Introduction
IL-25 (IL-17E) belongs to a family of IL-17 cytokines (IL17A to F) that are structurally related but with distinct functions1–3. There has been increasing evidence for the important role of IL-25 in driving Th2 pathway responses implicated in multiple immune-mediated conditions, including asthma and allergy development and severity4–6. In animal models, IL-25 and its receptor, IL-17RB, are increased following parasitic infection7,8, chronic allergen exposure6,9–13 and respiratory syncytial virus (RSV) infection14–16. In humans, allergen provocation increases expression of IL-25 and its receptor in the asthmatic bronchial submucosa11.
Potential sources of IL-25 in human include lung epithelial cells, bronchial endothelial cells, eosinophils, basophils and mast cells11,17,18. Activated eosinophils and basophils from normal and atopic subjects were found to secrete bioactive IL-25, which augments the functions of Th2 memory cells8,12,18,19. Therefore, IL-25 is likely involved in the innate pathway that promotes Th2 cytokine responses in allergic and asthmatic airway inflammation.
Several cell populations express the IL-25 receptor, IL-17RB, such as eosinophils, T lymphocytes, mast cells, bronchial endothelial and lung epithelial cells11,17. Previous research by our group has defined a unique population of IL-25-responsive granulocytes that were termed Type 2 Myeloid cell (T2M)10. The T2M cells express a set of defining cell surface markers, including IL-17RB, CD11b, CD16, and CD177. This distinctive population of granulocytes responds to IL-25 stimulation with production of type 2 cytokines (IL-4 and IL-13). In mice, the adaptive transfer of T2M cells reconstituted an IL-25-mediated allergic response in IL-17RB −/− animals. In addition, these cells appeared to be corticosteroid resistant, as high-dose steroid treatment did not reduce the IL-25-induced, T2M cell-associated pulmonary response. In humans, peripheral T2M cell numbers were found to be elevated in a small set of 9 moderate to severe asthma patients compared to control non-asthmatics10. Intracellular staining of this human granulocyte population showed expression of IL-4 and IL-13. Therefore, T2M cells may potentially play a pro-inflammatory role in asthma and contribute to the severity of the response.
The role and relative importance of the T2M cell to asthma severity is currently unknown. In this study, we sought to investigate the presence of IL-17RB+ granulocytes and T2M cell population in the peripheral blood of asthma subjects, and determine associations with clinical parameters. Our hypothesis was that both T2M cells and IL-17RB+ granulocytes are correlated with airflow obstruction and asthma control.
Methods
Subjects
Potential subjects, age 18 and above, with a physician-diagnosis of current asthma were recruited. Information regarding asthma clinical history and treatment, as well as other medical comorbidities including allergic rhinitis was obtained through patient interview and chart review. The diagnosis of allergic rhinitis was confirmed through review of previous skin tests and clinical history. Spirometry testing was performed on each patient in accordance with ATS guideline20. Subjects also provided a measure of current asthma control through the Asthma Control Test (ACT) score21. Exclusion criteria included smoking within the past six months, any significant cardiopulmonary disease other than asthma, and current use of any chronic immune suppressant administered systemically (including systemic corticosteroids). Adult volunteers without asthma and with similar exclusion criteria served as control subjects. All subjects provided written informed consent, and the study was approved by the University of Michigan IRB.
Granulocyte isolation from human peripheral blood
Peripheral blood was obtained from study subjects (20 ml). The erythrocyte layer was removed following Ficoll-Paque (GE healthcare) separation. Granulocytes were isolated from the erythrocyte layer following erythrocyte sedimentation with 5% dextran (Sigma) at room temperature for 30 minutes, followed by RBC lysis with 0.2% NaCl. The separated granulocytes were then washed twice with FACS buffer (PBS with 0.5% FBS).
Immunofluorescent staining and flow cytometry analysis
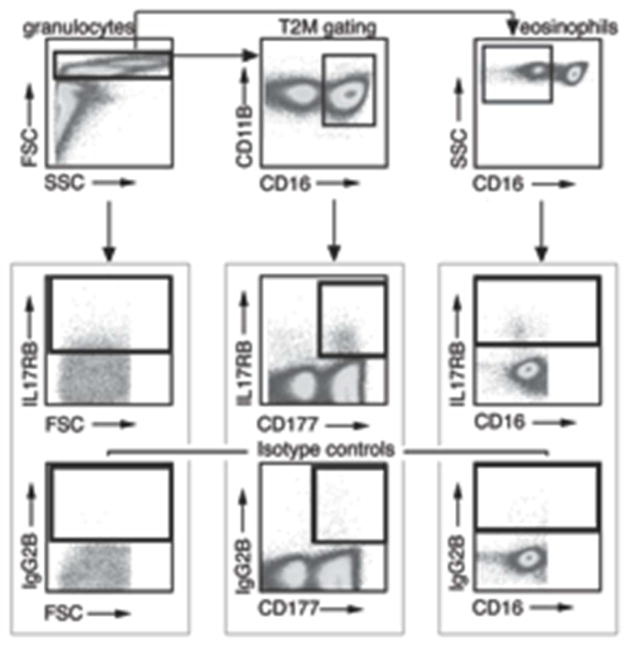
For cell surface marker staining, 1 million granulocytes were resuspended in total of 200 ul FACS buffer. FcR was blocked using purified human IgG at 1:500 final dilution (Sigma) for 15 minutes at 4°C. Cells were then incubated for 30 minutes in the dark with the following anti-human monoclonal antibodies: CD-177 FITC, CD-11b PerCP-Cy5.5, CD-16 Biotin conjugated with Pacific Orange, IL-17RB PE and isotype mouse IgG2b PE (all with 1:200 dilution, except for 1:100 dilution with IL-17RB antibody). All antibodies were from Biolegend, except for anti-human IL-17RB and IgG2b isotype (R&D System). After washing with FACS buffer, cells were resuspended in 1% paraformaldehyde and stored in 4°C. Data were collected on a BD Biosciences LSR II flow cytometer, with six parameters acquired: linear forward angle light scatter, linear side-angle light scatter, log PE, log FITC, log pacific orange, and log PerCP/Cy5.5. Data was processed with FlowJo software (Tree Star). Expression of IL-17RB, T2M and eosinophil were analyzed using step-wise gating (Fig 1).
Fig. 1.
Granulocytes are isolated from peripheral blood samples donated by asthmatic (n=26) and non-asthmatic (n=15) volunteers and analyzed with flow cytometry. Flow diagram shows the gating strategies for IL-17RB+ granulocytes, T2M (CD11B+CD16+CD177+IL-17RB+ granulocytes), and IL-17RB+ eosinophils (IL-17RB+CD16-granulocytes). Granulocytes were selected first and then subsequently gated for the subgroups. The bottom panel shows correspondent isotype control for each cell type.
Statistics
The significance of the differences between asthmatic and control groups was analyzed by using a non-parametric T-test. Correlations between the percentage of specific cell populations and FEV1/FVC, FEV1 and ACT were analyzed using a Pearson correlation analysis. Sample size calculations were based upon our previous study of 9 asthmatic subjects. Based on an absolute difference of 2% for T2M percentage between asthmatic subjects and healthy controls, we calculated that we would need 38 subjects in a 2:1 asthma:healthy ratio to evaluate for differences in T2M and IL-17RB+ cells in a population of more severe asthma subjects. This sample size was also felt to be adequate to determine correlations in clinical parameters, T2M cells, and IL-17RB+ cells.
Results
A total of 41 subjects were enrolled in the study (26 asthmatics and 15 control subjects). A summary of subject characteristics can be found in Table 1. Almost all asthmatic subjects (92%) were using an inhaled corticosteroid on a daily basis, and approximately half (56%) of them had used systemic steroid in the past year. The asthmatic subjects were significantly more likely to have allergic rhinitis compared to controls (92% vs 20%, p <0.001).
Table 1.
Subject characteristics.
| Asthma (n = 26) | Control (n = 15) | p value | |
|---|---|---|---|
| Gender, Female, n (%) | 20 (77%) | 9 (60%) | 0.17 |
| Age (SD) | 48.00 (13.89) | 40.71 (11.40) | 0.10 |
| Race | 0.01 | ||
| White, n (%) | 22 (84.6) | 11 (73.3) | |
| Black, n (%) | 4 (16) | 0 | |
| Asian, n (%) | 0 | 4 (26.7) | |
| Allergic rhinitis, n (%) | 24 (92) | 3 (20.0) | < 0.001 |
| Age at asthma diagnosis (SD) | 25.88 | (18.43) | |
| Current ICS use, n (%) | 24 (92) | ||
| Used systemic steroid in past year, n (%) | 14 (56%) | ||
| Average courses of systemic steroid in past year (SD) | 2.8 (3.1) | ||
| ACT score (SD) | 18.9 (4.2) | ||
| FEV1 percent predicted (SD) | 92.60 (13.64) | ||
| FEV1/FVC percent predicted (SD) | 96.56 (10.43) |
ICS = inhaled corticosteroid steroid.
IL-17RB+ granulocytes and type 2 myeloids
Among peripheral granulocytes, the overall percentage of cells expressing the IL-25 receptor (IL-17RB+ cells) was significantly higher in asthmatic subjects compared to control subjects (p = 0.03) (Figure 2A). Furthermore, when examining the percentage of IL-17RB+ granulocytes that were of the T2M subtype (IL17Rb+CD177+CD11b+CD16+), asthmatic subjects had significantly higher numbers than that of control non-asthmatics (p = 0.03) (Figure 2B).
Fig. 2.
Results of flow cytometry analysis of peripheral blood samples donated by asthmatic (n=26) and non-asthmatic (n=15) volunteers. (A) Percentage of IL-17RB+ granulocytes from asthmatic compared with control subjects. (*P=0.03). (B) Percentage of T2M cells from asthmatic compared with control subjects. (*P=0.03). Percentage of T2M is expressed as percentage of IL-17RB+ cells among CD11b+CD16+CD177+ granulocytes. All data are presented as mean ± s.e.m.
Clinical correlation of IL-17RB+ granulocytes and T2M in asthmatic subjects
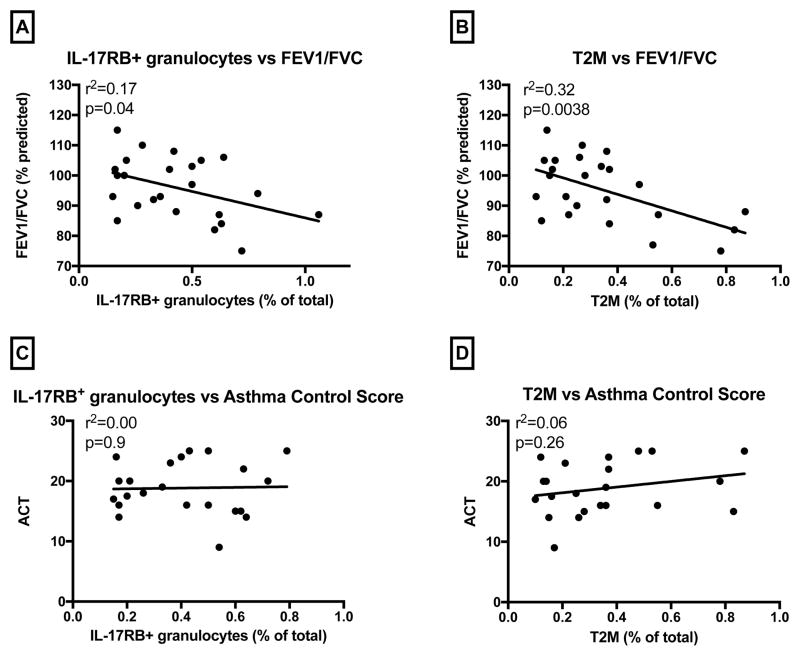
We sought to identify any relationship between traditional asthma parameters and IL-17RB+ granulocytes or T2M cells. As shown in Figure 3A, among asthmatic subjects, a higher percentage of IL-17RB+ granulocytes was associated with lower percent-predicted FEV1/FVC (r2 = 0.17, p = 0.043). For T2M cells, we again found that the percentage of T2M was negatively correlated with percent-predicted FEV1/FVC (r2 = 0.32, p = 0.0038) (Figure 3B). There was no correlation between FEV1 and percentage of IL-17RB+ granulocytes or T2M cells (Data not shown). Likewise, there was no correlation between subjective symptoms reflected through ACT scores and T2M cells or IL-17RB+ granulocytes (p = 0.9 for IL-17RB+ granulocytes and p = 0.26 for T2M cells) (Figures 3C and 3D).
Fig. 3.
Among asthmatic subjects (n=26), correlations were analyzed between cell percentage and spirometry parameter or ACT (asthma control test score). (A) Correlation analysis of IL-17RB+ granulocytes with FEV1/FVC. (r2=0.17, p=0.043). (B) Correlation analysis of T2M cells with FEV1/FVC. (r2=0.32, p=0.0038). (C) Correlation analysis of IL-17RB+ granulocytes with ACT. (r2=0, p=0.9). (D) Correlation analysis of T2M cell with ACT. (r2=0.06, p=0.26).
Correlation of eosinophils with clinical disease
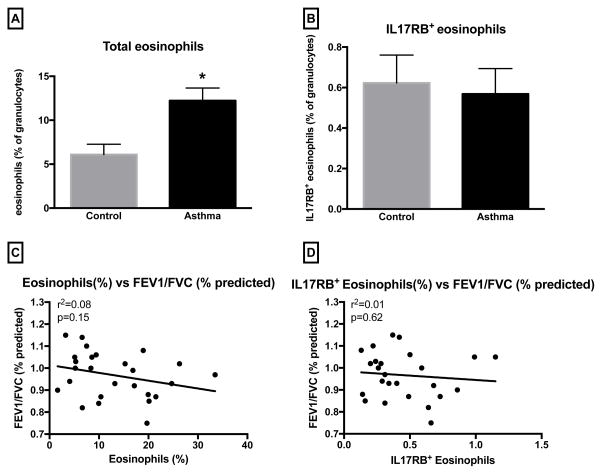
Eosinophils are known to be an important subset of granulocytes involved in asthma, and were investigated in this study as well. Compared to non-asthmatic controls, the percentage of eosinophils among granulocytes were higher in asthmatics (Figure 4A) (p=0.002). However, among eosinophils, the percentage of cells that expressed IL-17RB was similar between asthmatics and controls (Figure 4B). Among asthmatic subjects, the percentage of eosinophil was not correlated with FEV1/FVC (Figure 4C). Furthermore, the percentage of IL-17RB+ eosinophils did not correlate with FEV1/FVC (Figure 4D) nor with FEV1 or ACT scores (data not shown).
Fig. 4.
Percentage of eosinophil and eosinophil IL-17RB expression were analyzed in peripheral blood granulocytes from asthmatic (n=26) and non-asthmatic volunteers (n=15). (A) Percentage of eosinophils in granulocytes from asthmatics compared with control subjects. (*P=0.002). (B) Percentage of IL-17RB+ eosinophils in granulocytes from asthmatics compared with control subjects. (P=0.73). (C) Correlation analysis of eosinophil percentage with FEV1/FVC. (r2=0.08, P=0.15). (D) Correlation analysis of IL-17RB+ eosinophil percentage with FEV1/FVC. (r2=0.01, P=0.62).
Discussion
As a chronic inflammatory disease of the airways, allergic asthma involves the interplay of many cells and molecular pathways. IL-25 and its receptor, IL17-RB, have been described as important regulators of type 2 immunity. Previous research has suggested that lung epithelial cell-derived IL-25, along with IL-33 and TSLP (thymic stromal lymphopoietin), initiate and augment Th2-type airway inflammation and remodeling, leading to pathogenesis and complications of asthma22,23. Other investigators have found that IL-17RB was expressed in asthmatic airway on various cells such as eosinophils, mast cells, and endothelial cells11.
In our previous study, a small group of 9 asthmatic patients were found with elevated expression of IL-17RB on peripheral granulocytes as well as T2M cells (IL17RB+CD11b+CD16+CD177+ granulocytes), compared to non-asthmatic controls. The present study supports and extends the previous observations by verifying that the percentage of IL-17RB+ granulocytes, and T2M cells, are increased in asthmatics as compared to healthy controls. We included an expanded number of 26 asthmatic subjects, and asthma severity was also better characterized. Among the subjects, nearly all were on inhaled steroid (92%), and more than half needed systemic steroid in the past year (56%), indicating more severe asthma.
More importantly, the current study provides novel data that IL-17RB+ granulocytes and T2M cells correlate with the severity of airway obstruction (percent predicted FEV1/FVC). Our earlier observations that T2M cells produce Th2-type cytokines, IL-4 and IL-1310, may help to explain these findings. Specifically, IL-13 induces many features of allergic lung disease, including airway hyperresponsiveness, goblet cell metaplasia, and mucus hyper secretion, which all contribute to airway obstruction24–27. Previous studies on IL-25 and its cellular targets have mostly focused on local airway pathology, through methods of bronchial biopsy or cell culture of asthmatic lung tissue11,18,28. The current study investigated the effects of IL-25 on systemic inflammation in asthma through analysis of peripheral granulocytes. Overall, we found correlations of specific markers and cell populations in peripheral blood with the degree of airway obstruction. This study did not show a correlation with the subjective symptomatology scoring as in ACT. It may be that IL-17RB correlates more with baseline airway remodeling, airflow obstruction and hyperresponsiveness, rather than currently perceived symptom control. Future studies examining the correlation of T2M or IL-17RB+ cells with airway hyperresponsiveness through methacholine challenge may prove useful to further explore this relationship. Eosinophils are thought to be an important factor related to airway damage through the release of various toxic granule proteins19,29. In the analysis of granulocytes, we also assessed eosinophil percentage and used flow cytometry gating to identify the subset of eosinophils that were IL-17RB+. Similar to previous studies30–32, the percentage of eosinophils was higher in asthmatics. However, among eosinophils, the percentages of cells that expressed IL-17RB were similar between asthmatics and controls. Furthermore, there was no correlation between percentage of IL-17RB+ eosinophils with pulmonary function parameters (FEV1/FVC or FEV1). Thus, the IL-17RB+ granulocytes responsible for the inverse correlation between FEV1/FVC and IL-17RB positive cells appear to be a distinct granulocytic population rather than the eosinophil – and the T2M cells may play a role in this relationship. These findings provided insight into a distinct asthmatic endotype. Among patients who expressed high IL-17RB, there may not be an eosinophil-predominant pathophysiology. Previous studies have suggested that eosinophilic airway inflammation predicts a better response to inhaled corticosteroids, whereas noneosinophilic asthma is less responsive33,34. Interestingly, high-dose dexamethasone treatment did not reduce the IL-25-induced T2M pulmonary response in a previous murine study10.
Neutrophilic asthma has been described as a unique endotype35. T2M cells appear to be a steroid-resistance cell population, similar to neutrophils. However, the exact cellular designation of the T2M cell is not fully resolved. While the overall surface receptor expressions are distinct based upon microarray profile (suggesting the T2M is a separate subpopulation of granulocytes), the relationship between T2M and neutrophil is still being elucidated.
There are limitations to this study. Bronchoscopy and lung biopsy were not performed, and the findings of the peripheral blood may not accurately reflect the cellular physiology of the lung. We were unable to define steroid resistance among patients with limited data from chart review, although the average number of systemic steroid courses in the past year among our asthmatic subjects was 2.8, indicating a more severe asthma population. Due to our sample size, asthma subjects were not stratified by asthma severity or use of oral corticosteroids. Future studies with a larger cohort may be able to detect an interaction effect for the T2M cell based on such strata. Furthermore, this is a cross-section study, and cause-effect cannot be conclusively stated in regards to whether higher expression of IL-17RB or T2M may cause steroid resistance. A future prospective study may be helpful to find the relationship. An important consideration for our study was that the patients who came to our asthma clinic were in a relatively stable condition. Patients with acute asthma exacerbation, such as those who present to emergency room or urgent care centers, would have a greater upregulation of inflammatory cells and cytokines. The most common cause of severe exacerbations has been identified as respiratory viral infections36. Our laboratory has recently identified that RSV infection elicits enhanced IL-25 production and increases in T2M type cells into the lungs of allergic animals in an IL-17RB dependent manner. The same study also showed inhibition of IL-25 or deletion of IL-17RB reduced T2M cells attenuated the exacerbation responses16. Thus, future studies examining these parameters in asthmatic patients may provide further insight into the role of IL-17RB+ granulocytes in the progression of disease.
In summary, granulocytes expressing IL-17RB, and the T2M cells (a subset of IL-17RB+ granulocytes) are upregulated in asthmatics compared to controls, and are correlated with clinical measures of airway obstruction. IL-17RB+ granulocytes and T2M cells may serve as biomarkers and tools for clinical assessment and diagnosis, and as potential therapeutic targets. Further investigations on a larger scale, in various clinical settings, would be important and helpful.
Acknowledgments
Funding Source: Partially supported by NIH grant NIH RO1 A1036302
Abbreviations
- T2M
Type 2 Myeloid
- ACT
Asthma Control Test
- TSLP
thymic stromal lymphopoietin
- Th2
T helper 2
Footnotes
Trial Registration: None
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23(7):1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21(6):413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteleone G, Pallone F, Macdonald TT. Interleukin-25: a two-edged sword in the control of immune-inflammatory responses. Cytokine Growth Factor Rev. 2010;21(6):471–475. doi: 10.1016/j.cytogfr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Wisniewski JA, Borish L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy Asthma Proc. 2011;32(2):83–94. doi: 10.2500/aap.2011.32.3428. [DOI] [PubMed] [Google Scholar]
- 5.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10(4):225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamachi T, Maezawa Y, Ikeda K, Iwamoto I, Nakajima H. Interleukin 25 in allergic airway inflammation. Int Arch Allergy Immunol. 2006;140(Suppl 1):59–62. doi: 10.1159/000092713. [DOI] [PubMed] [Google Scholar]
- 7.Harvie M, Camberis M, Le Gros G. Development of CD4 T Cell Dependent Immunity Against N. brasiliensis Infection. Front Immunol. 2013;4:74. doi: 10.3389/fimmu.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203(4):1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory LG, Jones CP, Walker SA, et al. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax. 2013;68(1):82–90. doi: 10.1136/thoraxjnl-2012-202003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen BC, Budelsky AL, Baptist AP, Schaller MA, Lukacs NW. Interleukin-25 induces type 2 cytokine production in a steroid-resistant interleukin-17RB+ myeloid population that exacerbates asthmatic pathology. Nat Med. 2012;18(5):751–758. doi: 10.1038/nm.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrigan CJ, Wang W, Meng Q, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. The Journal of allergy and clinical immunology. 2011;128(1):116–124. doi: 10.1016/j.jaci.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Dolgachev V, Petersen BC, Budelsky AL, Berlin AA, Lukacs NW. Pulmonary IL-17E (IL-25) production and IL-17RB+ myeloid cell-derived Th2 cytokine production are dependent upon stem cell factor-induced responses during chronic allergic pulmonary disease. J Immunol. 2009;183(9):5705–5715. doi: 10.4049/jimmunol.0901666. [DOI] [PubMed] [Google Scholar]
- 13.Angkasekwinai P, Park H, Wang YH, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204(7):1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegle JS, Hansbro N, Herbert C, et al. Early-life viral infection and allergen exposure interact to induce an asthmatic phenotype in mice. Respir Res. 2010;11:14. doi: 10.1186/1465-9921-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiko GE, Phipps S, Angkasekwinai P, Dong C, Foster PS. NK cell deficiency predisposes to viral-induced Th2-type allergic inflammation via epithelial-derived IL-25. J Immunol. 2010;185(8):4681–4690. doi: 10.4049/jimmunol.1001758. [DOI] [PubMed] [Google Scholar]
- 16.Petersen BC, Dolgachev V, Rasky A, Lukacs NW. IL-17E (IL-25) and IL-17RB promote respiratory syncytial virus-induced pulmonary disease. Journal of leukocyte biology. 2014 doi: 10.1189/jlb.0913482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng D, Xue Z, Yi L, et al. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. American journal of respiratory and critical care medicine. 2014;190(6):639–648. doi: 10.1164/rccm.201403-0505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YH, Angkasekwinai P, Lu N, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. The Journal of experimental medicine. 2007;204(8):1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terrier B, Bieche I, Maisonobe T, et al. Interleukin-25: a cytokine linking eosinophils and adaptive immunity in Churg-Strauss syndrome. Blood. 2010;116(22):4523–4531. doi: 10.1182/blood-2010-02-267542. [DOI] [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. The European respiratory journal. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.Thomas M, Kay S, Pike J, et al. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Primary care respiratory journal : journal of the General Practice Airways Group. 2009;18(1):41–49. doi: 10.4104/pcrj.2009.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunological reviews. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambrecht BN, Hammad H. Asthma: the importance of dysregulated barrier immunity. European journal of immunology. 2013;43(12):3125–3137. doi: 10.1002/eji.201343730. [DOI] [PubMed] [Google Scholar]
- 24.Blanchard C, Mishra A, Saito-Akei H, Monk P, Anderson I, Rothenberg ME. Inhibition of human interleukin-13-induced respiratory and oesophageal inflammation by antihuman-interleukin-13 antibody (CAT-354) Clin Exp Allergy. 2005;35(8):1096–1103. doi: 10.1111/j.1365-2222.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang G, Volk A, Petley T, et al. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine. 2004;28(6):224–232. doi: 10.1016/j.cyto.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Webb DC, Mahalingam S, Cai Y, Matthaei KI, Donaldson DD, Foster PS. Antigen-specific production of interleukin (IL)-13 and IL-5 cooperate to mediate IL-4R alpha-independent airway hyperreactivity. Eur J Immunol. 2003;33(12):3377–3385. doi: 10.1002/eji.200324178. [DOI] [PubMed] [Google Scholar]
- 27.Mattes J, Yang M, Siqueira A, et al. IL-13 induces airways hyperreactivity independently of the IL-4R alpha chain in the allergic lung. J Immunol. 2001;167(3):1683–1692. doi: 10.4049/jimmunol.167.3.1683. [DOI] [PubMed] [Google Scholar]
- 28.Corrigan CJ, Wang W, Meng Q, et al. T-helper cell type 2 (Th2) memory T cell-potentiating cytokine IL-25 has the potential to promote angiogenesis in asthma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1579–1584. doi: 10.1073/pnas.1014241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang W, Smith SG, Beaudin S, et al. IL-25 and IL-25 receptor expression on eosinophils from subjects with allergic asthma. International archives of allergy and immunology. 2014;163(1):5–10. doi: 10.1159/000355331. [DOI] [PubMed] [Google Scholar]
- 30.Sanz ML, Parra A, Prieto I, Dieguez I, Oehling AK. Serum eosinophil peroxidase (EPO) levels in asthmatic patients. Allergy. 1997;52(4):417–422. doi: 10.1111/j.1398-9995.1997.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 31.Rao R, Frederick JM, Enander I, Gregson RK, Warner JA, Warner JO. Airway function correlates with circulating eosinophil, but not mast cell, markers of inflammation in childhood asthma. Clin Exp Allergy. 1996;26(7):789–793. [PubMed] [Google Scholar]
- 32.Durham SR, Loegering DA, Dunnette S, Gleich GJ, Kay AB. Blood eosinophils and eosinophil-derived proteins in allergic asthma. J Allergy Clin Immunol. 1989;84(6 Pt 1):931–936. doi: 10.1016/0091-6749(89)90391-6. [DOI] [PubMed] [Google Scholar]
- 33.Berry M, Morgan A, Shaw DE, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62(12):1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacci E, Cianchetti S, Bartoli M, et al. Low sputum eosinophils predict the lack of response to beclomethasone in symptomatic asthmatic patients. Chest. 2006;129(3):565–572. doi: 10.1378/chest.129.3.565. [DOI] [PubMed] [Google Scholar]
- 35.Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Systemic upregulation of neutrophil alpha-defensins and serine proteases in neutrophilic asthma. Thorax. 2011;66(11):942–947. doi: 10.1136/thx.2010.157719. [DOI] [PubMed] [Google Scholar]
- 36.Liao H, Yang Z, Yang C, et al. Impact of viral infection on acute exacerbation of asthma in out-patient clinics: a prospective study. Journal of thoracic disease. 2016;8(3):505–512. doi: 10.21037/jtd.2016.02.76. [DOI] [PMC free article] [PubMed] [Google Scholar]