INTRODUCTION
Anaphylaxis is a systemic allergic reaction that can be fatal.1 Several studies in different populations have reported increasing incidence rates of anaphylaxis over time.2–5 Clinical criteria for the diagnosis of anaphylaxis were poorly defined until 2006 when the National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network (NIAID/FAAN) diagnostic criteria for anaphylaxis were established. 6
One of the feared aspects of anaphylaxis is its recurrence. Previous studies report frequent recurrence of anaphylaxis, particularly in patients with multiple triggers7. Identification of those at greatest risk for recurrent anaphylaxis may enable patients to be more vigilant and prepared in order to mitigate the risk.
Despite the dangers of recurrent anaphylactic reactions, there are few studies on the incidence of and risk factors for recurrence, and there have been no population-based studies.7,8, 9 This study was designed to measure the rate and risk factors for recurrent anaphylactic reactions in the population of Olmsted County, Minnesota from 2001 to 2010.
METHODS
Study design and setting
We conducted a population-based observational cohort study among residents of Olmsted County between 2001 and 2010. We identified patients with anaphylaxis from the Rochester Epidemiology Project (REP), a medical record linkage system connecting the records of all health care providers in Olmsted County, Minnesota. 10–14 Every patient’s diagnosis was coded with either the codes of the Hospital Adaptation of the International Classification of Diseases (HICDA), Second Edition, or the codes of the International Classification of Diseases (ICD-9), Ninth Edition. This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Patients were included in the study only if they were residents of Olmsted County and had granted written permission for review of their medical records.
Selection of participants
All patients with codes representing anaphylactic shock, anaphylactic shock caused by food, anaphylactic shock not classified elsewhere, and anaphylactic shock after sting were obtained (HICDA 09894110, 09994110-2, ICD9 995.6, 995.60-69, 999.4, 999.41-42, 999.49, 995.0, V13.81). Additionally, three sub-lists with diagnoses representing potential anaphylactic reactions were generated. Twenty percent of the charts from each subgroup were chosen for review by random number sampling in order to identify additional cases of anaphylaxis. The sub-lists are as follows:
Diagnosis of venom, bee sting, or toxic effect of venom (HICDA 09894120-4, 09894210-1, 09894310, ICD9 V15.06, E905.0-.9, 989.5)
Diagnosis of allergy, foodstuff; adverse effect, food; dermatitis caused by food taken internally; or toxic effect of specific food (HICDA 06928540-2, 06928610-1, 34608120, ICD9 692.5, 693.1, 995.7, V15.01-.05, 988, 988.0-2, 988.8-.9)
Diagnosis of medication reactions (ICD9 995.2, 995.20-.21, 995.27, 999.4, 999.41-.42, 999.49)
The record of each patient was reviewed to confirm the diagnosis of anaphylaxis and to identify recurrences within the study period, using the NIAID/FAAN criteria to identify positive cases (Appendix Table I). Only cases that met the criteria for anaphylaxis were included in the study. Patients evaluated in the emergency department (ED), primary care clinics, allergy clinic, urgent care, and hospital ward were included. This methodology is similar to a prior study of anaphylaxis in Olmstead County which utilized REP resources.14, 15
Data collection, variables and outcome measures
The health records of all included patients were independently reviewed and abstracted by 2 previously trained investigators (CB, SL); data were extracted in duplicate for a sample of the cohort to ensure quality and consistency and to reduce bias. A calibration of the abstractors was performed after the duplicate abstraction, and inter-observer agreement was measured. The abstractor (CB) and principal investigator (SL) met frequently to review and discuss coding rules. Quality assurance of abstracted data abstracted was performed on a weekly basis. Data were entered into a REDCap (Research Electronic Data Capture, Vanderbilt University, Nashville, TN) database. A data abstraction form was created specifically for this study and was tested prior to starting data retrieval. This data abstraction tool was hosted at Mayo Clinic, and was constructed to support the collection of data for research studies by providing an interface for data collection, tracking data manipulation, automating the export of data to statistical software, and allowing the import of data from external sources. 16
Data were collected on demographic variables, past medical history, signs and symptoms, clinical presentation, clinical management, trigger, and allergy follow up. The inciting allergens of contrast and latex were combined with other triggers. For those who developed either index or recurrent anaphylaxis, we reviewed the charts and determined access to epinephrine prior to arrival to the health care setting. The total number of recurrent reactions within the study period for each patient was recorded. Recurrence of anaphylaxis was defined as the recurrence of symptoms meeting NIAID/FAAN criteria during study period. Biphasic reactions were excluded based on the duration of symptom recurrence and re-exposure to the trigger after the initial reaction. If the variable of interest was not mentioned in the records, including notes by the ED physician, allergy clinic, primary care appointment, and/or nurse, it was left as a missing value.
Statistical analysis
We present the variables collected with frequency counts and percentages. We report the rate of recurrent events per 100 person-years. Associations of features with time to recurrence were evaluated using Cox proportional hazards regression models and summarized with hazard ratios (HR) and 95% confidence intervals (CIs). The duration of follow-up was calculated from the index date of anaphylaxis to the date of first recurrence, the date of last follow-up if the patient was lost to follow-up prior to 12/31/2010, or 12/31/2010 if the patient was still being followed on that date. A multivariable model was developed using stepwise selection with the p-value for a feature to enter or leave the model set to 0.05. The modeling was done on a per-patient basis to account for those with multiple recurrences. Statistical analyses were performed using version 9.3 of the SAS software package (SAS Institute; Cary, NC). All tests were two-sided and p-values <0.05 were considered statistically significant.
RESULTS
Inter observer agreement
Twenty charts were reviewed by a trained medical assistant (CB) and the principal investigator (SL), and inter-rater agreement was very good, with an overall agreement of 95% and kappa of 0.88 (95% CI 0.64 to 1.00).
Participants
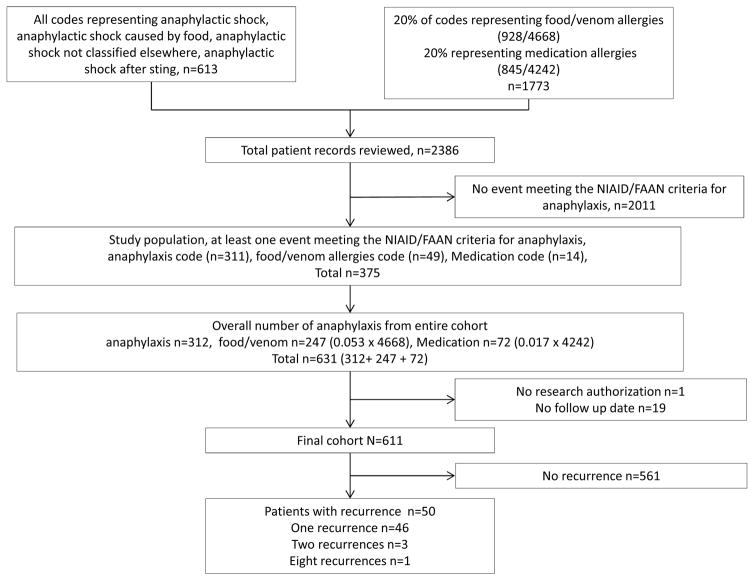
Using the resources of the Rochester Epidemiology Project, we identified 631 patients who had at least one anaphylactic reaction that met NIAID/FAAN criteria (Figure 1) in Olmsted County between January 2001 and December 2010. One patient did not have research authorization following the initial phase of the study. Nineteen patients who did not have follow-up after the index date of anaphylaxis were excluded, leaving 611 patients for the recurrence analysis. Features of interest for the 611 patients are summarized in Table I and Supplemental Table I.
Figure 1.
Flow chart of selection process
Table I.
Summary of features at index date of anaphylaxis, N=611
| Feature | N (%) |
|---|---|
| Age at index anaphylaxis | |
| Pediatric | 148 (24) |
| Adult | 463 (76) |
| Sex | |
| Women | 299 (49) |
| Men | 312 (51) |
| Race/ethnicity (N=562)* | |
| White | 476 (85) |
| Hispanic | 1 (<1) |
| Black | 18 (3) |
| Asian/Pacific Islander | 38 (7) |
| Other | 29 (5) |
| Visit setting (N=610) | |
| Emergency department | 467 (77) |
| Urgent care | 33 (5) |
| Clinic | 57 (9) |
| Allergy clinic | 40 (7) |
| Other | 13 (2) |
| Disposition (N=606) | |
| Home | 449 (74) |
| Observation unit | 72 (12) |
| Hospital ward | 57 (9) |
| Intensive care unit | 28 (5) |
| Past medical history | |
| Asthma | 101 (17) |
| Allergic rhinitis | 61 (10) |
| Atopic dermatitis | 21 (3) |
| Hives | 28 (5) |
| Inciting allergen | |
| Food | 223 (37) |
| Medication | 130 (21) |
| Contrast | 6 (1) |
| Latex | 1 (<1) |
| Venom | 146 (24) |
| Unknown | 68 (11) |
| Other | 37 (6) |
| Mucocutaneous symptom | 594 (97) |
| Gastrointestinal tract symptom | 121 (20) |
| Cardiovascular symptom | 222 (36) |
| Respiratory tract symptom | 482 (79) |
| Steroid provided as therapy | 392 (64) |
| Epinephrine provided as therapy | 288 (47) |
| Location of first epinephrine (N=283) | |
| Self/home | 22 (8) |
| EMS/resuscitation team | 26 (9) |
| Emergency department | 208 (74) |
| Intensive care unit/hospital ward | 5 (2) |
| Other | 22 (8) |
| Access to epinephrine autoinjector | 113 (18) |
Sample sizes for features with missing data or for subsets of interest are indicated in italics in parentheses.
Descriptive data
The median age of this cohort at first visit was 31 years (IQR, 18–45 years), including 148 (24%) children (age <18 years). Two hundred and ninety-nine (49%) of the patients were female, and 476 (85%) were Caucasian. On the first visit, 101 (17%) patients had a history of asthma, 61 (10%) allergic rhinitis, 21 (3%) atopic dermatitis, and 28 (5%) a history of hives. Table I shows the patient encounter setting and inciting trigger. Four hundred sixty-seven (467/610=77%) patients were seen in the ED, 40 (7%) were seen in the allergy clinic, 57 (9%) were seen in a non-allergist/immunologist outpatient clinic, 33 (5%) were seen in urgent care, and 13 (2%) were either seen elsewhere or in an unspecified location.
As shown in Table I and Supplemental Table I, an inciting trigger was identified in 543 (89%) of the cases. Two hundred and twenty-three (223/611=36%) patients had an inciting trigger of food, of which the largest proportion was peanut or tree nut (91/611, 15% overall). One hundred thirty (21%) patients had a medication as a trigger, including 45 (7% overall) antibiotics. Insect venom was the inciting trigger in 146 (24%) reactions, with bee stings accounting for 112 (18%) of the reactions.
Treatment and follow up of the 1st reaction are summarized in Table I and Supplemental Table I. Steroid therapy was administered in 392 (64%) of the reactions, and epinephrine was administered in 288 (47%) of the reactions. Of the 288 anaphylactic reactions, 283 had records of the locations of epinephrine administration, and 74% (n=208/283) of patients were treated with epinephrine in the ED. Four hundred and ninety-eight (82%) patients did not have access to an epinephrine auto-injector when their symptoms began. Four hundred and forty-nine (449/606=74%) patients were dismissed home after treatment in the outpatient setting, and 85 (14%) were admitted to either a hospital ward or the ICU. After the first reaction, 289 (289/611=47%) patients followed up with an allergist/immunologist, who identified a trigger in 74% (n=213/289) of cases.
Recurrent Anaphylaxis
Fifty (8%) patients experienced recurrence at a mean of 1.8 years following the index date of anaphylaxis (median 0.6; IQR 0.1–4.1; range 1 day to 7.5 years). The mean duration of follow-up for the 561 patients who did not experience a recurrence was 3.7 years (median 3.1; IQR 1.3–5.7; range 1 day to 9.9 years). There were 46 patients who experienced one recurrence, three patients who experienced two, and one patient who experienced eight during the time period under study, for a total of 60 recurrences observed during 2290 patient-years of follow-up, resulting in a recurrence rate of 2.6 per 100 person-years. The mean age at the date of recurrence was 34.3 years (median 32; IQR 18–53; range 5–75). Steroid treatment was provided in 45 (75%) of the recurrent reactions, and epinephrine was provided in 39 (65%) of the recurrent reactions. Forty-four (73%) patients had epinephrine available during their recurrent reactions. Twenty-five (42%) patients with recurrence had visited an allergist/immunologist after their first reaction, and in 15 (60%) of the 25 cases, the allergist/immunologist identified the trigger on a visit prior to the recurrence. The remaining features collected for the 60 recurrences are summarized in Table II and Supplemental Table II.
Table II.
Summary of features at recurrence, N=60
| Feature | N (%) |
|---|---|
| Age at recurrence | |
| Pediatric | 14 (23) |
| Adult | 46 (77) |
| Sex | |
| Women | 33 (55) |
| Men | 27 (45) |
| Race/ethnicity | |
| White | 52 (87) |
| Asian/Pacific Islander | 7 (12) |
| Other | 1 (2) |
| Visit setting | |
| Emergency department | 52 (87) |
| Clinic | 1 (2) |
| Allergy clinic | 5 (8) |
| Other | 2 (3) |
| Disposition | |
| Home | 45 (75) |
| Observation unit | 4 (7) |
| Hospital ward | 4 (7) |
| Intensive care unit | 7 (12) |
| Inciting allergen | |
| Food | 20 (33) |
| Medication | 5 (8) |
| Contrast | 1 (2) |
| Venom | 7 (12) |
| Unknown | 17 (28) |
| Other | 10 (17) |
| Allergist saw patient after index anaphylaxis | 25 (42) |
| Inciting allergen identified by allergist (N=25) | 15 (60) |
| Allergy tests performed | 12 (20) |
| Mucocutaneous symptom | 57 (95) |
| Gastrointestinal tract symptom | 12 (20) |
| Cardiovascular symptom | 30 (50) |
| Respiratory tract symptom | 39 (65) |
| Steroid provided as therapy | 45 (75) |
| Epinephrine provided as therapy | 39 (65) |
| Location of first epinephrine (N=39) | |
| Self/home | 16 (41) |
| EMS/resuscitation team | 3 (8) |
| Emergency department | 18 (46) |
| Other | 2 (5) |
| Access to epinephrine autoinjector | 44 (73) |
Risk factor analysis
Univariable associations with recurrence are summarized in Table III. The multivariable model to evaluate time to recurrence is summarized in Table IV. A history of atopic dermatitis (HR 5.6 [95% CI 2.0–16.1]; p=0.001), the presenting symptoms of cough (HR 4.7 [2.1–10.7]; p<0.001), oral pruritus (HR 9.9 [4.3–23.2]; p<0.001), and receiving steroid therapy for the index reaction (HR 5.2 [2.3–11.7]; p<0.001) were significantly associated with recurrence, while the cardiovascular symptom of chest pain (HR 0.24 [0.07–0.79]; p=0.019) was associated with a decreased risk of recurrence after multivariable adjustment.
Table III.
Univariable associations with recurrence
| Feature | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Age at index anaphylaxis | 1.07 (0.93–1.24)* | 0.36 |
| Age at index anaphylaxis | ||
| Pediatric | 1.0 (reference) | |
| Adult | 1.36 (0.66–2.81) | 0.40 |
| Sex | ||
| Women | 1.0 (reference) | |
| Men | 0.58 (0.33–1.03) | 0.064 |
| Race/ethnicity (N=562) | ||
| White | 1.0 (reference) | |
| Non-White | 1.07 (0.50–2.29) | 0.85 |
| Past medical history | ||
| Asthma | 1.39 (0.71–2.71) | 0.34 |
| Allergic rhinitis | 1.86 (0.87–3.96) | 0.11 |
| Atopic dermatitis | 3.41 (1.21–9.61) | 0.020 |
| Hives | 2.12 (0.84–5.36) | 0.11 |
| Inciting allergen | ||
| Food | 1.0 (reference) | |
| Medication | 0.63 (0.28–1.41) | 0.26 |
| Venom | 0.34 (0.14–0.84) | 0.019 |
| Unknown | 1.14 (0.50–2.58) | 0.76 |
| Contrast, latex, and other | 1.58 (0.67–3.72) | 0.30 |
| Mucocutaneous symptom | ||
| Diffuse urticarial | 0.78 (0.43–1.41) | 0.40 |
| Local angioedema | 1.04 (0.55–1.96) | 0.90 |
| Oral pruritus | 6.03 (2.69–13.49) | <0.001 |
| Pruritus | 1.58 (0.79–3.16) | 0.20 |
| Flushing | 1.75 (0.90–3.43) | 0.10 |
| Local urticarial | 0.97 (0.53–1.78) | 0.93 |
| Conjunctivitis | 0.73 (0.04–12.25)† | 0.83 |
| Oropharyngeal edema | 2.35 (1.11–5.02) | 0.027 |
| Diffuse angioedema | 0.61 (0.08–4.38) | 0.62 |
| Diaphoresis | 0.50 (0.03–8.42)† | 0.63 |
| Chemosis/edema ocular conjunctiva | 0.85 (0.21–3.51) | 0.82 |
| Tightness or fullness of throat | 1.60 (0.94–2.86) | 0.082 |
| Rhinitis | 0.62 (0.09–4.53) | 0.64 |
| Any of above | 1.20 (0.17–8.74) | 0.85 |
| Gastrointestinal tract symptom | ||
| Emesis | 0.50 (0.16–1.60) | 0.24 |
| Nausea | 0.49 (0.12–2.02) | 0.32 |
| Abdominal cramping | 1.09 (0.27–4.50) | 0.90 |
| Diarrhea | 0.20 (0.01–3.35) | 0.26 |
| Any of above | 0.44 (0.18–1.12) | 0.086 |
| Cardiovascular symptom | ||
| Pre-syncope/lightheadedness | 0.98 (0.48–2.02) | 0.96 |
| Chest pain | 0.32 (0.10–1.03) | 0.056 |
| Hypotension | 0.44 (0.11–1.80) | 0.25 |
| Arrhythmia | 12.39 (0.71–216.78) | 0.085 |
| Syncope | 0.26 (0.04–1.92) | 0.19 |
| Orthostatic hypotension | 0.61 (0.04–10.33) | 0.73 |
| Any of above | 0.55 (0.29–1.03) | 0.062 |
| Respiratory tract symptom | ||
| Dyspnea/difficulty breathing | 1.30 (0.71–2.38) | 0.40 |
| Wheezing/bronchospasm | 1.07 (0.50–2.29) | 0.85 |
| Cough | 3.93 (1.75–8.80) | <0.001 |
| Hoarseness/raspy voice | 3.89 (1.98–7.65) | <0.001 |
| Cyanosis | 0.52 (0.03–8.70) | 0.65 |
| Stridor | 1.81 (0.25–13.12) | 0.56 |
| Aphonia | 3.01 (0.18–50.53) | 0.44 |
| Any of above | 1.51 (0.73–3.13) | 0.26 |
| Steroid provided as therapy | 4.30 (1.93–9.59) | <0.001 |
| Epinephrine provided as therapy | 1.44 (0.82–2.52) | 0.20 |
Hazard ratio represents a 10-year increase in age.
Hazard ratio estimated using Firth’s penalized maximum likelihood.
CI: Confidence interval
Table IV.
Multivariable model to predict recurrence
| Feature | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Atopic dermatitis | 5.60 (1.95–16.13) | 0.001 |
| Oral pruritus | 9.93 (4.25–23.21) | <0.001 |
| Chest pain | 0.24 (0.07–0.79) | 0.019 |
| Cough | 4.70 (2.06–10.71) | <0.001 |
| Steroid provided as therapy | 5.16 (2.28–11.67) | <0.001 |
CI: Confidence interval
DISCUSSION
Main findings
In this population-based epidemiologic study, we found that recurrent anaphylaxis occurred in eight percent of the patients during the 10-year study period (2.6 per 100 patient-years). The median time to recurrence was 0.6 years. We found an association between recurrence and a history of atopic dermatitis and the presenting symptoms of cough, oral pruritus, and receiving steroid therapy for the index reaction, while the cardiovascular symptom of chest pain was significantly associated with a decreased risk of recurrence.
Interpretation of results
Eight percent of our patient population experienced a recurrence during the course of our study. As might be expected, this percentage is lower than those reported in studies of allergy clinic patients.7, 9 Mullins reported that 43 percent of allergy clinic patients surveyed experienced a recurrence over a 5.5 year period. It is possible that patients with recurring reactions were more likely to follow up at an allergy clinic. Additionally, patients with more recurrences may have been more likely to participate in the survey.17 Another study conducted in an allergy clinic found a recurrence rate of 76 percent. However, this study was conducted prior to the introduction of the NIAID/FAAN criteria, and the definition utilized did not require multisystem involvement.18 A more recent study conducted in 2013 found that 22 percent of children who presented to the ED with allergic reactions to food experienced a recurrence of allergic reactions in a three-year period. However, this study did not specifically examine anaphylaxis, but instead looked for recurrence of any allergic symptoms, so a lower rate would be expected than if only patients with anaphylaxis were included.19 Thus, our finding of an eight percent recurrence rate of anaphylaxis over a ten-year period in a population-based cohort is lower than rates reported in previously published literature. However, sampling and referral bias are likely to have been present in studies performed in allergy clinics compared to this population-based epidemiologic study.
Patients who experienced recurrent reactions typically experienced the recurrence in a relatively short time period, as the median time between the index and recurrent anaphylaxis was about seven months, with an interquartile range between 1 month and 4 years. These findings support the need for patients to immediately begin carrying an epinephrine auto-injector and to seek expedited outpatient allergist/immunologist follow up for further evaluation to confirm the diagnosis and establish the trigger whenever possible.
A history of atopic dermatitis was associated with an increased risk of recurrence in multivariable analysis. Mullin et al reported in their survey-based study in Australia that female sex was associated with increased recurrence, yet history of asthma or atopic dermatitis were not.17 Kemp et al reported that the patients presenting to the allergy clinic had a higher prevalence of atopic dermatitis (37%), and hypothesized that mast cell and basophils of atopic individuals are more active than non-atopic individual.18 These findings were not reported by Vetander et al, who reported known food allergy and previously prescribed epinephrine were associated with ED revisit among children.19 While specific risk factors have not been identified consistently in previous studies,17–19 atopy has been shown to be a risk factor for severe allergic reactions.20 In addition, the presenting symptoms of cough, oral pruritus, and steroid therapy for the index reaction remained significantly associated with recurrence, while the cardiovascular symptom of chest pain was associated with a decreased risk of recurrence. These have not been identified in prior studies. 17–19 Despite statistically significant results, there is not enough data to implicate the prediction of future recurrence for those with positive risk factors. Further studies are needed to confirm or dispute these findings.
One encouraging finding was an increase in epinephrine availability at the time of the recurrent reaction relative to the first reaction. While only 18 percent of patients had an epinephrine auto-injector available at the time of the index reaction, 73 percent of the patients that had a recurrence had an auto-injector available. Those patients with first time anaphylaxis may not have had a reason to carry epinephrine previously. Self-injectable epinephrine availability is a crucial life-saving treatment for the management of anaphylaxis. Although availability appears to be increasing, improvements are still needed. 21
Limitations and strengths
Our study has several potential limitations. First, we used ICD-9 and HICDA codes for identification of patients, so we may have missed some patients with anaphylaxis who were miscoded. Second, the study is a based on medical record review, and data collected is therefore limited to the information in the medical record. Third, we analyzed all patients with anaphylaxis diagnoses, but only a sampling of the patients with diagnoses of sting, allergic reactions, or medication reactions, which could introduce selection bias. Fourth, decision making regarding diagnosis and management was left to the clinical team caring for the patients; thus, there may be bias intrinsic to practice variation, and it is difficult to capture all elements influencing decision making. Fifth, data abstractors were not blinded to the outcomes measured or study objectives. Sixth, this study was conducted in a region with a predominantly White population, and the results may not be generalizable to other populations. Finally, multiple variables were tested for associations, and external validation is needed to confirm these findings.
Despite these limitations, this is the first study describing the recurrence of anaphylaxis in a population-based cohort. This study utilized a medical records linkage system to track each patient’s progress throughout a ten-year period. This enabled us to review visits to any clinic within the county, providing a comprehensive capture of each patient’s medical history and outcomes. It is also noteworthy that we used NIAID/FAAN criteria to define anaphylaxis in our cohort; the use of objective criteria likely increased the accurate identification of index and recurrent events.
Implication for clinical practice and policy
Eight percent of the patients experienced anaphylaxis recurrence within 10 years, (recurrence rate of 2.6 per 100 person-years) with most patients having the first recurrence within four years. These findings reinforce guidelines that recommend that patients be prescribed and carry self-injectable epinephrine and follow up promptly with an allergist/immunologist to help confirm the diagnosis and likely trigger. Fear of recurrent anaphylaxis can be a stressful burden on a patient and his or her family or caregivers, particularly if a trigger is unknown. Increased anxiety due to anaphylactic reactions has been associated with an increase in future reactions. 22 Understanding the overall risk of recurrence as well as specific associated risk factors will enable healthcare providers to more accurately educate and prepare patients regarding their risk of future recurrence and potentially avoid recurrences and adverse outcomes.
Unanswered questions and future research
Very few studies have been dedicated to investigating recurrence in anaphylaxis patients, and this is the first to be conducted since the establishment of the NIAID/FAAN criteria in 2006. 23 More discrete data on the recurrence of anaphylaxis and identifying the causative factors can benefit the patients and clinicians. In order to gain a better understanding of factors that can lead to recurrent anaphylaxis, larger studies are needed.
As a conclusion, we found that eight percent of patients with anaphylaxis experienced a recurrence of within ten years, and half of these experience a recurrence within 7 months. The risk of recurrence increased with a history of atopic dermatitis and the presence of mucocutaneous or respiratory symptoms during index reaction. Our findings can be used for risk-stratification of patients and underscore the importance of early patient access to self-injectable epinephrine and referral to an allergist/immunologist for additional testing and education.24
Supplementary Material
Acknowledgments
Funding source
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
We appreciate Waqas Gilani, MD, and Dante Lucas Souza from Mayo Clinic for assistance in chart review.
Abbreviations
- REP
Rochester Epidemiology Project
- ICD
International Classification of Diseases
- NIAID/FAAN
National Institutes of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network
- CI
Confidence interval
- ED
Emergency department
Footnotes
Trial registration
Not applicable
Conflict of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Annals of emergency medicine. 2006;47:373–80. doi: 10.1016/j.annemergmed.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Decker WW, Campbell RL, Manivannan V, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122:1161–5. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheikh A, Hippisley-Cox J, Newton J, Fenty J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J R Soc Med. 2008;101:139–43. doi: 10.1258/jrsm.2008.070306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tejedor-Alonso M, Moro-Moro M, Mosquera Gonzales M, et al. Increased incidence of admissions for anaphylaxis in Spain 1998–2011. Allergy. 2015 doi: 10.1111/all.12613. [DOI] [PubMed] [Google Scholar]
- 5.Turner P, Gowland M, Sharma V, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012. J Allergy Clin Immunol. 2015;135:956–63. doi: 10.1016/j.jaci.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373–80. doi: 10.1016/j.annemergmed.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Mullins RJ. Anaphylaxis: risk factors for recurrence. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2003;33:1033–40. doi: 10.1046/j.1365-2222.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 8.Gold MS, Sainsbury R. First aid anaphylaxis management in children who were prescribed an epinephrine autoinjector device (EpiPen) The Journal of allergy and clinical immunology. 2000;106:171–6. doi: 10.1067/mai.2000.106041. [DOI] [PubMed] [Google Scholar]
- 9.Kemp SF, Lockey RF, Wolf BL, Lieberman P. Anaphylaxis. A review of 266 cases. Archives of internal medicine. 1995;155:1749–54. doi: 10.1001/archinte.155.16.1749. [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic proceedings. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings. 2012;87:1202–13. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. International journal of epidemiology. 2012;41:1614–24. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Rocca WA. Use of a Medical Records Linkage System to Enumerate a Dynamic Population Over Time: The Rochester Epidemiology Project. American Journal of Epidemiology. 2011;173:1059–68. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SHE, Lohse CM, Gilani W, Chamberlain AM, Campbell RL. Trends, characteristics and incidence of anaphylaxis in 2001–2010: A population study. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.04.029. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decker WW, Campbell RL, Manivannan V, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. The Journal of allergy and clinical immunology. 2008;122:1161–5. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PT, Thielke R, Payne R, Gonzalez J, Conde NJ. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullins R. Anaphylaxis: risk factors for recurrence. Clin Exp Allergy. 2003;33:1033–40. doi: 10.1046/j.1365-2222.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 18.Kemp SL, Wolf R, Lieberman Phil P. Anaphylaxis: a review of 266 cases. Archives of Internal Medicine. 1995;155:1749–54. doi: 10.1001/archinte.155.16.1749. [DOI] [PubMed] [Google Scholar]
- 19.Vetander ML, Hakansson N, Lilja G, et al. Recurrent reactions to food among children at paediatric emergency departments. Clin Exp Allergy. 2013;44:113–20. doi: 10.1111/cea.12203. [DOI] [PubMed] [Google Scholar]
- 20.Sampson H. Anaphylaxis and Emergency Treatment. Pediatrics. 2003;111:1601–8. [PubMed] [Google Scholar]
- 21.Simons FE. Epinephrine auto-injectors: first-aid treatment still out of reach for many at risk of anaphylaxis in the community. Ann Allergy Asthma Immunol. 2009;102:403–9. doi: 10.1016/S1081-1206(10)60512-1. [DOI] [PubMed] [Google Scholar]
- 22.Manassis K. Managing Anxiety Related to Anaphylaxis in Childhood: A Systematic Review. Journal of Allergy. 2012;2012 doi: 10.1155/2012/316296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson HA, Munoz-Furlong A, Bock SA, et al. Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol. 2005;115:584–91. doi: 10.1016/j.jaci.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477–80. e1–42. doi: 10.1016/j.jaci.2010.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.