Abstract
Herpes simplex virus (HSV) uses the cell adhesion molecule nectin-1 as a receptor to enter neurons and epithelial cells. The viral glycoprotein D (gD) is used as a non-canonical ligand for nectin-1. The gD binding site on nectin-1 overlaps with a functional adhesive site involved in nectin-nectin homophilic trans-interaction. Consequently, when nectin-1 is engaged with a cellular ligand at cell junctions, the gD binding site is occupied. Here we report that HSV gD is able to disrupt intercellular homophilic trans-interaction of nectin-1 and induce a rapid redistribution of nectin-1 from cell junctions. This movement does not require the receptor’s interaction with the actin-binding adaptor afadin. Interaction of nectin-1 with afadin is also dispensable for virion surfing along nectin-1-rich filopodia. Cells seeded on gD-coated surfaces also fail to accumulate nectin-1 at cell contact. These data indicate that HSV gD affects nectin-1 locally through direct interaction and more globally through signaling.
Keywords: herpes simplex virus, glycoprotein, nectin-1, viral entry, cell adhesion, receptor
INTRODUCTION
Several viruses use cell-cell adhesion molecules from the immunoglobulin (Ig) superfamily as receptors to enter target cells (Dermody et al., 2009). In many cases, the interaction with the viral ligand requires the disruption of the receptor trans-interaction at intercellular contacts (Stehle and Dermody, 2004; Walters et al., 2002). Human viruses such as herpes simplex virus (HSV), measles virus, reoviruses and coxsackie virus (CVB) also require the interaction with cell adhesion molecules to direct virions to the endocytic pathway (Barton et al., 2001; Coyne et al., 2007; Muhlebach et al., 2011; Stiles and Krummenacher, 2010; Stiles et al., 2008; Stiles et al., 2010). Often, initiation or execution of endocytosis may require additional cell surface molecules such as integrins (Coyne and Bergelson, 2006; Gianni and Campadelli-Fiume, 2011; Maginnis et al., 2006; Meier and Greber, 2004; Stiles and Krummenacher, 2010). HSV uses nectin-1 as its main receptor to infect human epithelial cells in culture and neurons ex vivo (Galen et al., 2006; Geraghty et al., 1998; Huber et al., 2001; Linehan et al., 2004; Simpson et al., 2005; Tiwari et al., 2008). In mice, nectin-1 is the main receptor for virus spread to neurons and is responsible for the occurrence of mucocutaneous lesions (Knebel-Morsdorf, 2016; Kopp et al., 2009; Petermann et al., 2015; Rahn et al., 2015; Taylor et al., 2007). HSV can also use two other unrelated receptors, HVEM (herpesvirus entry mediator) and modified 3-O-sulfated-heparan sulfate (3-OS-HS), to enter certain cells (Krummenacher et al., 2004; Montgomery et al., 1996; Shukla et al., 1999; Tiwari et al., 2005; Tiwari et al., 2007). In the absence of nectin-1, HVEM only allows limited spread of HSV to ganglia in mice (Petermann et al., 2015; Taylor et al., 2007) and is necessary for HSV-1, not HSV-2, pathogenesis in the eye (Karaba et al., 2012).
The envelope glycoprotein D (gD) is the viral ligand for nectin-1, HVEM and 3-OS-HS (Krummenacher et al., 1998; Shukla et al., 1999; Whitbeck et al., 1997). Receptor binding to gD induces conformational changes to recruit and activate gH/gL and gB (Atanasiu et al., 2007; Avitabile et al., 2007; Eisenberg et al., 2012; Lazear et al., 2008; Whitbeck et al., 2006). HVEM and nectin-1 bind to different sites on gD but both receptors induce similar conformational changes to activate gD (Connolly et al., 2003; Krummenacher et al., 2005; Lazear et al., 2014; Spear et al., 2006). Membrane fusion can occur at the cell surface in a pH-independent manner (e.g. in Vero cell, primary neurons) but in most cases membrane fusion follows virion endocytosis (Clement et al., 2006; Milne et al., 2005; Nicola, 2016; Nicola and Straus, 2004). The route of HSV entry depends on the cell type but not necessarily on the gD receptor (Delboy et al., 2006; Milne et al., 2005; Nicola and Straus, 2004). Furthermore, fusion with a vesicular membrane occurs in a low-pH-dependent manner in some but not all cell types (Nicola et al., 2005; Nicola et al., 2003). Interaction of gD with nectin-1 or HVEM causes receptor internalization, which triggers virus endocytosis in cells where this route of entry is preferred (Stiles and Krummenacher, 2010; Stiles et al., 2008; Stiles et al., 2010). Virus endocytosis also benefits from the interaction of gH/gL with integrins (Gianni et al., 2013). Integrins are also involved in internalization of reoviruses which use JAM (junctional adhesion molecule) as a receptor (Maginnis et al., 2006). Later during infection, gD de novo expression leads to down-regulation of nectin-1 in infected cells (cis) (Krummenacher et al., 2003). Furthermore, expression of gD at the plasma membrane leads to down-regulation of nectin-1 and HVEM from the surface of adjacent cells (trans) following a transient interaction at cell-cell contacts (Stiles and Krummenacher, 2010; Stiles et al., 2008; Stiles et al., 2010).
Nectins and nectin-like molecules are a family of cell adhesion molecules that contain three extracellular Ig-like domains (one distal V-domains and two proximal C domains) (Mandai et al., 2015). Nectins are homodimers at the cell surface and trans-interact with members of the family in homophilic and heterophilic fashion (Miyahara et al., 2000; Sakisaka et al., 2007). Specificities of ligands within the nectin family allow a large set of trans-interactions that reflects particular intercellular junctions (Rikitake et al., 2012). For instance nectin-1 interacts with itself (homophilic trans-interaction) at epithelial adherens junctions (AJ) and with nectin-3 at neuronal synapses (Mizoguchi et al., 2002). Importantly, nectin-1 trans-interaction with a ligand is necessary for its accumulation at cell contacts (Krummenacher et al., 2003; Miyahara et al., 2000). Upon accumulation to junctions, nectin-1 recruits the actin-binding adaptor afadin (AF-6), which in turn helps recruits cadherin and several junctional molecules to cell-cell contacts (Takai et al., 2008). Although afadin interaction with nectin-1 is not required for infection or gD-mediated internalization of nectin-1 (Stiles and Krummenacher, 2010), it may affect virus spread (Keyser et al., 2007; Sakisaka et al., 2001). Interestingly, formation of actin-based filopodia can be induced by HSV in certain cells and these protrusions are likely involved in macropinocytosis of viral particles (Clement et al., 2006). Actin-remodeling small GTPases Rac1 and cdc42 are activated during HSV entry into epithelial cells (Hoppe et al., 2006) and gD binding activates cdc42 in neurons (De Regge et al., 2006).
Nectins dimerize through their V-domain using a canonical adhesive interface that involves β-strands C-C′-C″-F-G and intervening loops (Harrison et al., 2012). Remarkably, the gD binding site overlaps with this functional interface (Di Giovine et al., 2011; Narita et al., 2011; Zhang et al., 2011). Consequently, gD interferes with nectin-1 adhesive function. Indeed, soluble gD prevents nectin-1-mediated cell aggregation (Krummenacher et al., 2002; Sakisaka et al., 2001). Unlike cellular ligands, which are only known to cause nectin-1 accumulation at cell contacts and recruitment of afadin, gD binding at cell contacts induces nectin-1 endocytosis and dissociation from afadin (Stiles and Krummenacher, 2010; Stiles et al., 2008).
To investigate the specific response induced by gD, we first analyzed nectin-1 localization immediately upon binding to gD. By using purified soluble gD ectodomains we were able to intensify and magnify the effects that the virion glycoprotein may have locally at the site of infection. The very rapid redistribution of nectin-1 from the cell contacts reflects the disruption of the nectin-1 homophilic trans-interaction by gD. In addition to this direct steric effect, exposure of cells to purified gD immobilized on glass surfaces prevented the accumulation of nectin-1 at cell contacts. This suggests that a limited interaction with gD may cause a more extensive response in the cell that affects nectin-1 distribution. We further show that the relocalization of gD does not requiring interaction with the actin-binding afadin and that this interaction is also dispensable for virus surfing along filopodia. Nectin-1 redistribution is specific to gD and reinforces the concept that HSV uses gD as a non-canonical ligand for nectin-1 despite binding to a natural functional site on the receptor.
MATERIALS AND METHODS
Cell lines
Construction of cell lines derived from mouse melanoma B78H1 cells to constitutively express WT nectin-1 (B78H1-C10) or GFP-tagged nectin-1 (B78H1-CG23, -CXG10, -NGC12, -N1VB, -NGC-389) was previously described (Krummenacher et al., 2003; Krummenacher et al., 2002; Stiles and Krummenacher, 2010). These cell lines were grown in Dulbecco modified Eagle’s Medium (DMEM) containing 5% fetal calf serum (FCS) and penicillin/streptomycin plus 0.5 mg/ml G418. Human endometrial epithelial cells (ECC-1, a kind gift from Dr B. Herold) cells were maintained in DMEM-F12 medium supplemented with 10% fetal calf serum. ECC-1 cells were transfected with plasmid pCK495 encoding human nectin-1α tagged with EGFP at the N-terminus (Krummenacher et al., 2003). Cells were selected as a polyclonal population, named ECC-1-N1G, in culture medium supplemented with G418 (1/mg/ml) and subsequently maintained in medium containing 0.5 mg/ml G418.
Proteins and antibodies
Soluble proteins were purified from Sf9 cells infected with recombinant baculovirus. gD(306t) contains the full ectodomain of gD from HSV-1 KOS and gD(285t) corresponds to a shorter form of gD with higher affinity for nectin-1 (Rux et al., 1998; Sisk et al., 1994). Soluble gD was purified by immunoaffinity using monoclonal antibody (MAb) DL6 as previously described. Mutant gD(3C-38C)306t and gD(3C-38C)285t have been described previously (Lazear et al.). Nectin-1(346t) contains the ectodomain of human nectin-1α tagged with 6-histidines at the C-terminus (Krummenacher et al., 1998). Nectin-1(245t) and nectin-1(143t) contains respectively the tagged V-C and V domains of nectin-1 (Krummenacher et al., 1999). Nectin-2(361t) contains the human nectin-2 ectodomain tagged with 6 histidine residues at the C-terminus (Lazear et al.). Soluble nectin ectodomains were purified by nickel affinity chromatography. MAb CK41 detects a conformation-dependent epitope on the V-domain of nectin-1 and prevents nectin-1 binding to gD and to nectin-1 itself (Krummenacher et al., 2000). Thus CK41 is thought to bind a functional site on nectin-1.
Treatment of cells with soluble protein
Cells were seeded on LabTek 3.5 cm glass bottom culture dishes and grown overnight. Cells in 2 ml culture medium were placed in an INSTEC HCS60 heating stage for observation and imaging. Data were collected prior to and immediately after addition of soluble protein. Protein at the indicated final concentration was added to cell culture supernatant directly above the cells and allowed to diffuse. Images were collected using a Nikon Eclipse TE2000-U equipped with a Perkin-Elmer Spinning Disk Ultraview RS confocal scanner and a Hamamatsu ORCA-ER camera. A Melles-Griot Ionlaser system emitting at 488 and 568 nm was used to excite the fluorescence of GFP and AlexaFluor 594. Images were acquired and analyzed using the Perkin Elmer Ultraview software.
Treatment of cells with immobilized protein
PBS solutions containing 10 μg/ml gD(306t), 10 μg/ml gD(285t), 10 μg/ml gD(3-38C)306t, 10 μg/ml gD(3-38C)285t, 10 μg/ml nec1(346t), 14 μg/ml nec1(245t) or 28 μg/ml for the smaller nec1(143t) were added to glass coverslips and incubated for 2.5 h at room temperature. Coverslips were washed with culture medium containing 5% FCS. Cells were seeded on treated coverslips and allowed to adhere for various times. Medium was removed and cells were fixed with 3% paraformaldehyde in PBS for at least 30 min before quenching with 50 mM NH4Cl for 5 min at room temperature. The coverslips were rinsed three times with PBS and once with H2O and then mounted in ProLong Antifade solution.
Virus surfing
B78H1-C10 and B78H1-NGC-389 cells were grown overnight to be subconfluent on LabTek 3.5 cm glass bottom culture dishes. Virus KOS-K26 (Desai and Person, 1998) was added at an MOI of 50 pfu/cell and recorded over time. Images were collected using a Nikon Eclipse TE2000-U equipped with a Perkin-Elmer Spinning Disk Ultraview RS confocal scanner and a Hamamatsu ORCA-ER camera.
RESULTS
Rapid relocalization of nectin-1 at cell contacts in the presence of soluble gD
Since the gD binding site overlaps a nectin-1 functional site (Di Giovine et al., 2011; Zhang et al., 2011), we investigated how gD affects nectin-1 accumulation at cell contacts. We first used B78H1-derived cells as a model since B78H1 mouse melanoma cells do not express any endogenous HSV gD receptor (Miller et al., 2001). In B78-CG23 cells, nectin-1 tagged with a C-terminal GFP (nectin-1-GFP) is the only functional receptor for gD (Krummenacher et al., 2003). It is also a functional adhesion molecule, even though the cytosolic GFP tag prevents interaction with afadin (Krummenacher et al., 2003). Nectin-1-GFP accumulates on filopodia and at contact areas between cells where it forms a patchy pattern of zones of intense accumulation and zones of apparent exclusion (Fig. 1A, D, G, J). When B78-CG23 cells were exposed to low concentrations of the high-affinity gD(285t), the distribution of nectin-1 was rapidly affected (Fig. 1A–C). After 10 seconds, changes of pattern are limited (compare panels A and B, at areas indicated by arrow #1). Within two minutes following the addition of 5 μg/ml gD(285t), no recognizable pattern could be seen (Fig. 1C). Movie S1 (suppl. data) shows that relocalization of nectin-1 is essentially complete 40 seconds after adding 100 μg/ml gD(285t). gD(3C-38C) fails to bind nectin-1 due to a double mutation which blocks access to the nectin-1 binding site (Connolly et al., 2005). Accordingly, the non-binding gD(3C-38C)306t had no effect on nectin-1 localization, even at high concentration (Fig. 1E, F)(Movie S2). Since nectin-1 naturally binds to itself, the nectin-1 ectodomain (nec1(346t)) or the single adhesive V-domain (nec1(143t)) were tested. At high concentrations, neither soluble forms of nectin-1 affected nectin-1-GFP localization (Fig 1G–L). This suggests that, unlike gD, soluble nectin-1 did not compete with membrane anchored nectin-1. Because addition of GFP to the nectin-1 C-terminus prevents functional interaction with cytoplasmic afadin in B78-CG23 cells, we also tested the effect of gD on nectin-1 tagged with an N-terminal GFP (GFP-nectin-1) in B78-NCG12 cells. GFP-nectin-1 interacts with cytoplasmic afadin (Krummenacher et al., 2003) but was similarly redistributed upon addition of gD to B78-NGC12 cells (data not shown). This suggests that gD binding to a functional site of nectin-1 uniquely leads to rapid redistribution of nectin-1 from cell contacts. This effect does not require the connection of nectin-1 to the actin cytoskeleton mediated by afadin.
Figure 1. Relocalization of nectin-1-GFP in response to soluble gD.
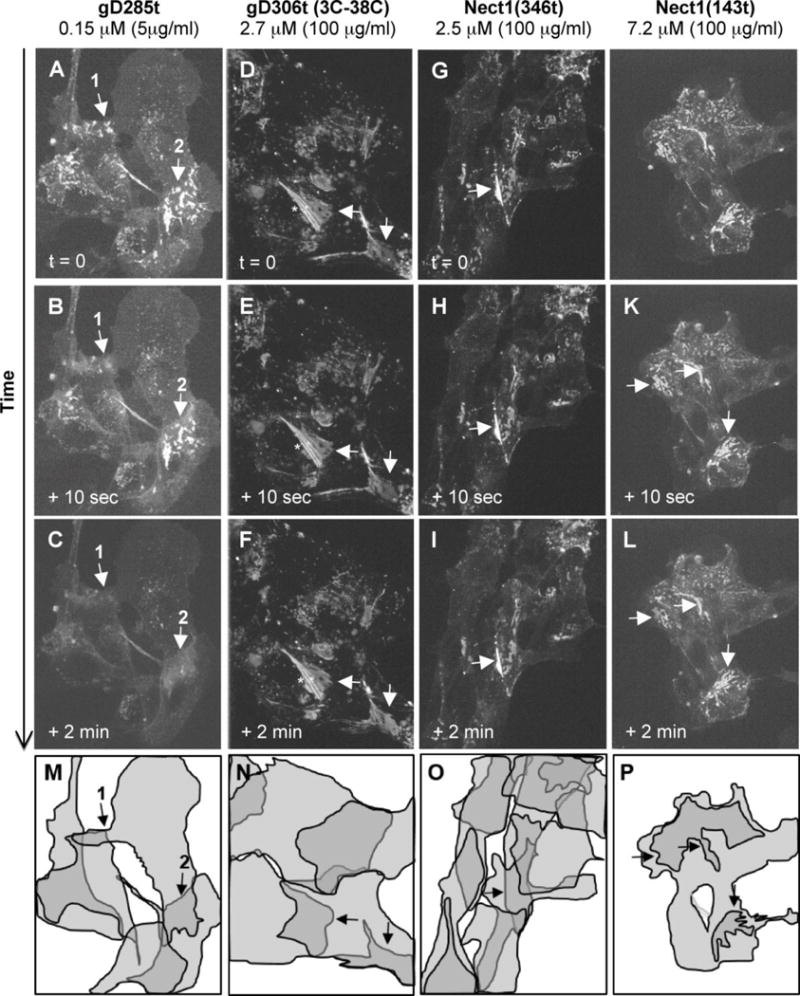
Localization of nectin-1-GFP in B78-CG23 cells is indicated at different time points after the addition of the indicated purified forms of gD. Panels A, D, G and J show the typical pattern of nectin-1-GFP accumulation at contacts between overlapping cells (arrows) prior to the addition of gD. Only soluble gD(285t) led to changes in this accumulation pattern (A–C). Soluble non-binding gD(3-38C)306t (D–F), soluble nectin-1 ectodomain (G–I) and soluble nectin-1 V-domain (J–L) do not affect nectin-1-GFP patterns over time. Total projections of Z-sections are shown at each time point. Striation indicated by and asterisk is due to this projection of non-overlapping sections. Panels M-O indicate the position of major cells in the corresponding fields. These shapes represent the best approximations from 3D projections of the multiple confocal z-sections. The darker areas represent cell overlaps. In panel P, the overall shape of the cell cluster and the darker areas of cell contacts are indicated.
Alternative splicing of the PVRL1 gene can generate two membrane-anchored isoforms of nectin-1 (α and β), which share the same ectodomain but have alternate trans-membrane regions and cytoplasmic tails. Nectin-1α, which was used so far in this study, possesses the C-terminal afadin binding site. In contrast, nectin-1β lacks this binding site and is naturally unable to bind afadin. Nevertheless, nectin-1β accumulates at cell contacts of B78H1 cells upon homophilic trans-interaction (Stiles and Krummenacher, 2010). Here, B78-N1BG cells expressing GFP-nectin-1β (Stiles and Krummenacher, 2010) were used to record the localization of the receptor before and immediately after addition of the soluble gD. For live cell microscopy, we used the full gD ectodomain (gD(306t)), which has a lower affinity for nectin-1 compared to gD(285t) (Krummenacher et al., 1998). Similar to the α isoform, GFP-nectin-1β accumulated on filopodia and at defined zones of the cell contact area (Fig. 2A). When gD(306t) was added, this pattern gradually dissolved and after 2 minutes the distribution of nectin-1 appeared homogenous (Fig. 2B–F, Movie S3). Upon close examination, it appears the dissolution of that pattern originates at the periphery of the contact area and proceeds towards its center. Concomitantly, accumulation of nectin-1 on filopodia also decreased. When soluble nectin-1(346t) is used under similar conditions no effect is seen (Movie S4). This control shows that the apparent fading of the signal upon addition of gD is not due to photobleaching of GFP. We took advantage of the contact zones with different intensities to analyze the changes of redistribution. Regions with low and high accumulation of nectin-1-GFP were selected to monitor changes of fluorescence intensities before and immediately after the addition of soluble gD(306t) (Fig. 2G, H). A loss of intensity in the bright zone was concomitant with an increase of brightness in the originally dark zone. This change is reproducible. However, the decrease in brightness within an area of contact is not necessarily of the same magnitude as the increase of brightness in another area, because bright and dark areas are not necessarily equal in surface. However, brightness across reaches similar levels across the contact area at the end of the recording, as suggested by the images (Fig. 2G; Suppl. movie S3). The movement of the cells further complicates the analysis (notably of the kinetics of redistribution) as the contact areas can vary over time. To avoid over interpretation of quantification based on confocal sections, we used arbitrary units in our measurements over time and omitted numbers in graphs of figure 2H. This suggests that gD induces a fast redistribution of nectin-1 on the plasma membrane at or near cell-cell contact sites. Because nectin-1 needs to trans-interact with a membrane-anchored ligand on opposite cells to accumulate at cell contacts, this dissipation is consistent with soluble gD disrupting the functional homophilic trans-interaction of nectin-1 (Krummenacher et al., 2002; Sakisaka et al., 2001). Consequently, nectin-1 bound to soluble gD is free to move on the cell surface (Fig. 2I). This characteristic of gD was also tested in epithelial cells expressing a GFP-nectin-1 which was able to interact with cytoplasmic afadin. Human endometrial epithelial cells (EEC-1) were stably transfected to express GFP-nectin-1α and sorted to generate the polyclonal line ECC-1-N1G. In these cells GFP-nectin-1 readily accumulates at cell contacts (Fig. 3A.). Upon addition of gD(285t), we also observed a redistribution of nectin-1 away from the cell junctions. In these cells gD appears less efficient since a distinct amount of nectin-1 remains at cell contacts. Although gD is seen at cell junctions (Fig. 3B inset), the relative inefficiency of gD to induce large scale redistribution of nectin-1 may be due to the difficulty that gD may encounter to reach nectin-1 in established junctions. Altogether, these data demonstrate that gD is able to displace nectin-1 ligands at cell contacts.
Figure 2.
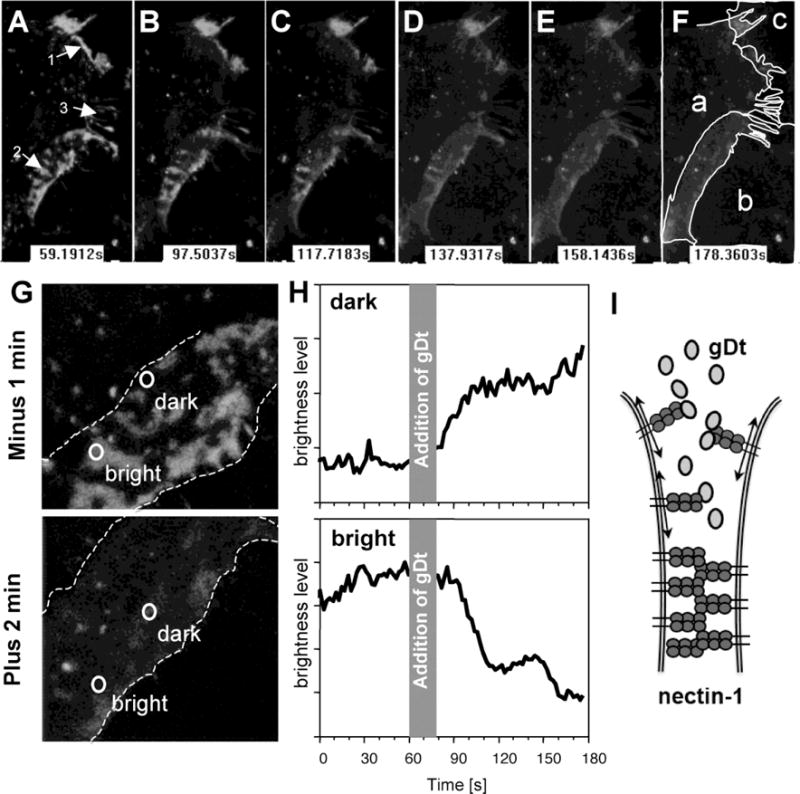
Dissipation of nectin-1 from cell contacts induced by soluble gD. A–F. B78H1-N1BG cells expressing nectin-1β-GFP were observed by confocal live cell microscopy and recorded before (A) and during 2 min following addition of soluble gD (B–F). Purified ectodomain gD(306t) was added (100 μg/ml) after 60 sec of recording. Nectin-1-GFP is visible at cell contacts (1–2) and on filopodia (3) before addition of gD. Nectin-1 forms a typical pattern of nectin-1 at areas of contact between cells a and b (2). Light and dark zones are rapidly lost and the contact area appears a pale gray. The circles delineating regions of interests appear to be located differently in the picture frame as they followed cells moving towards each other. Recording started at time t=0 sec, stopped at t=60 sec for addition of gD and resumed at t=77 sec. At each indicated time a stack of six z-sections is shown. Shape of cells a-d is shown on panel F. Panels A-F are black and white snapshots form live recording (Supplementary Movie S3) with enhanced brightness and contrast. All panels were treated similarly to avoid artefacts. G. Magnification of contact area between cells a and b to show the localization of regions of interest where nectin-1-GFP accumulates (bright) or is excluded (dark). The area of overlap is delineated by dashed lines. Snapshots were collected 60 sec prior to the addition of gD or 100 sec after recording resumed. H. Quantification of GFP intensity over time in regions of interest indicated in panel G was performed over time using supplementary movie S3. The gray area indicates the period of time when recording was paused during the addition of gD. Arbitrary units are used. I. Model of infiltration and binding of soluble gD leading to lateral diffusion of nectin-1-GFP.
Figure 3.
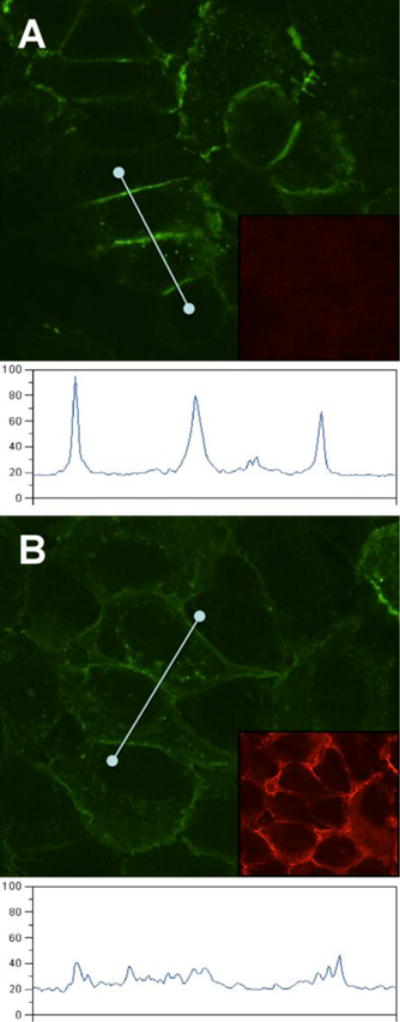
Redistribution of GFP-nectin-1 from junction of epithelial cells. A. detection of GFP-nectin-1a accumulating at junctions between human ECC-1-NIG cells. B. After incubation with soluble gD(285t) (1 μM) for 2h, the intensity of GFP at cell junctions is decreased. Under each condition, an intensity profile following a straight line through several cell junctions is shown. Images were captured under similar conditions. Intensity units are arbitrary. The insets show gD immunostaining with polyclonal serum R7 followed by Alexa594-coupled secondary antibody.
Exposure to immobilized gD causes a relocalization of nectin-1
The relocation of nectin-1 from cell junctions by soluble gD reflects the ability of gD to disrupt the nectin-1 trans-dimers. Here, we wanted to test whether gD can affect nectin-1 location without intercalating between cells at junctions. It has been shown that the spreading of L-cells was affected by immobilized nectin-1 ligands on the support surface (Kawakatsu et al., 2002). Thus we tested whether gD may also globally affect the distribution of nectin-1 when immobilized on glass coverslips. B78-CXG10 cells which express nectin-1-GFP (Krummenacher et al., 2003) were allowed to attach and spread on the glass coverslip surface before fixing and observation of GFP. When coverslips were pre-treated with PBS only, nectin-1-GFP showed its characteristic accumulation at cell-contact (Fig. 4A). In contrast, when gD(285t) was immobilized on the surface, nectin-1 failed to accumulate at cell contacts, even though gD was not sterically intercalating between cells (Fig 4B). A milder effect was seen with the low affinity gD(306t) (Fig. 4B). The specificity was confirmed with the use of gD(3C-38C) that does not bind nectin-1. Cells exposed to this form of gD still accumulated nectin-1-GFP at cell contacts (Fig. 4D, E). Nectin-1-GFP in B78-CXG10 cells does not interact with endogenous afadin. However, the same effect is observed using B78-NGC12 cells expressing GFP-nectin-1 that interacts with afadin and B78-CG23, where nectin-1GFP does not interact with afadin (data not shown). This suggests that the response is not mediated through afadin. We further compared the effect of immobilized gD with that of various nectin-1 constructs. Again, a lack of accumulation of nectin-1 at cell contacts was seen when gD(285t) was present on the glass surface (Fig. 5B). In contrast, nectin-GFP was not visibly perturbed when any one of the nectin-1 constructs was immobilized on glass (Fig 5D–F). The soluble nectin-2 ectodomains (i.e. nec2(361t)) which does not interact with nectin-1 was used as a negative control and showed no effect. This suggests that gD may have an extensive effect in the location of nectin-1 and induces a different response than nectin-1 itself.
Figure 4.
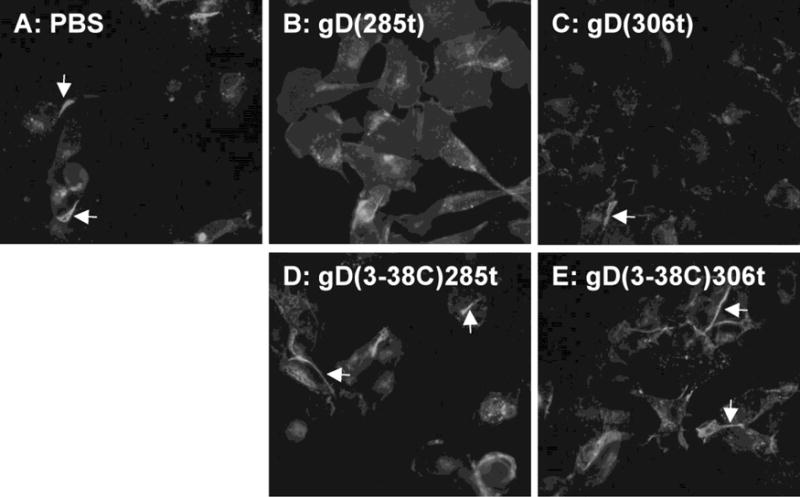
Inhibition of accumulation of nectin-1 at cell contact by immobilized gD. B78-CXG10 cells expressing nectin-1-GFP were seeded for two hours on glass that had been pretreated with PBS or the indicated purified forms of gD. Cells were fixed after 2h. Arrows indicate accumulation of nectin-1-GFP at cell contacts.
Figure 5.
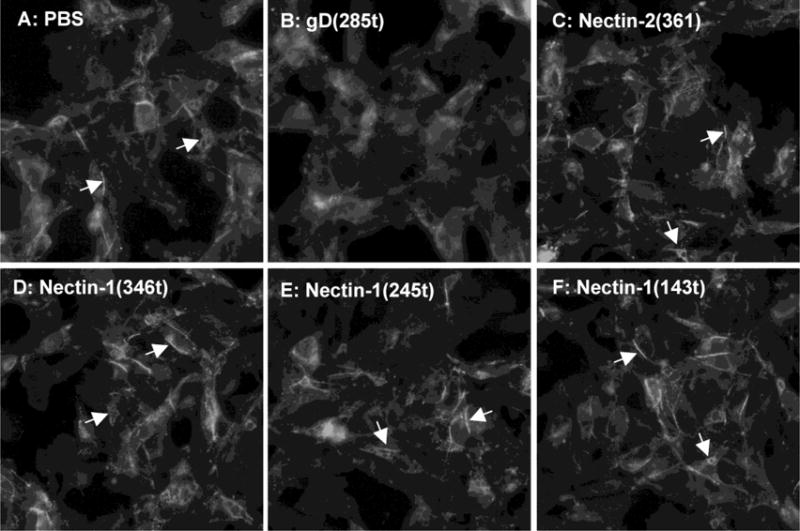
Accumulation of nectin-1 at cell contact in the presence of immobilized purified nectin-1. B78-CXG10 cells expressing nectin-1-GFP were seeded for two hours on glass that had been pretreated with PBS or the indicated purified proteins. Cells were cultures overnight before fixation. Arrows indicate accumulation of nectin-1-GFP at cell contacts.
Virus surfing on nectin-1-expressing cells
HSV, like other viruses, has been shown to move along cell filopodia and the speed of this movement is consistent with actin-based motors (Clement et al., 2006; Lehmann et al., 2005; Oh et al., 2010). Since nectin-1 is abundant on filopodia, we investigated whether interaction of nectin-1 cytoplasmic tail with the actin-binding afadin was required for surfing. We first used C10 cells, which only have human nectin-1α as a receptor for HSV. In these cells nectin-1 binds to afadin (Krummenacher et al., 2002). When HSV-K26, which has its capsid tagged with GFP (Desai and Person, 1998), was added to cells, it rapidly attached to the surface. Virions that bound to distal regions of filopodia moved directionally towards the cell body and accumulated at the base of filopodia (Fig. 6). We then used B78-NGC-289 cells which express a truncated form of human nectin-1α lacking the cytoplasmic tail and its afadin binding site (Stiles and Krummenacher, 2010). This receptor, which is functional for entry and for adhesion, is also tagged with GFP at its N-terminus. Again, when HSV-K26 is added to these cells, virions are seen moving along filopodia (Fig. 7). Both virions move towards the same cell (upper left side of images in figure 7), where virions are already accumulating and where active membrane ruffling is observed. This indicates that the association of nectin-1 with cytoplasmic adaptors, including the actin-binding afadin, is not required for virus surfing.
Figure 6.
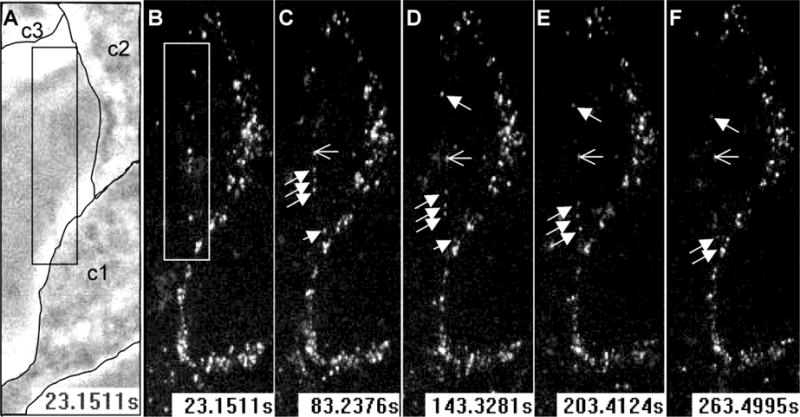
HSV surfing on filopodia of nectin-1 expressing cells. HSV-K26 with GFP-tagged capsid was added to C10 cells about 3 min prior to recording. Images show the space between two cells (boxed in A and B) connected by a thin filopodium, which itself is not visible in contrast image (panel A) Three particles (filled arrows) move at the same speed and reach the lower cell where virions are already accumulating. The open arrow shows a fixed capsid for reference. The contours of unstained cells (c1, c2 and c3) are outlined in panel A and became visible in fluorescence fields as they accumulate GFP-tagged virions (B–F). Stacks of 3 confocal Z-sections are shown at each time point in the GFP channel (B–F). Time from the start of recording is indicated in seconds.
Figure 7.
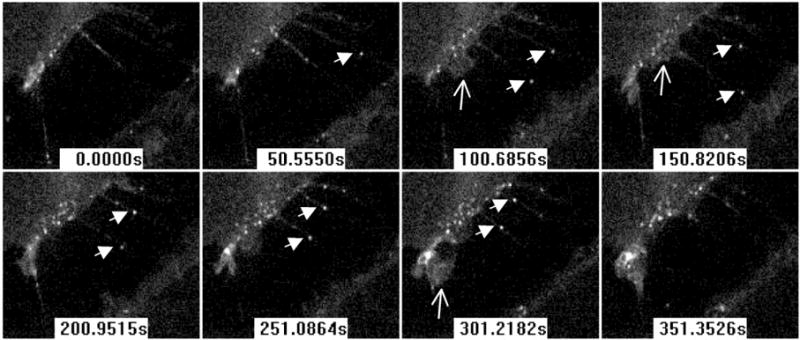
HSV surfing on filopodia of cells expressing GFP-nectin-1 without cytoplasmic tail. HSV-K26 with GFP-tagged capsid is added to B78-NGC-389 cells immediately prior to recording. Confocal images show the space between two cells connected by filopodia. Filled arrows indicate two moving capsids on two different filopodia. Open arrows indicate areas of ruffling membrane. Time from the start of recording is indicated in seconds.
DISCUSSION
It is remarkable that several viruses use nectins as receptors since these molecules may not be readily available on the surface of the cell (Geraghty et al., 1998; Mendelsohn et al., 1989; Muhlebach et al., 2011; Noyce et al., 2011; Noyce et al., 2013). Furthermore, viral ligands often share binding sites with the natural ligands for these adhesion molecules (Di Giovine et al., 2011; Harrison et al., 2012; Zhang et al., 2013). This implies that the viral ligand may need to displace natural ligands at cell contacts and disrupts receptors trans-interactions during entry. The reason for such a preference shown by many unrelated viruses is unclear. It may be to access areas with high density of receptors, to induce signaling that mimic cell adhesion or, for HSV and measles in particular, to spread directly from cell to cell. In any case, viruses using such receptors face several challenges that include reaching the receptor, accessing the binding site and possibly inducing endocytosis. The consequences of gD binding to nectin-1 to trigger the fusion machinery are well documented, however this binding is not sufficient to allow virus entry (Geraghty et al., 2001). This is important since this observation suggests that nectin-1 has an additional role in routing virions during entry and/or transducing signals that benefit the establishment of infection.
HSV binding to cells is sufficient to activate signaling pathways (MacLeod and Minson, 2010). A first pathway may activate PI3K to induce the formation of filopodia (Clement et al., 2006; Tiwari and Shukla, 2010) and a second pathway may trigger endocytosis of the virus (Gianni and Campadelli-Fiume, 2011; Stiles and Krummenacher, 2010; Stiles et al., 2010). HSV moves towards the cell body by riding along these actin-rich filopodia (figures 6, 7 and reference (Clement et al., 2006; Oh et al., 2010)). Oh et al. (Oh et al., 2010) reported that cdc42, which can be activated by gD binding to nectin-1 (De Regge et al., 2006), had only a limited role in HSV surfing. That study also showed that gB alone allows surfing of quantum dots, presumably through interaction with heparan sulfate proteoglycans (Oh et al., 2010). However, since nectin-1 is enriched on filopodia and is able to connect to the actin cytoskeleton via the adaptor afadin, we asked whether this connection was required for HSV surfing. HSV movement appeared similar on filopodia of B78H1 derived cells whether the available nectin-1 was able or not to connect to the actin cytoskeleton, this indicates that HSV does not take advantage of the receptor connection to the actin cytoskeleton to move towards or on the cell surface.
Since HSV uses a cell adhesion molecule as a receptor, it is important for the virus to access its receptor at cell junctions. This is supported by the observation that disruption of cell junctions with calcium chelators exposes nectin-1 and facilitates HSV entry (Yoon and Spear, 2002). Furthermore, the overlapping positions of the gD binding site and the canonical nectin-1 dimerization site posits that a dissociation of nectin-1 dimers at must occur during virus entry, or spread, at cell junctions. Since a direct observation of this local virus-induced dissociation is difficult to observe, we used soluble gD to magnify this effect throughout cell contact surfaces. The soluble glycoprotein diffuses more readily than virions and allows the visualization of nectin-1 dissociation by increasing receptor motility on the plasma membrane. Using this model we observed that the extent and rapidity of nectin-1 relocalization was dependent on the concentration and affinity of gD. The affinity of the gD ectodomain (gD(306t)) for soluble nectin-1 is in the micromolar range but can be increased by truncating its C-terminus which masks part of the nectin-1 binding site. As a result gD(285t) binds faster to nectin-1 thereby increasing its affinity 50 to 100 fold. The affinity of nectin-1 homo-dimerization was determined as 17.5 mM (Harrison et al., 2012). This may explain why a relatively high concentration of gD(306t) was needed. Although it is difficult to relate the active concentration of soluble gD with the amount of gD on the virion envelope required for entry, the local concentration of gD on the viral envelope may be sufficient to disrupt nectin-1 trans-interaction and localization locally at the site of entry. A close investigation of the effects of virion gD at the local site of interaction with nectin-1 is needed. The global redistribution of nectin-1, which is magnified by soluble gD, is unlikely to extend to the whole junction in the presence of limited number of virions. Yet, the ability of gD to induce nectin-1 motility suggests that it may occur locally at the site of virion binding. We believe that this capacity is critical for the virus to use nectin-1 receptors that are engaged in homophilic interactions at cell junctions.
The use of soluble gD aims at magnifying effects on receptors for direct and kinetics observations. The concentrations used cannot be directly compared to a concentration of gD during an infection but may mimic local concentration of virion gD at the site of binding. The largescale effect on junctions may not be achieved during infection but our data reflects relocalization of nectin-1 bound by virions during infection and spread. This relocation is thought to be a prelude to virus internalization by endocytosis (Stiles and Krummenacher, 2010). Adenoviruses use a soluble form of their receptor-binding fiber protein to disrupt CAR trans-interaction and facilitate dissemination of virus through epithelium (Walters et al., 2002). There is no evidence that HSV uses or requires this mechanism to disseminate through an epithelial tissue.
Relatively little is known about cell responses induced by HSV gD binding to nectin-1. Like nectin-1 natural ligands, gD activates small GTPases involved in actin-remodeling (De Regge et al., 2006; Hoppe et al., 2005; Hoppe et al., 2006; Ogita and Takai, 2006) but gD appears unique in triggering nectin-1 internalization rather than accumulation at cell contacts (Stiles and Krummenacher, 2010). Interestingly, the majority of GFP-nectin-1 was down-regulated from the surface of B78 cells early during entry. Since those cells overexpress nectin-1, the extent of down-regulation achieved by relatively few virions (50 pfu/cells), suggested that HSV may induce a response leading to internalization of nectin-1 molecules not directly interacting with gD. Consistent with that observation, here we see that, cells exposed to gD adsorbed on support surface failed to accumulate nectin-1 at cell contacts. Soluble gD, but not soluble nectin-1, triggers this response through interaction with cell surface nectin-1. This again sets gD apart from natural ligands for nectin-1, however this response may be triggered naturally under physiological circumstances that have not yet been observed. This study shows that HSV gD is adapted to disrupt nectin-1 trans-interaction at cell contacts and to induce receptor relocalization away from junctions as a prelude to virus endocytosis. This highlight the dual nature of gD which mimics the binding of a natural nectin ligand but induces an alternate response in the cell for the benefit of the virus during entry.
Supplementary Material
B78H1-N1BG cells expressing nectin-1β-GFP were recorded for 1 min before and during 2 min following the addition of soluble purified gD ectodomain, gD(285t) (100 μg/ml). Nectin-1-GFP is visible at cell contacts and on filopodia before the addition of gD. Nectin-1-GFP forms a typical pattern of nectin-1 at areas of contact (bottom right quadrant) which is rapidly lost after gD(285t) is added. Recording started at time t=0 sec, stopped at t=60 sec for addition of gD and resumed at t=77 sec. A projection of six z-sections is shown.
B78H1-N1BG cells expressing nectin-1β-GFP were recorded for 1 min before and during 2 min following the addition of soluble purified nectin-1 ectodomain nectin-1(346t) (100 μg/ml). Nectin-1-GFP is visible at cell contacts before the addition of gD and its distribution remains unchanged in the presence of soluble nectin-1. Recording started at time t=0 sec, stopped at t=60 sec for addition of gD and resumed at t=78 sec. A projection of six z-sections is shown.
B78H1-N1BG cells expressing GFP-nectin-1α were recorded for 1 min before and during 2 min following the addition of soluble purified gD truncation, gD(285t) (100 μg/ml). GFP-Nectin-1 forms a typical pattern of nectin-1 at areas of contact (right side of screen) which is rapidly lost after gD(285t) is added. Recording started at time t=0 sec, stopped at t=60 sec for addition of gD and resumed at t=84 sec. A projection of z-sections is shown.
B78H1-CG23 cells expressing nectin-1α-GFP were recorded for 1 min before and during 2 min following the addition of soluble purified gD truncation, gD(3-38C)285t (100 μg/ml). Nectin-1-GFP forms a typical pattern of nectin-1 at areas of contact (for instance bottom right region of screen) which is maintained after gD(3-38C)285t is added. Recording started at time t=0 sec, stopped at t=60 sec for addition of gD and resumed at t=83 sec. A projection of z-sections is shown. Since the z-sections are non-overlapping, striation are seen in some areas due to section stacking.
Research highlights VIRO-16-490.
Soluble forms of HSV gD induce nectin-1 relocalization at cell junctions.
Nectin-1 relocalization is rapid and specific to gD binding.
Exposure to immobilized HSV gD prevents of nectin-1 accumulation at cell contacts.
Virion surfing does not require nectin-1 connection to afadin and cytoskeleton.
Acknowledgments
This study was supported by Public Health Service grant AI-097171 to C.K. from the National Institute of Allergy and Infectious Diseases and by the College of Sciences and Mathematics at Rowan University. We thank Betsy Herold (A. Einstein College of Medicine) for the ECC-1 cell line. We are very grateful to Gary Cohen and Roselyn Eisenberg (UPENN) for reagents and discussions of the project. We appreciate the advice and helpful discussions from members of the Krummenacher and Cohen/Eisenberg laboratories.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atanasiu D, Whitbeck JC, Cairns TM, Reilly B, Cohen GH, Eisenberg RJ. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc Natl Acad Sci U S A. 2007;104:18718–18723. doi: 10.1073/pnas.0707452104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitabile E, Forghieri C, Campadelli-Fiume G. Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein. J Virol. 2007;81:11532–11537. doi: 10.1128/JVI.01343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody TS. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BY, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174:1009–1021. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SA, Landsburg DJ, Carfi A, Whitbeck JC, Zuo Y, Wiley DC, Cohen GH, Eisenberg RJ. Potential nectin-1 binding site on herpes simplex virus glycoprotein D. Journal of Virology. 2005;79:1282–1295. doi: 10.1128/JVI.79.2.1282-1295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Cohen GH, Eisenberg RJ. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD/HveA interface. J Virol. 2003;77:8127–8140. doi: 10.1128/JVI.77.14.8127-8140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Shen L, Turner JR, Bergelson JM. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2:181–192. doi: 10.1016/j.chom.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Regge N, Nauwynck HJ, Geenen K, Krummenacher C, Cohen GH, Eisenberg RJ, Mettenleiter TC, Favoreel HW. Alpha-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J Cell Biol. 2006;174:267–275. doi: 10.1083/jcb.200510156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delboy MG, Patterson JL, Hollander AM, Nicola AV. Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells. Virol J. 2006;3:105. doi: 10.1186/1743-422X-3-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody TS, Kirchner E, Guglielmi KM, Stehle T. Immunoglobulin superfamily virus receptors and the evolution of adaptive immunity. PLoS Pathog. 2009;5:e1000481. doi: 10.1371/journal.ppat.1000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P, Person S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol. 1998;72:7563–7568. doi: 10.1128/jvi.72.9.7563-7568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog. 2011;7:e1002277. doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen B, Cheshenko N, Tuyama A, Ramratnam B, Herold BC. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J Virol. 2006;80:12209–12218. doi: 10.1128/JVI.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty RJ, Fridberg A, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Use of chimeric nectin-1(HveC)-related receptors to demonstrate that ability to bind alphaherpesvirus gD is not necessarily sufficient for viral entry. Virology. 2001;285:366–375. doi: 10.1006/viro.2001.0989. [DOI] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Eisenberg RJ, Cohen GH, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Gianni T, Campadelli-Fiume G. alphaVbeta3-integrin relocalizes nectin1 and routes herpes simplex virus to lipid rafts. J Virol. 2011 doi: 10.1128/JVI.06689-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni T, Salvioli S, Chesnokova LS, Hutt-Fletcher LM, Campadelli-Fiume G. αvβ6- and αvβ8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLoS Pathog. 2013;9:e1003806. doi: 10.1371/journal.ppat.1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison OJ, Vendome J, Brasch J, Jin X, Hong S, Katsamba PS, Ahlsen G, Troyanovsky RB, Troyanovsky SM, Honig B, Shapiro L. Nectin ectodomain structures reveal a canonical adhesive interface. Nat Struct Mol Biol. 2012;19:906–915. doi: 10.1038/nsmb.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe S, Jaeger V, Schelhaas M, Knebel-Morsdorf D. HSV infection of epithelial cells: role of Rac1, cdc42 and VASP during entry. 30th International Herpesvirus Workshop; Turku, Fin. 2005. [Google Scholar]
- Hoppe S, Schelhaas M, Jaeger V, Liebig T, Petermann P, Knebel-Morsdorf D. Early herpes simplex virus type 1 infection is dependent on regulated Rac1/Cdc42 signalling in epithelial MDCKII cells. J Gen Virol. 2006;87:3483–3494. doi: 10.1099/vir.0.82231-0. [DOI] [PubMed] [Google Scholar]
- Huber MT, Wisner TW, Hegde NR, Goldsmith KA, Rauch DA, Roller RJ, Krummenacher C, Eisenberg RJ, Cohen GH, Johnson DC. Herpes Simplex Virus with Highly Reduced gD Levels Can Efficiently Enter and Spread between Human Keratinocytes. J Virol. 2001;75:10309–10318. doi: 10.1128/JVI.75.21.10309-10318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaba AH, Kopp SJ, Longnecker R. Herpesvirus entry mediator is a serotype specific determinant of pathogenesis in ocular herpes. Proc Natl Acad Sci U S A. 2012;109:20649–20654. doi: 10.1073/pnas.1216967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakatsu T, Shimizu K, Honda T, Fukuhara T, Hoshino T, Takai Y. Trans-interactions of nectins induce formation of filopodia and Lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J Biol Chem. 2002;277:50749–50755. doi: 10.1074/jbc.M209846200. [DOI] [PubMed] [Google Scholar]
- Keyser J, Lorger M, Pavlovic J, Radziwill G, Moelling K. Role of AF6 protein in cell-to-cell spread of Herpes simplex virus 1. FEBS Lett. 2007;581:5349–5354. doi: 10.1016/j.febslet.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Knebel-Morsdorf D. Nectin-1 and HVEM: cellular receptors for HSV-1 in skin. Oncotarget. 2016 doi: 10.18632/oncotarget.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp SJ, Banisadr G, Glajch K, Maurer UE, Grunewald K, Miller RJ, Osten P, Spear PG. Infection of neurons and encephalitis after intracranial inoculation of herpes simplex virus requires the entry receptor nectin-1. Proc Natl Acad Sci U S A. 2009;106:17916–17920. doi: 10.1073/pnas.0908892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Baribaud F, Ponce De Leon M, Baribaud I, Whitbeck JC, Xu R, Cohen GH, Eisenberg RJ. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology. 2004;322:286–299. doi: 10.1016/j.virol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Krummenacher C, Baribaud I, Eisenberg RJ, Cohen GH. Cellular localization of nectin-1 and glycoprotein D during herpes simplex virus infection. J Virol. 2003;77:8985–8999. doi: 10.1128/JVI.77.16.8985-8999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Baribaud I, Ponce de Leon M, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. Localization of a binding site for herpes simplex virus glycoprotein D on the herpesvirus entry mediator C by using anti-receptor monoclonal antibodies. J Virol. 2000;74:10863–10872. doi: 10.1128/jvi.74.23.10863-10872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Baribaud I, Sanzo JF, Cohen GH, Eisenberg RJ. Effects of herpes simplex virus on structure and function of nectin- 1/HveC. J Virol. 2002;76:2424–2433. doi: 10.1128/jvi.76.5.2424-2433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Rux AH, Whitbeck JC, Ponce de Leon M, Lou H, Baribaud I, Hou W, Zou C, Geraghty RJ, Spear PG, Eisenberg RJ, Cohen GH. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity while the third domain is involved in oligomerization of HveC. J Virol. 1999;73:8127–8137. doi: 10.1128/jvi.73.10.8127-8137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, Eisenberg RJ, Cohen GH, Wiley DC, Carfi A. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. Embo J. 2005;24:4144–4153. doi: 10.1038/sj.emboj.7600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear E, Carfi A, Whitbeck JC, Cairns TM, Krummenacher C, Cohen GH, Eisenberg RJ. Engineered disulfide bonds in herpes simplex virus type 1 gD separate receptor binding from fusion initiation and viral entry. J Virol. 2008;82:700–709. doi: 10.1128/JVI.02192-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear E, Whitbeck JC, Zuo Y, Carfi A, Cohen GH, Eisenberg RJ, Krummenacher C. Induction of conformational changes at the N-terminus of herpes simplex virus glycoprotein D upon binding to HVEM and nectin-1. Virology. 2014;448:185–195. doi: 10.1016/j.virol.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170:317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM, Richman S, Krummenacher C, Eisenberg RJ, Cohen GH, Iwasaki A. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J Virol. 2004;78:2530–2536. doi: 10.1128/JVI.78.5.2530-2536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod IJ, Minson T. Binding of herpes simplex virus type-1 virions leads to the induction of intracellular signalling in the absence of virus entry. PLoS One. 2010;5:e9560. doi: 10.1371/journal.pone.0009560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maginnis MS, Forrest JC, Kopecky-Bromberg SA, Dickeson SK, Santoro SA, Zutter MM, Nemerow GR, Bergelson JM, Dermody TS. Beta1 integrin mediates internalization of mammalian reovirus. J Virol. 2006;80:2760–2770. doi: 10.1128/JVI.80.6.2760-2770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Rikitake Y, Mori M, Takai Y. Nectins and nectin-like molecules in development and disease. Curr Top Dev Biol. 2015;112:197–231. doi: 10.1016/bs.ctdb.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Meier O, Greber UF. Adenovirus endocytosis. J Gene Med. 2004;6(Suppl 1):S152–163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Miller CG, Krummenacher C, Eisenberg RJ, Cohen GH, Fraser NW. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol Ther. 2001;3:160–168. doi: 10.1006/mthe.2000.0240. [DOI] [PubMed] [Google Scholar]
- Milne RS, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J Virol. 2005;79:6655–6663. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T, Honda T, Kiyohara Y, Heo K, Higashi M, Tsutsumi T, Sonoda S, Ide C, Takai Y. Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol. 2002;156:555–565. doi: 10.1083/jcb.200103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, Leonard VH, Navaratnarajah CK, Frenzke M, Wong XX, Sawatsky B, Ramachandran S, McCray PB, Jr, Cichutek K, von Messling V, Lopez M, Cattaneo R. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480:530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita H, Yamamoto Y, Suzuki M, Miyazaki N, Yoshida A, Kawai K, Iwasaki K, Nakagawa A, Takai Y, Sakisaka T. Crystal structure of the cis-dimer of nectin-1: implications for the architecture of cell-cell junctions. J Biol Chem. 2011;286:12659–12669. doi: 10.1074/jbc.M110.197368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV. Herpesvirus entry into host cells mediated by endosomal low pH. Traffic. 2016 doi: 10.1111/tra.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, Hou J, Major EO, Straus SE. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol. 2005;79:7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77:5324–5332. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78:7508–7517. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson G, Tsao MS, Richardson CD. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011;7:e1002240. doi: 10.1371/journal.ppat.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce RS, Delpeut S, Richardson CD. Dog nectin-4 is an epithelial cell receptor for canine distemper virus that facilitates virus entry and syncytia formation. Virology. 2013;436:210–220. doi: 10.1016/j.virol.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Ogita H, Takai Y. Activation of Rap1, Cdc42, and rac by nectin adhesion system. Methods Enzymol. 2006;406:415–424. doi: 10.1016/S0076-6879(06)06030-7. [DOI] [PubMed] [Google Scholar]
- Oh MJ, Akhtar J, Desai P, Shukla D. A role for heparan sulfate in viral surfing. Biochem Biophys Res Commun. 2010;391:176–181. doi: 10.1016/j.bbrc.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann P, Thier K, Rahn E, Rixon FJ, Bloch W, Ozcelik S, Krummenacher C, Barron MJ, Dixon MJ, Scheu S, Pfeffer K, Knebel-Morsdorf D. Entry mechanisms of herpes simplex virus 1 into murine epidermis: involvement of nectin-1 and herpesvirus entry mediator as cellular receptors. J Virol. 2015;89:262–274. doi: 10.1128/JVI.02917-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn E, Petermann P, Thier K, Bloch W, Morgner J, Wickström SA, Knebel-Mörsdorf D. Invasion of Herpes Simplex Virus Type 1 into Murine Epidermis: An Ex Vivo Infection Study. J Invest Dermatol. 2015;135:3009–3016. doi: 10.1038/jid.2015.290. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Mandai K, Takai Y. The role of nectins in different types of cell-cell adhesion. J Cell Sci. 2012;125:3713–3722. doi: 10.1242/jcs.099572. [DOI] [PubMed] [Google Scholar]
- Rux AH, Willis SH, Nicola AV, Hou W, Peng C, Lou H, Cohen GH, Eisenberg RJ. Functional region IV of glycoprotein D from herpes simplex virus modulates glycoprotein binding to the herpes virus entry mediator. J Virol. 1998;72:7091–7098. doi: 10.1128/jvi.72.9.7091-7098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T, Ikeda W, Ogita H, Fujita N, Takai Y. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr Opin Cell Biol. 2007;19:593–602. doi: 10.1016/j.ceb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Taniguchi T, Nakanishi H, Takahashi K, Miyahara M, Ikeda W, Yokoyama S, Peng YF, Yamanishi K, Takai Y. Requirement of interaction of nectin-1alpha/HveC with afadin for efficient cell-cell spread of herpes simplex virus type 1. J Virol. 2001;75:4734–4743. doi: 10.1128/JVI.75.10.4734-4743.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- Simpson SA, Manchak MD, Hager EJ, Krummenacher C, Whitbeck JC, Levin MJ, Freed CR, Wilcox CL, Cohen GH, Eisenberg RJ, Pizer LI. Nectin-1/HveC Mediates herpes simplex virus type 1 entry into primary human sensory neurons and fibroblasts. J Neurovirol. 2005;11:208–218. doi: 10.1080/13550280590924214. [DOI] [PubMed] [Google Scholar]
- Sisk WP, Bradley JD, Leipold RJ, Stoltzfus AM, Ponce de Leon M, Hilf M, Peng C, Cohen GH, Eisenberg RJ. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J Virol. 1994;68:766–775. doi: 10.1128/jvi.68.2.766-775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PG, Manoj S, Yoon M, Jogger CR, Zago A, Myscofski D. Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry. Virology. 2006;344:17–24. doi: 10.1016/j.virol.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Stehle T, Dermody TS. Structural similarities in the cellular receptors used by adenovirus and reovirus. Viral Immunol. 2004;17:129–143. doi: 10.1089/0882824041310621. [DOI] [PubMed] [Google Scholar]
- Stiles KM, Krummenacher C. Glycoprotein D actively induces rapid internalization of two nectin-1 isoforms during herpes simplex virus entry. Virology. 2010;399:109–119. doi: 10.1016/j.virol.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles KM, Milne RS, Cohen GH, Eisenberg RJ, Krummenacher C. The herpes simplex virus receptor nectin-1 is down-regulated after trans-interaction with glycoprotein D. Virology. 2008;373:98–111. doi: 10.1016/j.virol.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles KM, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C. Herpes simplex virus glycoprotein D interferes with binding of herpesvirus entry mediator to its ligands through downregulation and direct competition. J Virol. 2010;84:11646–11660. doi: 10.1128/JVI.01550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Ikeda W, Ogita H, Rikitake Y. The Immunoglobulin-Like Cell Adhesion Molecule Nectin and Its Associated Protein Afadin. Annu Rev Cell Dev Biol. 2008;24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, Ware CF, Pfeffer K, Miyoshi J, Takai Y, Spear PG. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Clement C, Scanlan PM, Kowlessur D, Yue BY, Shukla D. A role for herpesvirus entry mediator as the receptor for herpes simplex virus 1 entry into primary human trabecular meshwork cells. J Virol. 2005;79:13173–13179. doi: 10.1128/JVI.79.20.13173-13179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Oh MJ, Kovacs M, Shukla SY, Valyi-Nagy T, Shukla D. Role for nectin-1 in herpes simplex virus 1 entry and spread in human retinal pigment epithelial cells. Febs J. 2008;275:5272–5285. doi: 10.1111/j.1742-4658.2008.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Shukla D. Phosphoinositide 3 kinase signalling may affect multiple steps during herpes simplex virus type-1 entry. J Gen Virol. 2010;91:3002–3009. doi: 10.1099/vir.0.024166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Shukla SY, Yue BY, Shukla D. Herpes simplex virus type 2 entry into cultured human corneal fibroblasts is mediated by herpesvirus entry mediator. J Gen Virol. 2007;88:2106–2110. doi: 10.1099/vir.0.82830-0. [DOI] [PubMed] [Google Scholar]
- Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110:789–799. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- Whitbeck JC, Peng C, Lou H, Xu R, Willis SH, Ponce de Leon M, Peng T, Nicola AV, Montgomery RI, Warner MS, Soulika AM, Spruce LA, Moore WT, Lambris JD, Spear PG, Cohen GH, Eisenberg RJ. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the TNFR superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitbeck JC, Zuo Y, Milne RSB, Cohen GH, Eisenberg RJ. Stable association of herpes simplex virus with target membrane is triggered by low pH in the presence of the gD receptor HVEM. Journal of Virology. 2006;80:3773–3780. doi: 10.1128/JVI.80.8.3773-3780.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M, Spear PG. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J Virol. 2002;76:7203–7208. doi: 10.1128/JVI.76.14.7203-7208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Yan J, Lu G, Guo Z, Fan Z, Wang J, Shi Y, Qi J, Gao GF. Binding of herpes simplex virus glycoprotein D to nectin-1 exploits host cell adhesion. Nat Commun. 2011;2:577. doi: 10.1038/ncomms1571. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lu G, Qi J, Li Y, He Y, Xu X, Shi J, Zhang CW, Yan J, Gao GF. Structure of measles virus hemagglutinin bound to its epithelial receptor nectin-4. Nat Struct Mol Biol. 2013;20:67–72. doi: 10.1038/nsmb.2432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
B78H1-N1BG cells expressing nectin-1β-GFP were recorded for 1 min before and during 2 min following the addition of soluble purified gD ectodomain, gD(285t) (100 μg/ml). Nectin-1-GFP is visible at cell contacts and on filopodia before the addition of gD. Nectin-1-GFP forms a typical pattern of nectin-1 at areas of contact (bottom right quadrant) which is rapidly lost after gD(285t) is added. Recording started at time t=0 sec, stopped at t=60 sec for addition of gD and resumed at t=77 sec. A projection of six z-sections is shown.
B78H1-N1BG cells expressing nectin-1β-GFP were recorded for 1 min before and during 2 min following the addition of soluble purified nectin-1 ectodomain nectin-1(346t) (100 μg/ml). Nectin-1-GFP is visible at cell contacts before the addition of gD and its distribution remains unchanged in the presence of soluble nectin-1. Recording started at time t=0 sec, stopped at t=60 sec for addition of gD and resumed at t=78 sec. A projection of six z-sections is shown.
B78H1-N1BG cells expressing GFP-nectin-1α were recorded for 1 min before and during 2 min following the addition of soluble purified gD truncation, gD(285t) (100 μg/ml). GFP-Nectin-1 forms a typical pattern of nectin-1 at areas of contact (right side of screen) which is rapidly lost after gD(285t) is added. Recording started at time t=0 sec, stopped at t=60 sec for addition of gD and resumed at t=84 sec. A projection of z-sections is shown.
B78H1-CG23 cells expressing nectin-1α-GFP were recorded for 1 min before and during 2 min following the addition of soluble purified gD truncation, gD(3-38C)285t (100 μg/ml). Nectin-1-GFP forms a typical pattern of nectin-1 at areas of contact (for instance bottom right region of screen) which is maintained after gD(3-38C)285t is added. Recording started at time t=0 sec, stopped at t=60 sec for addition of gD and resumed at t=83 sec. A projection of z-sections is shown. Since the z-sections are non-overlapping, striation are seen in some areas due to section stacking.


