (Fig. 1, Table 1). There were interactive effects of native plant communities and nutrient enrichment on leaf mass, number of nodes, and stem length of H. vulgaris. Nutrient enrichment significantly increased these three traits when H. vulgaris grew alone, but had little effect when it grew with the native plant community (Fig. 1D,E and G, Table 1).
Figure 1.
Effects of plant community and nutrient level on the growth of H. vulgaris (mean ± SE): (A) total mass; (B) root mass; (C) stem mass; (D) leaf mass; (E) number of nodes; (F) petiole length; (G) stem length; and (H) leaf area. See Table 1 for ANOVA summaries.
Table 1. Summary of ANOVAs for the effects of plant community and nutrient level on the growth of Hydrocotyle vulgaris.
| Traits | Plant community (P) | Nutrient (N) | P × N | |||
|---|---|---|---|---|---|---|
| F1,20 | P | F1,20 | P | F1,20 | P | |
| Total mass | 10.72 | 0.004 | 3.60 | 0.072 | 3.25 | 0.087 |
| Root mass | 8.77 | 0.008 | 1.34 | 0.261 | 3.37 | 0.081 |
| Stem mass | 3.28 | 0.085 | 10.28 | 0.004 | 2.93 | 0.103 |
| Leaf massa | 14.14 | 0.001 | 7.36 | 0.013 | 4.72 | 0.042 |
| No. of nodes | 7.73 | 0.012 | 8.49 | 0.009 | 5.05 | 0.036 |
| Petiole lengthb | 0.65 | 0.429 | 11.19 | 0.003 | 1.61 | 0.692 |
| Stem lengtha | 9.42 | 0.006 | 7.66 | 0.012 | 4.69 | 0.043 |
| Leaf areaa | 7.66 | 0.012 | 9.55 | 0.006 | 3.44 | 0.079 |
Values are in bold if P < 0.01 and in italics if P < 0.05. See Fig. 1 for graphical representation of data.
aIndicates log-transformed data.
bIndicates square root-transformed data.
Responses of communities, functional groups, and species
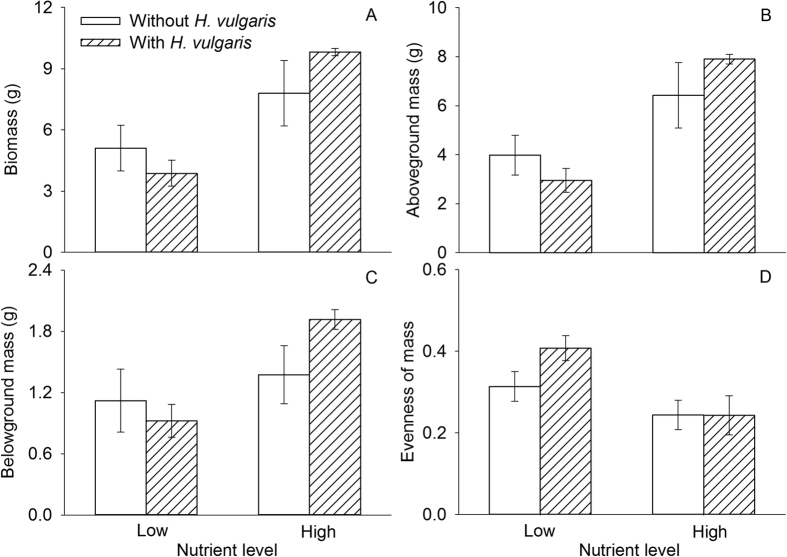
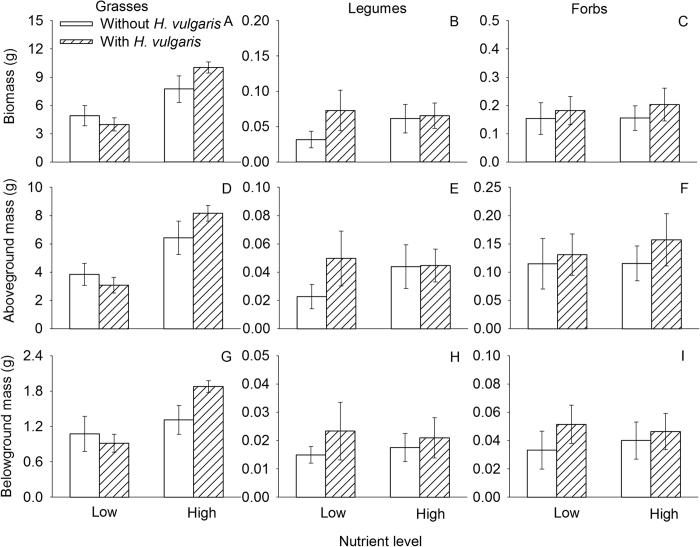
Invasion by H. vulgaris significantly affected none of the indexes of the native community, but nutrient enrichment significantly increased its biomass and decreased its evenness (Fig. 2A–D, Table 2A). Grasses were the dominant functional group of the native communities and their mass constituted over 90% mass of the native communities (Fig. 3). Invasion by H. vulgaris affected none of the parameters of any of the functional groups (Table 3). Nutrient enrichment significantly increased biomass (total, aboveground, and underground) of grasses, but had no effect on that of legumes or forbs.
Figure 2.
Effects of H. vulgaris invasion and nutrient level on measure of the native plant communities (mean ± SE): (A) total mass; (B) aboveground mass; (C) belowground mass; and (D) evenness of mass. See Table 2A for ANOVA summaries.
Table 2. Summary of ANOVAs for the effects of Hydrocotyle vulgaris invasion and nutrient level on biomass and evenness of the native communities (A) and biomass of each native species (B).
| Traits | Invasion (I) | Nutrient (N) | I × N | |||
|---|---|---|---|---|---|---|
| F1,20 | P | F1,20 | P | F1,20 | P | |
| (A) Native plant communities | ||||||
| Total massb | 0.06 | 0.808 | 17.84 | 0.001 | 3.05 | 0.100 |
| Aboveground massb | 0.01 | 0.942 | 20.41 | <0.001 | 3.17 | 0.094 |
| Underground mass | 0.55 | 0.467 | 7.35 | 0.015 | 2.58 | 0.128 |
| Evenness of mass | 1.49 | 0.240 | 9.39 | 0.007 | 1.53 | 0.233 |
| (B) Native species | ||||||
| Setaria viridis | 0.05 | 0.819 | 19.21 | <0.001 | 2.25 | 0.152 |
| Festuca arundinacea | 0.01 | 0.930 | 0.48 | 0.495 | 0.76 | 0.397 |
| Poa pratensis | 0.03 | 0.859 | 2.46 | 0.136 | 0.75 | 0.398 |
| Bromus inermis | 0.31 | 0.588 | 0.25 | 0.623 | 1.60 | 0.224 |
| Trifolium repens | 1.17 | 0.293 | 0.02 | 0.889 | 0.36 | 0.556 |
| Astragalus sinicus | 4.78 | 0.049 | 0.10 | 0.756 | 0.20 | 0.661 |
| Plantago asiatica | 1.89 | 0.188 | 0.84 | 0.374 | 0.14 | 0.711 |
| Oxalis corniculata | 1.12 | 0.305 | 0.93 | 0.350 | 0.00 | 0.986 |
Figure 3. Effects of H. vulgaris invasion and nutrient level on biomass of functional groups (mean ± SE).
(A,B and C) total mass; (D,E and F) aboveground mass; and (G,H and I) belowground mass. See Table 3 for ANOVA summaries.
Table 3. Summary of ANOVAs for the effects of Hydrocotyle vulgaris invasion and nutrient level on biomass of each functional group.
| Traits | Invasion (I) | Nutrient (N) | I × N | |||
|---|---|---|---|---|---|---|
| F1,20 | P | F1,20 | P | F1,20 | P | |
| (A) Grasses | ||||||
| Total mass | 0.54 | 0.470 | 22.82 | <0.001 | 3.01 | 0.100 |
| Aboveground mass | 0.40 | 0.533 | 25.61 | <0.001 | 2.69 | 0.117 |
| Underground mass | 1.10 | 0.307 | 9.63 | 0.006 | 3.51 | 0.077 |
| (B) Legumes | ||||||
| Total mass | 1.13 | 0.302 | 0.27 | 0.609 | 0.75 | 0.397 |
| Aboveground mass | 0.87 | 0.364 | 0.30 | 0.590 | 0.78 | 0.388 |
| Underground mass | 0.69 | 0.416 | 0.00 | 0.991 | 0.12 | 0.732 |
| (C) Forbs | ||||||
| Total mass | 0.54 | 0.473 | 0.05 | 0.832 | 0.03 | 0.856 |
| Aboveground mass | 0.52 | 0.479 | 0.11 | 0.741 | 0.10 | 0.753 |
| Underground mass | 0.86 | 0.366 | 0.00 | 0.953 | 0.21 | 0.655 |
Values are in bold if P < 0.01 and in italics if P < 0.05. See Fig. 3 for data.
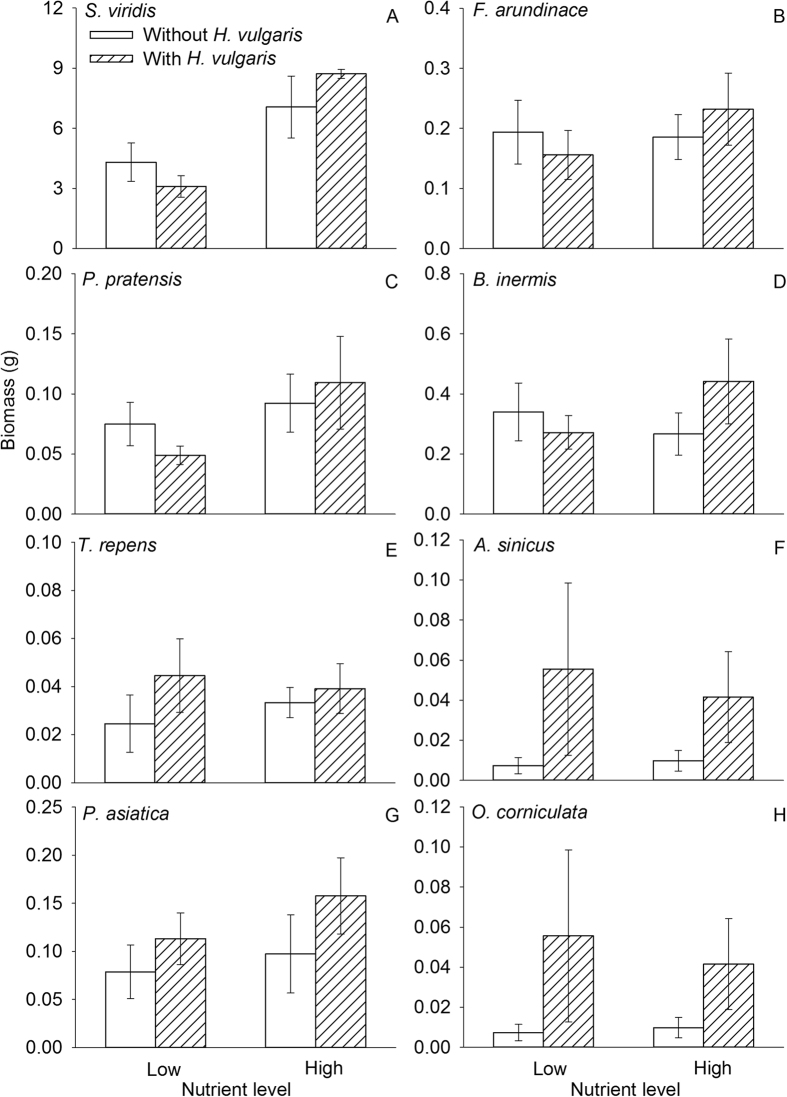
At the species level, S. viridis exhibited the highest mass (Fig. 4A). Invasion by H. vulgaris had no effect on biomass of any native species, while nutrient enrichment significantly increased biomass of S. viridis (Fig. 4, Table 2B). Neither invasion nor nutrient enrichment had a significant effect on seedling establishment of any species, except for A. sinicus whose seedling establishment increased due to the presence of H. vulgaris (Supplementary Table S1 and Figure S1).
Figure 4. Effects of H. vulgaris invasion and nutrient level on total mass of each of eight species (mean ± SE).
See Table 2B for ANOVA summaries.
Interactions between H. vulgaris and the native plant community
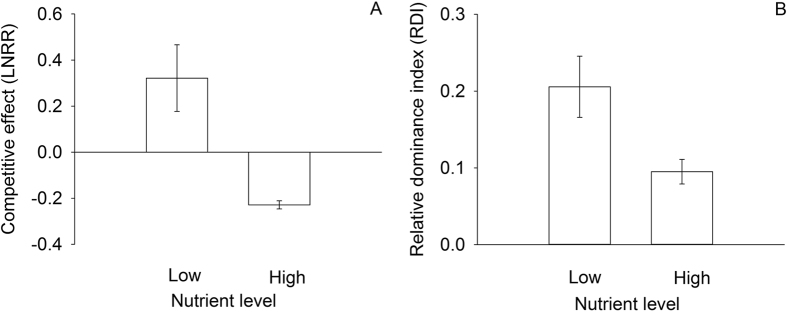
Nutrient enrichment significantly decreased the community-level competitive effect of H. vulgaris (F1,10 = 14.27, P = 0.005) and relative dominance index of H. vulgaris (F1,10 = 6.61, P = 0.027; Fig. 5). Nutrient enrichment can even invert the competitive effect from positive to negative (Fig. 5).
Figure 5.
Effects of nutrition level on (A) competitive effect and (B) relative dominance index of H. vulgaris (mean ± SE).
Discussion
The presence of the terrestrial plant community and nutrient enrichment had opposite effects on the growth and establishment of H. vulgaris. There were also significant interaction effects of these two factors on leaf mass, number of nodes, and stem length of H. vulgaris. In particular, high nutrient availability enhanced the growth of H. vulgaris when growing without a native plant community, but had no effect when growing with the native plant community. Our results thus contradict with those of previous studies, which have typically demonstrated that the invasion success of exotic plants into native plant communities can be enhanced by the enrichment of growth-limiting nutrients27,35,36. There are several potential explanations for this phenomenon. First, biological interactions among species may limit the establishment and spread of introduced species17,37. The introduced and native species may affect each other by directly competing for soil nutrients, light, water, and physical space38. As such, in the high nutrient level treatment, the native species S. viridis can capture more light before interspecific competition can suppress its growth, thus developing a higher and denser canopy than H. vulgaris and shading H. vulgaris and thereby preventing its spread39. Alternatively, the spread of stolons and root development at nodes of H. vulgaris may have been limited to vacant spaces between native plants, which were diminished by the vigorous growth of the resident root systems under high nutrient conditions29. Previous studies have suggested that root phenotypic plasticity within native communities enables native plants to persist in the context of plant invasion or changing resource levels40. Furthermore, S. viridis can interfere with and suppress the normal root growth of forbs by releasing nonspecific allelochemicals into the rhizosphere20. A combination of these factors may explain the observed results.
Overall, H. vulgaris invasion had no effect on the recipient native plant community in terms of biomass or seeding establishment for all species except for A. sinicus. This result indicates that the resident vegetation community could resist invasion. Nutrient enrichment promoted biomass of the native communities and decreased also their evenness (Fig. 2). The decreased community evenness was because nutrient enrichment mainly increased biomass of S. viridis and had no significant effect on biomass of other species. We found no interaction effect of H. vulgaris invasion and nutrient availability on biomass of native communities, agreeing with previous findings29. However, most previous studies have shown that plant invasion reduces community biomass41,42,43. This discrepancy may reflect differences in characteristics of invasive species or composition of native communities.
We found that nutrient enrichment increased the productivity of the grasses, mainly S. viridis, while had little impact on that of the other two functional groups (legumes and forbs). Grasses, especially S. viridis, produced the majority of total biomass of the community, suggesting that grasses were the dominant functional group and S. viridis was the dominant species in this community. Grasses were more sensitive to nutrient addition and able to obtain more nutrients under the enrichment treatment, as observed in other studies9,10,44,45. As such, the resistance of the community to invasion may be attributed to the functional identity of resident competitors. It is likely that the fast-growing, native grasses and the dense canopy formed by them, especially that of S. viridis39, may have helped the community resist competition from H. vulgaris. The absence of interactive effects of these two factors suggests that such differential functional responses to invasion are not dependent on resource availability46.
The interaction between the native plant community and H. vulgaris was mediated by the nutrient availability. Specifically, nutrient enrichment inverted the competitive effect of H. vulgaris from positive to negative, such that H. vulgaris only had a detrimental effect on the growth of the plant community when nutrient levels were low. This suggests that changes in nutrient availability altered the competition pattern between the native plant community and H. vulgaris, although the influence of H. vulgaris invasion was limited. This positive or negative competitive effect suggests that H. vulgaris can decrease or increase biomass of the plant community according to nutrient availability. One possible explanation for this facilitation of the community by H. vulgaris under high nutrient availability is that exorbitantly high nutrient availability might inhibit plant growth by changing physical and chemical soil properties, causing a nutrient imbalance and reducing photosynthesis by hindering the absorption of Ca2+ and Mg2+ 47,48. The addition of H. vulgaris may have helped to reduce these disruptive nutrient resources and therefore reduce the possible negative effects of high nutrients so that the invaded plant community tended to gain more biomass than the uninvaded community. As shown in a previous study, S. viridis grown in close proximity to Eupatorium adenophorum accumulates more biomass than S. viridis grown in the native control soil49. Such impacts may explain the facilitation of the native community by H. vulgaris under high nutrient availability.
Moreover, high nutrient availability reduced the relative dominance index of H. vulgaris grown in the native plant community. This result suggests that the interspecific competitive ability of H. vulgaris was relatively high under low nutrient availability and the ability of the native plant community to resist invasion was relative high under high nutrient availability. Many studies have shown that environmental conditions can change the intensity of interspecific interactions50,51. Moreover, the interaction between resources and competition may increase the capacity of native plants to resist exotic invasive species by reducing the availability of other resources28. Thus, it may be difficult for H. vulgaris to spread in a nutrient-rich and species-rich native plant community.
Our results indicate that intrinsic community attributes and nutrient availability can affect the impacts of invasion. Specifically, native species, especially the grass S. viridis, may enhance community resistance to invasion by H. vulgaris. Accordingly, increases in nutrient deposition due to future global change may not promote H. vulgaris invasion into such plant communities, especially those dominated by S. viridis. Because our experiment did not test the physiological and biochemical parameters of H. vulgaris and the plant community, the potential mechanisms mediating these interactions between H. vulgaris and the plant community remain to be explored. Therefore, further studies should be designed to examine the mechanism underlying the interaction between H. vulgaris and nutrients available in the rhizosphere to fully understand how nutrient availability affects the invasion process of H. vulgaris or other similar clonal plants. In the present study, the effects of H. vulgaris invasion on species interactions could not be verified for lack of a monoculture community treatment for each species. To separate the effects on the growth of individual species from effects on competitive interactions among species, it is essential to further construct monocultures containing a single species.
Methods
Study species
Hydrocotyle vulgaris L. (Apiaceae) is a perennial clonal herb52. It commonly occurs in bogs, valleys, and dune grasslands. It was introduced to China as an ornamental plant in the 1990 s and is now considered to be a species with high potential invasiveness53. Each ramet, which consists of a leaf and adventitious roots, may be formed by a node along stolons52,54. In the field, H. vulgaris can produce extensive shoot systems and experience heterogeneous micro-environments created by either resource availability or aggregations of neighboring plants54. H. vulgaris plants used in this experiment were collected from a wetland in the suburbs of Hangzhou, Zhejiang Province, China and were propagated vegetatively in a greenhouse at Forest Science Co. Ltd. of Beijing Forestry University.
The constructed plant communities consisted of a suite of species commonly found in the steppe of northern China55. The plant communities consisted of eight species drawn from a random pool. They were classified into three functional groups: four grasses (Setaria viridis, Festuca arundinacea, Poa pratensis, and Bromus inermis), two legumes (Trifolium repens and Astragalus sinicus), and two forbs (Plantago asiatica and Oxalis corniculata).
Experimental design
We set up three plant arrangement treatments, i.e. H. vulgaris alone, a native plant community without H. vulgaris, and a native plant community with H. vulgaris, crossed with two nutrient addition treatments (i.e., a low or high nutrient level). Each of the six treatment combinations was replicated six times and distributed randomly amongst 36 plastic containers (length, 40 cm; width, 40 cm; depth, 40 cm) filled with 24 L of a 1:1:1 (v/v/v) mixture of quartz sand, vermiculite, and peat. On July 4, 2014, 80 seeds of each of the eight native species were sown uniformly into each container in the treatments with the native plant community with or without H. vulgaris. One week later, we planted nine ramets of H. vulgaris in the treatment with both the native plant community and H. vulgaris, and the treatment with H. vulgaris only (no native plant community). Each H. vulgaris ramet was composed of a node and a leaf, and was about 10 cm in height.
For the low-nutrient treatment, 100 mL of 0.3 g L−1 water-soluble fertilizer (20:20:20, N:P:K; Peters Professional; Everris, Geldermalsen, The Netherlands) was added once every two weeks. The low-nutrient treatment consisted of the application of 1.5 g nitrogen (N) and 1.5 g phosphorous (P) m−2 year−1. The low-nutrient treatment was designed to simulate filed conditions56. In the high-nutrient treatment nutrient concentrations were five times as high as those in the low-nutrient treatment (i.e., 7.5 g N and 7.5 g P m−2 year−1). The nutrient concentrations in the high nutrient treatment were based on previous research in grasslands57.
Measurements
On September 6, 2014, we harvested the surviving plants of H. vulgaris in each container and counted the number of seedlings of each native species. We counted the total number of stem nodes and measured both petiole length and total stem length of H. vulgaris. Leaf area of H. vulgaris was measured using WinFOLIA (Pro2004a, Regent Instruments, Québec, Canada). All H. vulgaris were separated into three parts: leaves, stems, and roots. All plant parts were separately oven-dried at 70 °C for at least 48 hours and then weighed. The surviving plants of each of the forbs in each container were separated into shoots and roots, similarly dried, and weighed. Then, we calculated the sum of the mass, aboveground mass, and belowground mass of all native species as biomass measures of the native plant community. We also calculated biomass of each functional group (grasses, legumes and fobs).
Data analysis
We calculated the Pielou evenness index as H/lnS, where H is the Shannon–Wiener diversity index based on the proportional final dry mass and S is the number of species. H was calculated as: H = −∑Pi ln (Pi) (i = 1, 2, …S), where S is the number of plant community species and Piis biomass of species i divided by the sum of biomass for all eight species in the community58.
The competitive effect of H. vulgaris was calculated as: competitive effect = ln (biomass of the native community not invaded by H. vulgaris/biomass of the native community invaded by H. vulgaris)32,59. A positive value suggests competition between H. vulgaris and the native plant community, while a negative value indicates that H. vulgaris invasion promotes the growth of the community59. We also calculated the dominance of H. vulgaris as: the relative dominance index = biomass of H. vulgaris/total biomass of all plants in the invaded community60.
We performed two-way ANOVAs to test effects of plant community (H. vulgaris alone vs. the native community with H. vulgaris) and nutrient level (low or high) on total mass, root mass, stem mass, leaf mass, number of node, petiole length, stem length, and leaf area of H. vulgaris. We also used two-way ANOVAs to examine effects of invasion (native plant communities without vs. with H. vulgaris) and nutrient level (low or high) on total biomass, above- or belowground biomass, evenness of the native plant community, biomass of each functional group, and biomass of each plant species. A one-way ANOVA was performed to test the effect of nutrient level (low or high) on the competitive effect and relative dominance index of H. vulgaris.
Data were transformed to natural log or square root before analysis when necessary to remove heteroscedasticity. Leaf mass, stem length, and leaf area of H. vulgaris were transformed using the natural log transformation, and petiole length of H. vulgaris as well as total mass and aboveground mass of plant communities were transformed using the square root transformation. All data analyses were conducted with SPSS 19.0 (IBM Corp., Armonk, NY, USA).
Additional Information
How to cite this article: Liu, L. et al. Nutrient enrichment alters impacts of Hydrocotyle vulgaris invasion on native plant communities. Sci. Rep. 6, 39468; doi: 10.1038/srep39468 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This research was supported by the Fundamental Research Funds for the Central Universities (2015ZCQ-BH-01), the National Natural Science Foundation of China (31470475 and 41303057), and the National Key Research and Development Program (2016YFC1201100).
Footnotes
Author Contributions H.Q., B.-C.D., and H.-L.L. designed the experiment, H.Q., X.-Q.B., and L.L. (Lin Li) conducted the experiment. L.L. (Lin Liu), H.Q. and H.-L.L. performed the statistical analysis, L.L. (Lin Liu), H.Q., F.-D. L., G.-C.L., and H.-L.L. wrote the manuscript.
References
- Mack R. N. et al. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710 (2000). [Google Scholar]
- Yu H., Yu F. H., Miao S. L. & Dong M. Holoparasitic Cuscuta campestris suppresses invasive Mikania micrantha and contributes to native community recovery. Biol. Conserv. 141, 2653–2661 (2008). [Google Scholar]
- Vorsino A. E. et al. Modeling Hawaiian ecosystem degradation due to invasive plants under current and future climates. Plos One 9, e95427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville N., Vanderhoeven S., Gruber W. & Meerts P. Invasion by Fallopia japonica increases topsoil mineral nutrient concentrations. Ecoscience 14, 230–240 (2007). [Google Scholar]
- Colomer-Ventura F. et al. Contemporary evolution of an invasive plant is associated with climate but not with herbivory. Funct. Ecol. 29, 1475–1485 (2015). [Google Scholar]
- Yu F. H., Wang N., Alpert P., He W. M. & Dong M. Physiological integration in an introduced, invasive plant increases its spread into experimental communities and modifies their structure. Am. J. Bot. 96, 1983–1989 (2009). [DOI] [PubMed] [Google Scholar]
- Wang N. et al. Clonal integration affects growth, photosynthetic efficiency and biomass allocation, but not the competitive ability, of the alien invasive Alternanthera philoxeroides. Ann. Bot. 101, 671–678 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y. B. et al. United we stand, divided we fall: a meta-analysis of experiments on clonal integration and its relationship to invasiveness. Oecologia 171, 317–327 (2013). [DOI] [PubMed] [Google Scholar]
- Song M. H. et al. Different responses to availability and form of nitrogen in space and time explain species coexistence in an alpine meadow community after release from grazing. Global Change Biol. 18, 3100–3111 (2012). [DOI] [PubMed] [Google Scholar]
- Song M. H. & Yu F. H. Reduced compensatory effects explain the nitrogen-mediated reduction in stability of an alpine meadow on the Tibetan Plateau. New Phytol. 207, 70–77 (2015). [DOI] [PubMed] [Google Scholar]
- Siemann E. & Rogers W. E. The role of soil resources in an exotic tree invasion in Texas coastal prairie. J. Ecol. 95, 689–697 (2007). [Google Scholar]
- Li H. L. et al. Nitrogen level changes the interactions between a native (Scirpus triqueter) and an exotic species (Spartina anglica) in coastal China. Plos One 6, e25629. (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberling J. M. & Fridley J. D. Resource-use strategies of native and invasive plants in Eastern North American forests. New Phytol. 200, 523–533 (2013). [DOI] [PubMed] [Google Scholar]
- Daniel S. et al. Unraveling the life history of successful invaders. Science 337, 580–583 (2012). [DOI] [PubMed] [Google Scholar]
- Davidson A. M., Jennions M. & Nicotra A. B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 14, 419–431 (2011). [DOI] [PubMed] [Google Scholar]
- Lonsdale W. M. Global patterns of plant invasions and the concept of invasibility. Ecology 80, 1522–1536 (1999). [Google Scholar]
- Derivera C. E., Ruiz G. M., Hines A. H. & Jivoff P. Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology 86, 3364–3376 (2005). [Google Scholar]
- Richards C. L. & Pigliucci M. Plasticity in salt tolerance traits allows for invasion of novel habitat by Japanese knotweed S. L. (Fallopia japonica and F. xbohemica, Polygonaceae). Am. J. Bot. 95, 931–942 (2008). [DOI] [PubMed] [Google Scholar]
- Huber-Sannwald E. & Durham S. Effects of nutrient patches and root systems on the clonal plasticity of a rhizomatous grass. Ecology 79, 2267–2280 (1998). [Google Scholar]
- Bais H. P., Weir T. L., Perry L. G., Gilroy S. & Vivanco J. M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266 (2006). [DOI] [PubMed] [Google Scholar]
- Vitousek P. M. & Hooper D. U. The effects of plant composition and diversity on ecosystem processes. Science 277, 1302–1305 (1997). [Google Scholar]
- Young S. L., Barney J. N., Kyser G. B., Jones T. S. & Ditomaso J. M. Functionally similar species confer greater resistance to invasion: implications for grassland restoration. Restor. Ecol. 17, 884–892 (2009). [Google Scholar]
- Blumenthal D. Interrelated causes of plant invasion. Science 310, 243–244 (2005). [DOI] [PubMed] [Google Scholar]
- Davis M. A., Philip G. J. & Ken T. Fluctuating resources in plant communities: a general theory of invasibility. J. Ecol. 88, 528–534 (2000). [Google Scholar]
- Fetene M. Intra- and inter-specific competition between seedlings of Acacia etbaica and a perennial grass (Hyparrenia hirta). J. Arid. Environ. 55, 441–451 (2003). [Google Scholar]
- Kiehl K., Esselink P. & Bakker J. P. Nutrient limitation and plant species composition in temperate salt marshes. Oecologia 111, 325–330 (1997). [DOI] [PubMed] [Google Scholar]
- Leishman M. R. & Thomson V. P. Experimental evidence for the effects of additional water, nutrients and physical disturbance on invasive plants in low fertility Hawkesbury Sandstone soils, Sydney, Australia. J. Ecol. 93, 38–49 (2005). [Google Scholar]
- Going B. M., Hillerislambers J. & Levine J. M. Abiotic and biotic resistance to grass invasion in serpentine annual plant communities. Oecologia 159, 839–847 (2009). [DOI] [PubMed] [Google Scholar]
- Gooden B. & French K. Impacts of alien plant invasion on native plant communities are mediated by functional identity of resident species, not resource availability. Oikos 124, 298–306 (2014). [Google Scholar]
- Elser J. J. et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142 (2008). [DOI] [PubMed] [Google Scholar]
- Huang J. et al. Urbanization in China changes the composition and main sources of wet inorganic nitrogen deposition. Environ. Sci. Pollut. R. 22, 6526–6534 (2015). [DOI] [PubMed] [Google Scholar]
- He W. M., Yu G. L. & Sun Z. K. Nitrogen deposition enhances Bromus tectorum invasion: biogeographic differences in growth and competitive ability between China and North America. Ecography 34, 1059–1066 (2011). [Google Scholar]
- Bradley B. A., Blumenthal D. M., Wilcove D. S. & Ziska L. H. Predicting plant invasions in an era of global change. Trends. Ecol. Evol. 25, 310–318 (2010). [DOI] [PubMed] [Google Scholar]
- Seastedt T. R. & Suding K. N. Biotic constraints on the invasion of diffuse knapweed (Centaurea diffusa) in North American grasslands. Oecologia 151, 626–636 (2007). [DOI] [PubMed] [Google Scholar]
- Barger N. N., D’Antonio C. M., Ghneim T. & Cuevas E. Constraints to colonization and growth of the African grass, Melinis minutiflora, in a Venezuelan savanna. Plant Ecol. 167, 31–43 (2003). [Google Scholar]
- Sorrell B. K., Brix H., Fitridge I., Konnerup D. & Lambertini C. Gas exchange and growth responses to nutrient enrichment in invasive Glyceria maxima and native New Zealand Carex species. Aquat. Bot. 103, 37–47 (2012). [Google Scholar]
- Gestoso I., Arenas F. & Olabarria C. Feeding behaviour of an intertidal snail: Does past environmental stress affect predator choices and prey vulnerability? J. Sea. Res. 97, 66–74 (2015). [Google Scholar]
- Leger E. A. & Espeland E. K. Coevolution between native and invasive plant competitors: implications for invasive species management. Evol. Appl. 3, 169–178 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. G., Cronin S. A. & Paschke M. W. Native cover crops suppress exotic annuals and favor native perennials in a greenhouse competition experiment. Plant Ecol. 204, 247–259 (2009). [Google Scholar]
- Phillips A. J. & Leger E. A. Plastic responses of native plant root systems to the presence of an invasive annual grass. Am. J. Bot. 102, 73–84 (2015). [DOI] [PubMed] [Google Scholar]
- Maron J. L. & Marler M. Field-based competitive impacts between invaders and natives at varying resource supply. J. Ecol. 96, 1187–1197 (2008). [Google Scholar]
- Flory S. L. & Bauer J. T. Experimental evidence for indirect facilitation among invasive plants. J. Ecol. 102, 12–18 (2014). [Google Scholar]
- Michelan T. S., Thomaz S. M., Mormul R. P. & Carvalho P. Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Freshwater Biol. 55, 1315–1326 (2009). [Google Scholar]
- Robleto E. A., Kmiecik K., Oplinger E. S., Nienhuis J. & Triplett E. W. Trifolitoxin production increases nodulation competitiveness of Rhizobium etli CE3 under agricultural conditions. Appl. Environ. Microb. 64, 2630–2633 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Bao X. M., Liu X. J. & Zhang F. S. Impact of nitrogen addition on plant community in a semi-arid temperate steppe in China. J. Arid. Land. 4, 3–10 (2012). [Google Scholar]
- Shea K. & Chesson P. Community ecology theory as a framework for biological invasions. Trends. Ecol. Evol. 17, 170–176 (2002). [Google Scholar]
- Mcnulty S. G., Aber J. D. & Newman S. D. Nitrogen saturation in a high elevation New England spruce-fir stand. Forest Ecol. Manag. 84, 109–121 (1996). [Google Scholar]
- Persson H., Ahlström K. & Clemensson-Lindell A. Nitrogen addition and removal at Gårdsjön — effects on fine-root growth and fine-root chemistry. Forest Ecol. Manag. 101, 199–205 (1998). [Google Scholar]
- Xiao B., Zhou W., Liu W. X., Jiang Z. L. & Wan F. H. Feedback of Ageratina adenophora soil microbe on A. adenophora and native plants. J. Agr. Sci. Tech. 16, 151–158 (2014). [Google Scholar]
- Liu L., Dong B. C., Aplert P. & Yu F. H. 2016. Effects of soil substrate heterogeneity and moisture on interspecific competition between Alternanthera philoxeroides and four native species. J. Plant Ecol., doi: 10.1093/jpe/rtw052 (2016). [DOI] [Google Scholar]
- Zhang J. Y., Cheng G. W., Yu F. H., Krauchi N. & Li M. H. Intensity and importance of competition for a grass (Festuca rubra L.) and a legume (Trifolium pretense L.) vary with environmental changes. J. Integr. Plant Biol. 50, 1570–1579 (2008). [DOI] [PubMed] [Google Scholar]
- Dong B. C. et al. Soil heterogeneity affects ramet placement of Hydrocotyle vulgaris. J. Plant Ecol. 8, 91–100 (2015). [Google Scholar]
- Miao L. H., Ji M. C., Wang Y. Y., Qiao D. D. & Chen Y. C. Study on invasion risk of Hydrocotyle vulgaris as an alien species in wetlands. J. Zhejiang Uni. 37, 425–431 (2011). [Google Scholar]
- Dong B. C., Wang J. Z., Liu R. H., Zhang M. X. & Yu F. H. Effects of heterogeneous competitor distribution and ramet aggregation on the growth and size structure of a clonal plant. Plos One 8, 68557 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X. Vegetation atlas of China. (Scientific Press, Beijing, 2001). [Google Scholar]
- Ying Z. et al. Spatial and temporal variation of atmospheric nitrogen deposition in North China Plain. Acta Ecol. Sin. 26, 1633–1638 (2006). [Google Scholar]
- Kolb A., Alpert P., Enters D. & Holzapfel C. Patterns of invasion within a grassland community. J. Eco. 90, 871–881 (2002). [Google Scholar]
- Kent M. & Coker P. Vegetation description and analysis. Area 12, 76–77 (1992). [Google Scholar]
- Wang P., Lei J. P., Li M. H. & Yu F. H. Spatial heterogeneity in light supply affects intraspecific competition of a stoloniferous clonal plant. Plos One 7, e39105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y. B., Wang W. B., Feng Y. L., Zheng Y. L. & Gong H. D. Synergistic interactions of CO2 enrichment and nitrogen deposition promote growth and ecophysiological advantages of invading Eupatorium adenophorum in Southwest China. Planta 236, 1205–1213 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.