Abstract
Human mesenchymal stem cells (hMSCs) are a widely available and clinically relevant cell type with a host of applications in regenerative medicine. Current clinical expansion methods can lead to selective changes in hMSC phenotype potentially resulting from relatively undefined cell culture surfaces. Chemically defined synthetic surfaces can aid in understanding the influence of cell–material interactions on stem cell behavior. Here, a thin copolymer coating for hMSC culture on plastic substrates is developed. The random copolymer is synthesized by living free radical polymerization and characterized in solution before application to the substrate, ensuring a homogeneous coating and limiting the sample-to-sample variations. The ability to coat multiple substrate types and cover large surface areas is reported. Arg–Gly–Asp-containing peptides are incorporated into the coating under aqueous conditions via their lysine or cysteine side chains, resulting in amide and thioester linkages, respectively. Stability studies show amide linkages to be stable and thioester linkages to be labile under standard serum-containing culture conditions. In addition, chemically defined passaging of hMSCs using only ethylenediaminetetraacetic acid on polystyrene dishes is shown. After passage, the hMSCs can be seeded back onto the same plate, indicating potential reusability of the coating.
1. Introduction
Regulation of fundamental stem cell behavior using two dimensional synthetic templates in vitro is of immense importance in regenerative medicine.[1] In particular, human mesenchymal stem cells (hMSCs) are of great interest, as they undergo multilineage differentiation, are derived from adult tissues, and secrete a variety of soluble proteins that can have therapeutic effects.[2] Currently, there are over 300 clinical trials using MSCs (www.clinicaltrials.gov), for treatment of a wide variety of conditions such as multiple sclerosis, cartilage defects, and diabetes. Control over cell behaviors such as adhesion, proliferation, and differentiation may facilitate increased therapeutic applications of stem cells. Chemically defined growth of stem cells allows for quantifiable cell– material interactions and hence, control over cell behaviors. However, cells are traditionally grown on tissue culture polystyrene (TCPS), which is low cost, sterile, and semireusable, leading to its widespread use over other materials such as glass. TCPS undergoes rapid adsorption of proteins in biological fluid, creating a poorly defined surface for cell studies, where identity, density, and orientation of the proteins is unknown.[3] To better understand substrate factors influencing cell behavior, a substantial amount of research has focused on creating synthetic 2D substrates for chemically defined cell culture. These include self-assembled monolayers (SAMs), hydrogels, polymer brushes, thin films, and layer-by-layer films.[4] Many of these systems use poly(ethylene glycol) (PEG) to provide a “blank slate” background to cells and prevent nonspecific protein adsorption.[5] PEG is relatively easy to functionalize with biomolecules using a number of different chemistries.[6] For example, SAMs terminated with oligoethylene glycol chains and functionalized with specific peptides can present a powerful platform for regulating stem cell behavior, as they are formed easily, and the functionalization with peptides can be achieved using a wide variety of distinct chemistries.[7] However, the formation of SAMs often requires substrate specific chemistry, which cannot be implemented on plastic substrates.[8] The use of multiple substrate types is often desired, to explore additional factors such as effect of substrate stiffness on cell behavior or to utilize the optical clarity of the substrate for imaging.
Thus, it is highly desirable to have a chemically defined coating that is compatible with multiple substrate types and is stable over long term in cell culture conditions. Here, we report the development of a thin, crosslinked copolymer coating presenting the integrin-binding cell adhesion peptide Arg–Gly–Asp (RGD) to support hMSC culture. The copolymer can be spun onto plastic (e.g., polystyrene, polycarbonate), silicon, glass, and gold substrates. Specifically, we synthesized a polyethylene glycol methyl ether methacrylate-ran-glycidyl methacrylate-ran-vinyl azlactone, P(PEGMEMA-r-GMA-r-VDM) copolymer. The PEGMEMA provided a cytophobic background, GMA thermally crosslinked the coating and the VDM acted as a highly reactive unit to efficiently incorporate cell adhesion peptides. Cell adhesion peptides (cyclo Arg–Gly– Asp-d-Phe–Lys (cRGDfK) or cyclo Arg–Gly–Asp-d-Phe–Cys (cRGDfC)) were coupled in aqueous media to azlactone units through lysine (Lys) or cysteine (Cys) side chains. Polarization modulation-infrared reflection absorption spectroscopy (PMIRRAS) experiments confirmed that Lys or Cys side chains coupled to the polymer coating, resulting in amide or thioester linkages, respectively. The coatings supported the adhesion of both hMSCs and human embryonic stem cells. These coatings simultaneously meet multiple design criteria: simple application to multiple substrate types, compatibility with large area plastic substrates, ability to undergo rapid peptide attachment in aqueous solution at low peptide concentrations, and long-term stability in cell culture conditions. We investigated the relative stability of the thioester and the amide polymer–peptide linkages in cell culture conditions and found that although the thioester bond forms more efficiently, the amide bond had better stability. Finally, we demonstrate the ability to perform enzyme-free passaging of hMSCs using only ethylenediaminetetraacetic acid (EDTA), thus preserving the coating and allowing its reuse. These results show the potential of the coating for large area, chemically defined culture of adult and pluripotent stem cells.
2. Results and Discussion
2.1. Design, Synthesis, and Coating Formation
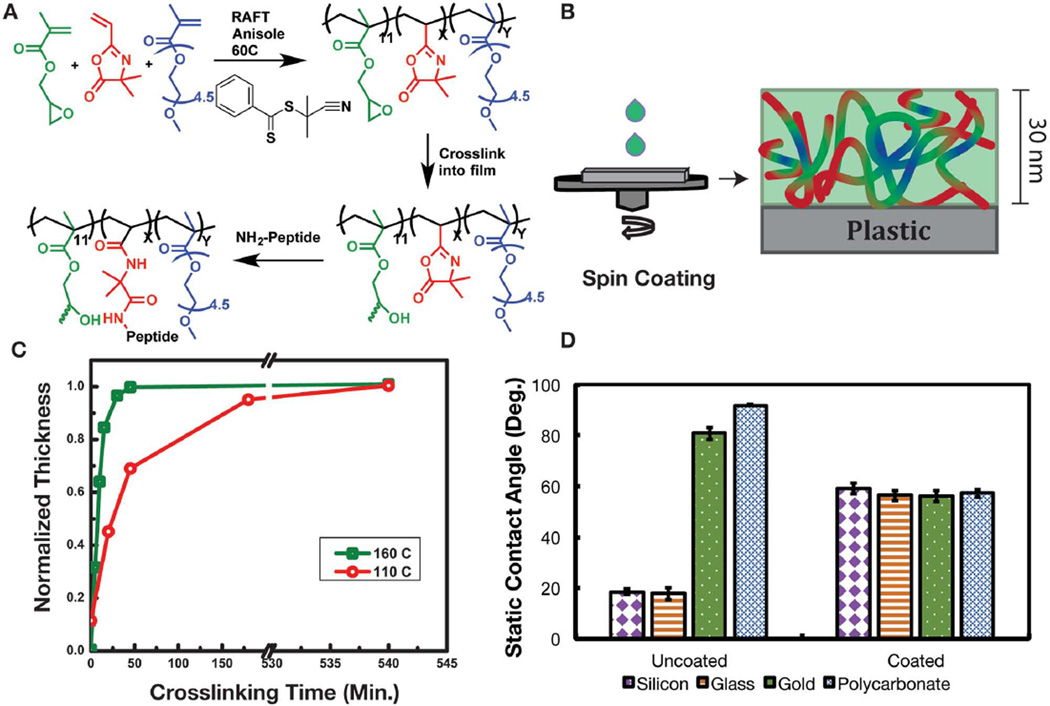
To test the copolymerization by living free radical methods we copolymerized just two monomers, namely, GMA and VDM. Atom transfer radical polymerization (ATRP), reversible addition fragmentation chain transfer (RAFT) polymerization, and conventional free radical polymerization using azobisisobutyronitrile (AIBN) were all effective in copolymerizing GMA and VDM. While this copolymer can in principle be used to introduce both peptides and PEG chains (to impart cell-adhesive function and resistance to nonSpecific protein adsorption, respectively) via ring opening of the VDM by the N-terminus of peptides or protein-resistant PEG–amines, quantification of the relative concentrations of peptide and PEG chains can be challenging. Moreover, the copolymer was not soluble in plastic compatible solvents such as water and ethanol. Thus, we introduced a third comonomer, PEGMEMA, which resulted in a copolymer soluble in ethanol, and limited nonSpecific protein adsorption (as blank coatings do not promote cell adhesion). The copolymer P(PEGMEMA-r-GMA-r-VDM) was synthesized by RAFT (Figure 1a) and the chemical composition was characterized by 1H NMR (Figure S1, Supporting Information) in solution. Multiple compositions with varied amounts of VDM and a fixed amount of GMA synthesized by RAFT polymerization are listed in Table S1 (Supporting Information). For this study we focused on the copolymer composition containing 24% azlactone, 11% GMA, and 65% PEGMEMA with a molecular weight of 43 094 Da and dispersity of 1.28. The relatively high molecular weight and low dispersity indicates that the reaction was well controlled by RAFT. The narrow dispersity also aids in accurate characterization of the relative amounts of VDM and GMA in solution. This advantage is unique over systems that are polymerized on the substrate surface, such as commercially available OptiChem and Synthemax surfaces.[9] We chose this particular composition with 24% peptide binding VDM units for cell studies, based on our previous work, in which the nonSpecific protein adsorption increased when the peptide binding units were over 30% of the composition.[10]
Figure 1.
A) Chemistry of polymerization by RAFT, and the crosslinking and post-functionalization process. B) Schematic depiction of the process of spin-coating the copolymer onto a substrate. C) Plot of the normalized coating thickness as a function of crosslinking time, at two temperatures 160 and 110 °C. D) Plot showing the static contact angle of uncoated substrates (silicon, glass, gold, and polycarbonate) and copolymer-coated substrates. Error bars represent the one standard deviation from the mean from five drops.
2.1.1. Optimization of Crosslinking
Coatings were fabricated by spin-coating the copolymer from ethanol solution (Figure 1b), a solvent tolerant of plastic substrates. For optimization of the crosslinking conditions, which required measurement of thickness by ellipsometry, silicon substrates were used. Figure 1c shows two different crosslinking temperatures, 160 and 110 °C. The coating was considered fully crosslinked when the thickness did not change after rinsing with solvent. Typically, crosslinking of the coating was completed within 45 min at 160 °C, while crosslinking took up to 3 h at 110 °C and up to 24 h at 85 °C. The ability to crosslink at lower temperatures makes the copolymer especially useful for application to surfaces with lower glass transition temperature, such as polystyrene cell culture dishes. The crosslinking dynamics observed here are generally consistent with previous reports. Typically, to crosslink coatings containing GMA units, the thermal annealing temperatures in the literature vary from 210 °C to as low as 70 °C.[11] However, in general longer times are required for full crosslinking at low temperatures.
2.1.2. Physical Stability of the Coating
During the process of cell culture, the coating is immersed in aqueous solution for extended timeframes; therefore, the coating itself must be physically stable under these conditions. Applications requiring extended stability include the maintenance of cell population and stem cell differentiation. Our studies show that while coatings were immersed in deionized (DI) water at 37 °C (Figure S2, Supporting Information), 92% of the coating thickness was retained over a 38 d period. These results confirm that 11% GMA in the copolymer provided sufficient stability to minimize and prevent uncrosslinked (i.e., physically entangled) chains from eluting out over time. It is noteworthy that only 11% of the total polymer functionality is used in crosslinking, leaving the remaining 89% to tailor Specific properties.
2.1.3. Demonstration of Compatibility with a Broad Range of Substrates
To demonstrate substrate compatibility, coatings were cast and crosslinked onto gold, silicon, glass, and polycarbonate, and the static water contact angles on coated and uncoated substrates were compared. The uncoated substrates had initial static water contact angles that varied from 20° to 95°. However, the contact angles of the coated substrates were similar (≈59°), and independent of the identity of the underlying substrate (Figure 1d). From this simple test we concluded that the coating is compatible with multiple substrate types. The 59° contact angle for the copolymer coated surfaces correlates well with the contact angle of 52° reported previously for 6 nm thick PEG brushes.[12]
2.2. Characterization of Azlactone Ring Opening by PM-IRRAS
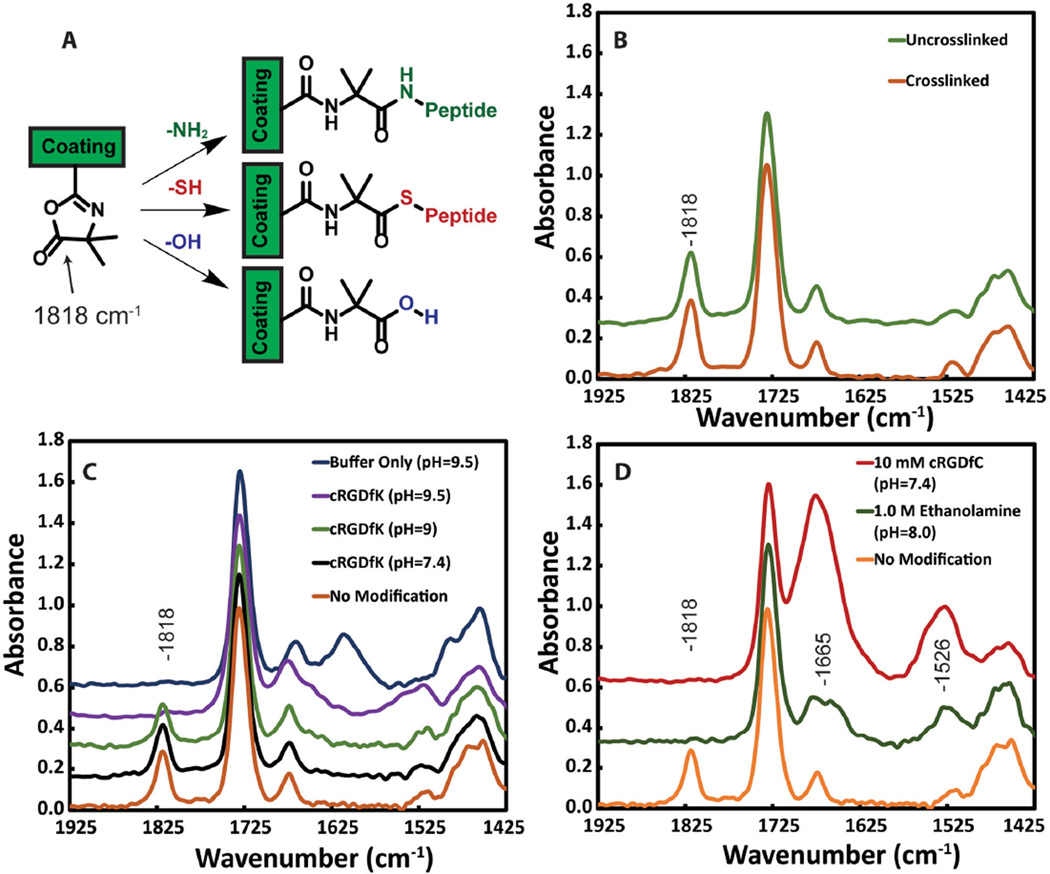
To quantify the reactivity and accessibility of the azlactone ring for nucleophiles (—OH, —SH, or —NH2, Figure 2a), we used PM-IRRAS. An efficient reaction should result in the disappearance of the prominent peak from the cyclic ester. First, to evaluate the effect of thermal crosslinking, if any, on the azlactone ring stability, we compared the crosslinked and noncrosslinked coatings (Figure 2b). Figure 2b shows that the cyclic ester peak at 1818 cm−1 from the azlactone ring is intact after crosslinking. This is consistent with other reports on high temperature stability (180 °C for 5 min) of the azlactone ring in the absence of nucleophiles.[13]
Figure 2.
PM-IRRAS analysis of copolymer coatings on gold substrates. A) Reaction of the azlactone ring with amines, thiols, or hydroxyls results in formation of an amide linkage, thioester linkage or ring hydrolysis, respectively and disappearance of the peak from the cyclic ester. B) Before and after crosslinking the peak at 1818 cm−1 remains intact, confirming that the azlactone ring does not open during the thermal crosslinking at 110 °C. C) Coatings after the reaction with cRGDfK peptide at various pH, in the presence of 1.5m sodium sulfate. D) Coatings after reaction with 1.0m ethanolamine (green) and 10 × 10−3 m cRGDfC peptide (red).
The coupling of a small molecule amine at high concentrations (1.0m ethanolamine) and pH 8.0 resulted in quantitative azlactone ring opening.[14] After 1 h of reaction at room temperature, the peak located at 1818 cm−1 (Figure 2d, green line) completely disappeared and new peaks at 1661 and 1525 cm−1 from amide linkages appeared.[13,15] This indicates that the azlactone functionality in the coating is accessible and can react with high concentrations of amines.
Coupling of larger molecules at lower concentrations was investigated using a cell adhesive peptide, namely, cRGDfK. Here, cRGDfK reacted through the primary amine on the Lys side chain with the azlactone and the reactivity was highly dependent on pH and ionic strength of the solution. At pH 7.4 and peptide concentrations of 10 × 10−3m or lower, the azlactone ring stayed intact at room temperature, even when reacted for 24 h (Figure 2c, black line). It is likely that at low concentration of the peptide, the reaction kinetics are slow and the PEG side chains pose a substantial steric barrier to reaction of the deprotonated amine with the azlactone ring. Typically, relatively high concentrations of amines (50–100 × 10−3 m) in organic solvents (e.g., dimethyl sulfoxide, tetrahydrofuran) have been used to open azlactone rings.[16] However, water-based coupling is necessary if plastic surfaces are to be used with this coating. Among the few reports on azlactone coupling in aqueous media, Coleman and co-workers have shown increased binding of Protein A to azlactone support beads in the presence of a high concentration of sodium sulfate at basic pH.[14] The enhanced reaction of amines with azlactone in the presence of sodium sulfate was attributed to increased interactions between the hydrophobic regions of the peptide and the azlactone rings.[17]
Thus, to facilitate amine coupling at a low concentration of 10 × 10−3 m, we added 1.5 m sodium sulfate and raised the pH (Figure 2c). The cRGDfK peptide reacted within 1 h once the pH was raised to 9.5. Coupling was confirmed by the disappearance of the azlactone peak at 1818 cm−1 and the appearance of amide peaks at 1661 and 1535 cm−1 (Figure 2c, purple line) from the peptide backbone. As a control, upon incubation of the coating in pH 9.5 buffer for 1 h in the absence of the peptides or amines, complete hydrolysis of the azlactone ring was observed, resulting in the emergence of a carboxylate peak at 1610 cm−1 (Figure 2c, blue line). This suggested a competition between amines and hydroxyl groups to ring open the azlactone at basic pH. Nevertheless, this optimized coupling condition (1.5 m sodium sulfate at pH 9.5) was used for the remainder of the studies using the cRGDfK, as it gave the best coupling efficiency at low peptide concentrations. We also investigated the reaction of a comparable cyclic RGD variant, cRGDfC, which opens azlactone rings through a thiol side group, forming a thioester linkage. The peptide was reacted for 1 h at 10 × 10−3 m in PBS at pH 7.4 (Figure 2d). Surprisingly, under these conditions the peak at 1818 cm−1 completely disappeared and two strong amide peaks appeared. The increased intensities of the amide peaks suggested that Cys reacts more rapidly and more completely with azlactones than Lys at low concentration and neutral pH. To our knowledge this is the first report of the reaction of thiols with azlactones on a polymer surface, even though the reaction is widely accepted in the literature.[18]
2.3. X-Ray Photoelectron Spectroscopy (XPS) Analysis of Coatings
XPS was used to quantify the reaction of cRGDfC and cRGDfK to the copolymer coating. In the coating, the elements carbon (C), oxygen (O), and nitrogen (N) are present (Figure S3, Supporting Information). Each azlactone ring has one nitrogen atom (N), which provided a marker to calculate the theoretical maximum peptide concentration (Equation (S1), Supporting Information). The concentration of the peptide used for coupling was varied from 0.084 to 8.4 × 10−3 m at a fixed reaction time of 1 h at room temperature. Results show that peptide concentrations as low as 0.084 × 10−3 m for cRGDfK and 0.84 × 10−3 m for cRGDfC (Figure S4, Supporting Information) can be used while maximizing the respective reaction efficiencies. Comparison of the percentages of cRGDfK and cRGDfC peptides (Figure S4, Supporting Information) coupled to the coating reconfirmed that the Cys coupling was more efficient than the Lys coupling, even with the optimized Lys coupling conditions outlined in the PM-IRRAS section above (10 × 10−3 m peptide with 1.5 m sodium sulfate at pH 9.5). Overall, the XPS data agrees with the PM-IRRAS data on the more quantitative reaction of azlactones with thiol over amine nucleophiles.
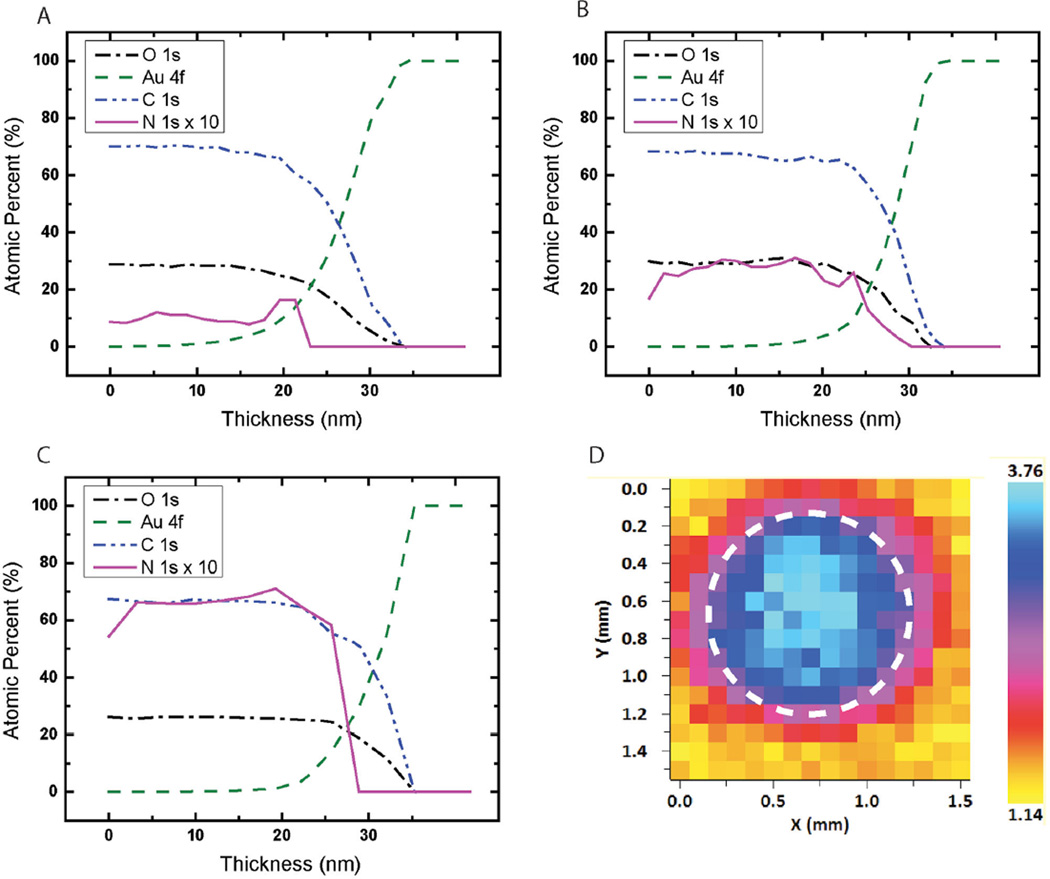
XPS typically samples only 10 nm into the depth of a surface. Since we first crosslinked the coating and subsequently coupled the peptides, the spatial distribution of the peptides throughout the thickness of the coating is of interest. The distribution was studied by depth profiling using large argon clusters, and spectra were acquired every 1.5 nm. In XPS depth profiling, the top layer of the material is continuously removed while XPS spectra are acquired. The azlactone content in an unmodified coating was nearly uniform throughout the thickness of the layer (Figure 3a). The increased nitrogen content after coupling (Figure 3b,c) was attributed to amide bonds from the peptides. In fact, the nitrogen signal was relatively well distributed throughout the 30 nm thickness of the coating, indicating that the peptides diffuse and couple uniformly throughout the depth of the coating.
Figure 3.
XPS depth profiling on A) an unmodified coating, B) a coating coupled with cRGDfK, and C) a coating coupled with cRGDfC. The nitrogen (N) atomic percent is multiplied by 10 for better visualization in graphs (A–C). D) XPS map of the atomic percent of N over a 1.5 mm2 area that has been patterned with a 1.1 mm diameter spot of cRGDfK peptide.
We utilized the XPS mapping function to map the atomic percent of nitrogen on the surface of the coating (Figure 3d) in order to visualize the localization of the cRGDfK peptide, which had been patterned with peptides using an elastomeric stencil. Elemental mapping of N (1s) atomic percent over the 1.5 mm2 area shows the center of the spot at 3.76% and the background area at 1.14%. These atomic percentages are in good agreement with the results from depth profiling studies (≈3.0% for cRGDfK and 1.0% for the blank coating) and show the feasibility of localizing the peptides to a small area on the coating. Patterning or spatial localization of the peptide allows for the efficient evaluation of multiple peptide conditions on the same surface.
2.4. Coatings for Controlled Stem Cell Adhesion
Cell adhesion experiments were conducted to test the effectiveness of the template as a cell culture substrate. We investigated hMSC adhesion to both cRGDfK and cRGDfC, protease-free passaging of hMSCs off of the coating, and the stability of the linkage between the coating and peptides in aqueous environments.
2.4.1. Stem Cell Attachment to Cyclic Peptides
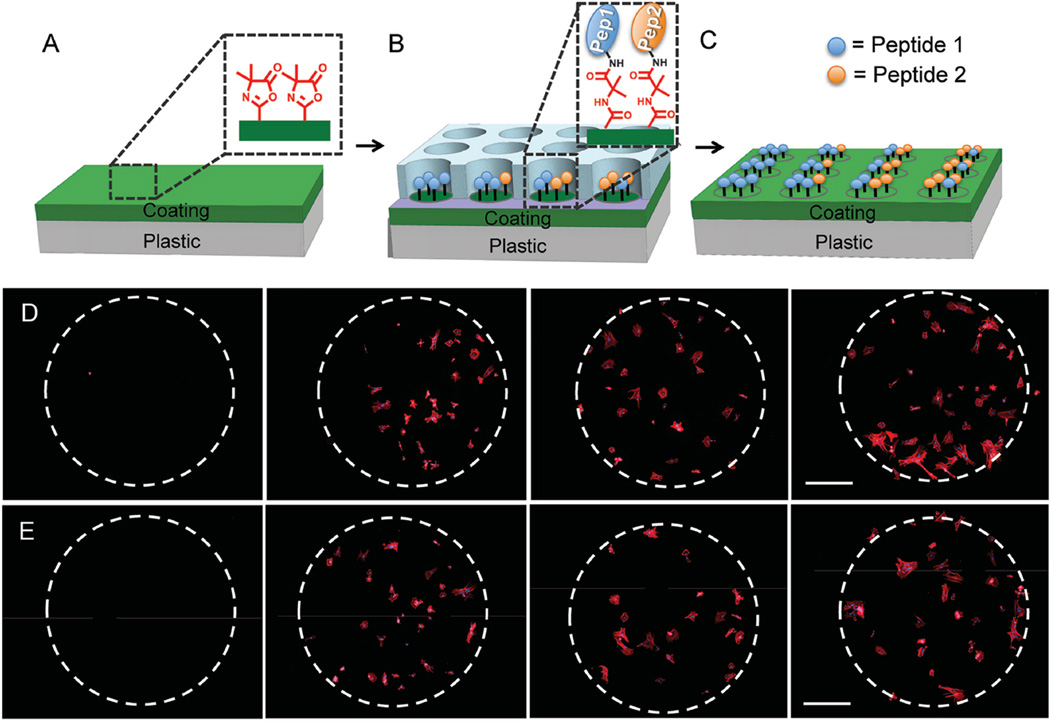
Synthetic substrates can provide unprecedented control over surface density and type of adhesion peptides accessible to stem cells. To demonstrate this capability, we used an elastomeric stencil to pattern multiple peptide combinations into 1.1 mm diameter spots on the coating (Figure 4a–c). Within each spot, a solution containing a mixture of adhesive and nonadhesive (“mutant”) peptide, with total peptide concentration in the solution held constant at 1 × 10−3 m was dispensed. This allows variation of the concentration of the adhesive peptide on the surface while keeping the total molar concentration of peptides (adhesive peptide + mutant, nonadhesive peptides) constant. Culture of hMSCs in alpha minimum essential medium (αMEM) containing 10% fetal bovine serum (FBS) showed minimal nonSpecific cell adhesion on spots containing only the nonadhesive cRADfK or cRADfC peptide (“0%” condition) or to the surrounding unmodified coating (outside the white dashed lines). Figure 4d,e shows attachment and spreading of hMSCs on patterned spots presenting increasing concentration of the adhesive peptide.
Figure 4.
A–C) The patterning process using an elastomeric stencil to create spots of peptide on the polymer coating. D) hMSC adhesion to 0%, 25%, 75%, and 100% cRGDfK (left to right) and E) 0%, 25%, 75%, and 100% cRGDfC. Here the nonadhesive mutant peptide (cRADfK or cRADfC, respectively) was mixed with the adhesive peptide, effectively lowering the amount of adhesive peptide on the surface. hMSCs are stained for the nuclei (blue) and the actin cytoskeleton (red). Scale bar represents 250 µm.
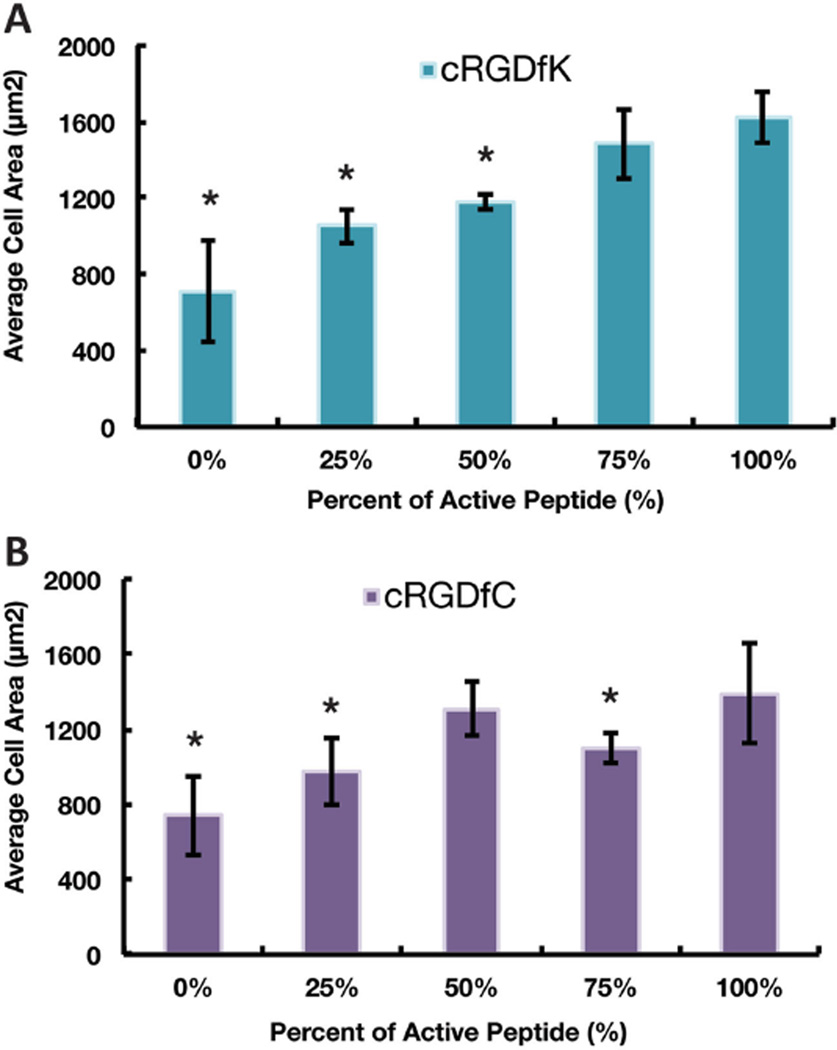
To quantify cell adhesion, hMSCs were allowed to attach for 20 h on large area samples (1 in. × 1 in.) before they were fixed and stained for the actin cytoskeleton. The measure of cell spreading represented by the hMSC projected cell area increases with the amount of RGD on cRGDfK functionalized coatings.[10,19] However, for cRGDfC functionalized coatings, the projected cell area saturates at about 50% peptide concentration (Figure 5). It should be noted that even though cRGDfC substrates are expected to have higher total peptide density than cRGDfK substrates based on reactivity at a given peptide concentration, the projected cell area on cRGDfC was not significantly higher. We postulate that this may be due to the labile nature of the thioester bond in the cell culture medium, preventing the formation of stable focal adhesions. This is discussed in the Section 2.4.3 in more detail.
Figure 5.
The average projected area of hMSCs on surfaces containing A) cRGDfK and B) cRGDfC. The “*” indicates a significant difference from the 100% active condition (p < 0.05). Error bars represent one standard deviation from the mean calculated from ten 10× images on two samples.
2.4.2. Chemically Defined Passaging of hMSCs
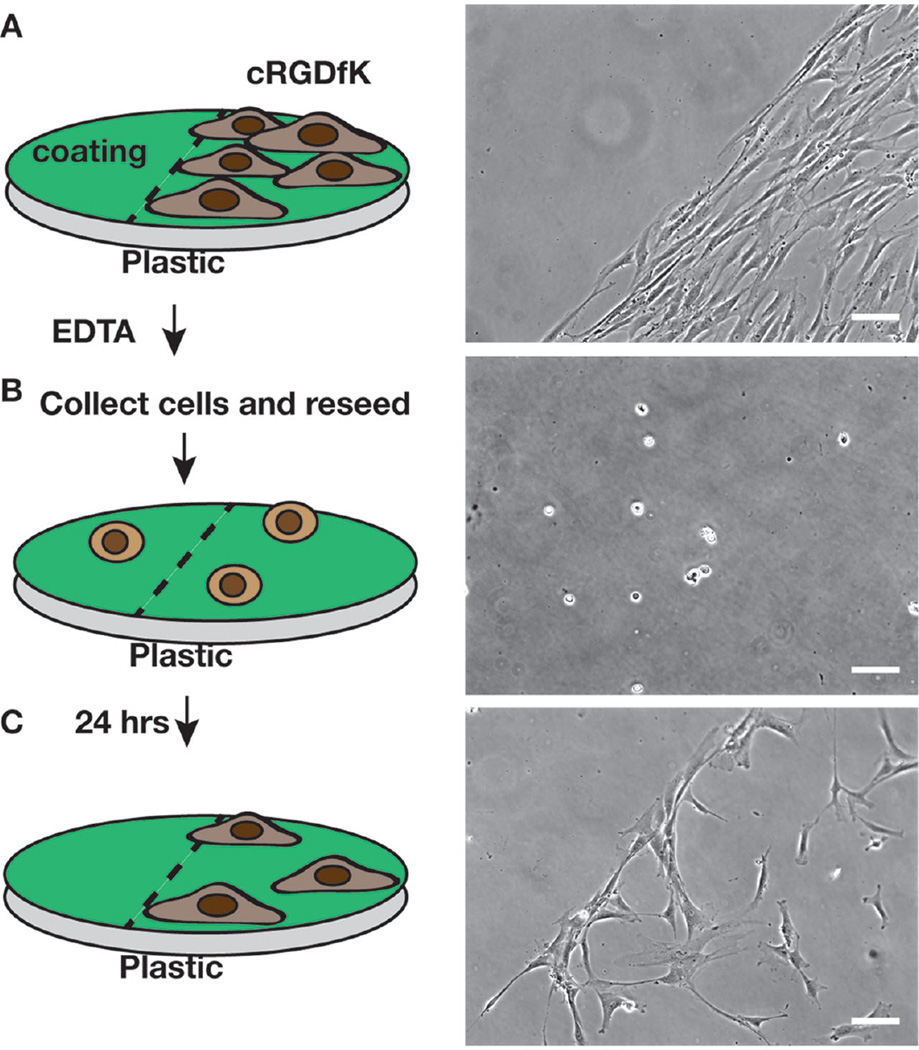
It is highly desirable to use chemically defined agents for passage of hMSCs, particularly when expanding hMSCs for clinical applications. We passaged hMSCs by first growing them on the cRGDfK presenting coating for 3 d in αMEM with 10% FBS, followed by Versene (a solution of 0.48 × 10−3 m EDTA in PBS) treatment to remove the cells from the surface (Figure 6). Successful reseeding onto the same substrate suggested that the substrate was unaltered by the Versene treatment, as cells adhered to the adhesive (cRGDfK) regions, but not to the nonadhesive (unmodified) regions. Furthermore, the observation that minimal hMSC adhesion occurred after reseeding onto the unmodified coating confirmed that the coating surface remained nonfouling after 3 d in standard cell culture conditions.
Figure 6.
On a plastic dish coated with the copolymer, the cRGDfK peptide was coupled to half the area using an elastomeric strip as a mask: A) hMSCs seeded at 1000 cells cm−2 were grown for 3 d and imaged. B) Cells were passaged using Versene (EDTA) solution, divided and reseeded onto the same surface. C) The cells were imaged again 24 h later. Scale bar represents 100 µm.
On the commonly used TCPS substrates, expansion of hMSCs without the use of proteases such as trypsin is not possible. Trypsin is a protease that can cleave arginine and lysine residues in peptide backbone and damage the chemically defined peptide–polymer surface.[20] Instead it is highly advantageous to use EDTA on stem cells because it is gentle, chemically defined, and xeno-free. However, to date EDTA alone has not been shown to be effective for passaging hMSCs on TCPS in the absence of proteases such as trypsin.[21] EDTA works by binding calcium and magnesium ions, and thereby interfering with integrin-mediated cell adhesion. The cRGDfK peptide used here is known to bind primarily to αvβ3 integrins and αvβ5 integrins.[22] We speculate that EDTA disrupts the binding of integrins to the adhesion peptides, thus triggering cell detachment. Therefore, the ability to grow hMSCs and passage them using a chemically defined solution on large plastic dishes may be uniquely enabling for hMSC culture and biomanufacturing applications.
2.4.3. Evaluation of Coating-Peptide Stability in Culture Conditions
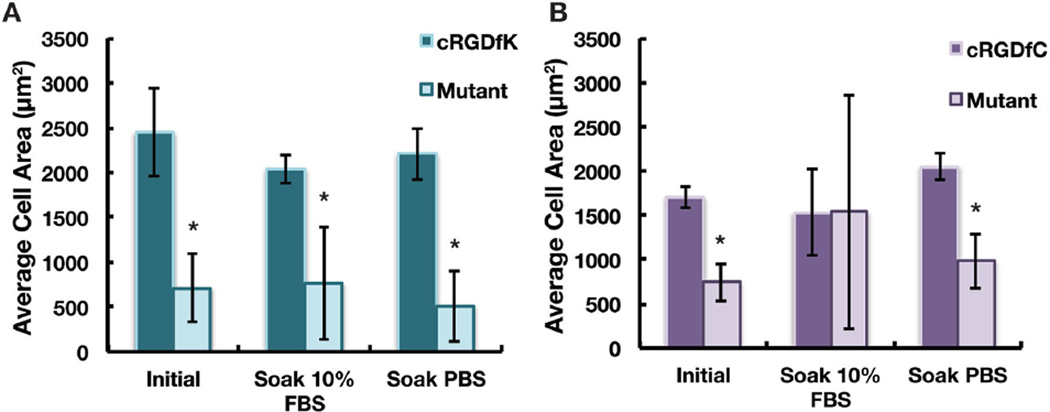
To assess the stability of the polymer–peptide linkage (either amide or thioester), the peptide-functionalized coatings were soaked in PBS for one week or αMEM + 10% FBS for two weeks. hMSCs were then seeded onto the presoaked coatings and the average hMSC area on spots patterned with either the adhesive peptide or the nonadhesive mutant peptide (Figure 7) was quantified. The coatings coupled through the amide linkage showed well spread cells on the adhesive RGD sequence, but not on the nonadhesive mutant sequence under both conditions tested (Figure 7a). Hence, the peptides bound through the amide linkage have extended stability under cell culture conditions at least out to two weeks. The peptides coupled through the thioester linkage were stable after soaking in PBS but not in αMEM + 10% FBS after one and two weeks (Figure S6, Supporting Information and Figure 7b). XPS analysis of coatings soaked in αMEM + 10% FBS for one and two weeks shows a 40% decrease in the cRGDfC peptide concentration at both time points compared to coatings prior to soaking (Figure S5, Supporting Information). In general, the labile nature of the thioester linkage can be attributed to simple hydrolysis, hydrolysis catalyzed by proteases present in the serum, and/or thiol displacement by primary amines.[23] Figure 7b suggests that displacement is more likely as soaking in αMEM + 10% FBS resulted in more hMSC spreading for the mutant peptide. If the peptide was hydrolyzed, the cell area would likely decrease. It is noteworthy that the labile nature of the thioester bond may ultimately be useful in the design of dynamic cell-responsive surfaces.
Figure 7.
Average area of hMSCs on coatings coupled with A) cRGDfK peptide to form an amide linkage or B) cRGDfC peptide, to form a thioester linkage. Patterned coatings were soaked in either αMEM + 10% FBS for two weeks before seeding of hMSCs or alternatively in PBS for one week before seeding cells. Cells were allowed to adhere for 7 h. Error bars represent one standard deviation from the mean on six spots. “*” denotes statistical significance between the mutant and the RGD sequence, p < 0.05.
2.4.4. Human Embryonic Stem Cell Attachment
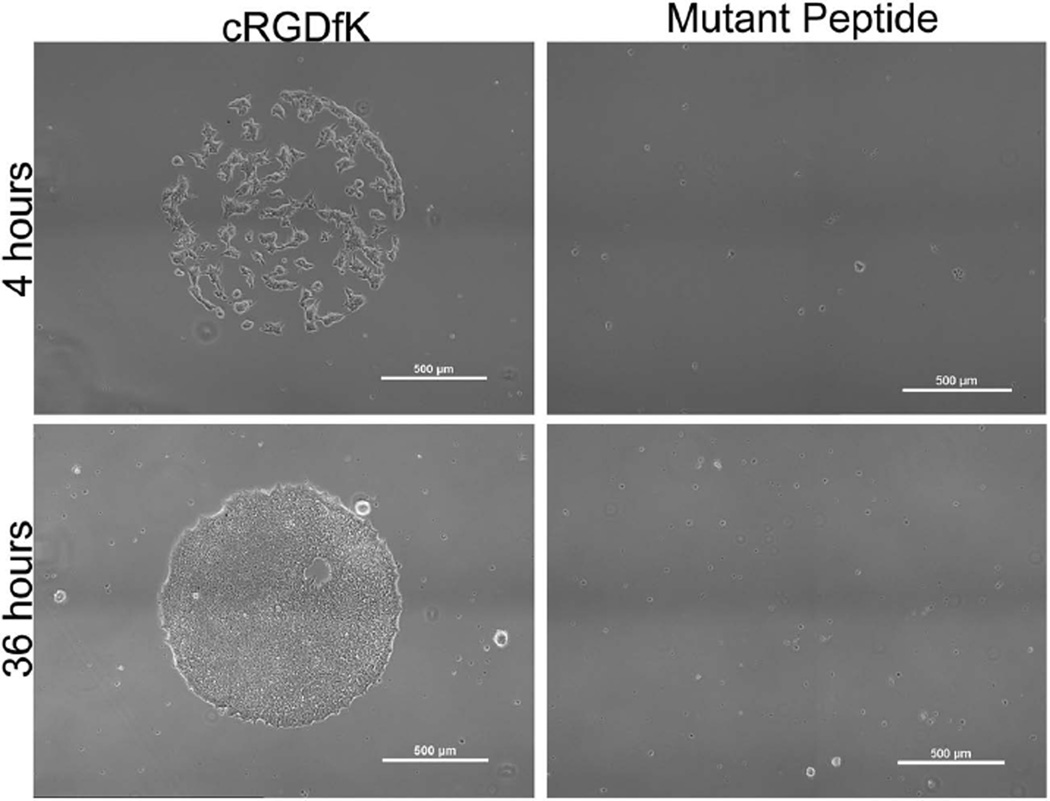
Human embryonic stem cells (hESCs) can also be readily cultured on coatings presenting cRGDfK peptide (Figure 8). hESCs remained on the surface for 4 d and were confluent on the spots by 36 h after seeding. The mutant control cRADfK peptide did not mediate adhesion of hESCs, confirming that the cells interacted Specifically with the active cRGDfK peptide. Although the hESC pluripotency after culture was not tested in this study, these initial results demonstrate the applicability of our coating for promoting adhesion of multiple human stem cell types. The use of a chemically defined coating for hESC culture may be valuable, as it is xeno-free unlike traditional coatings for hESC culture (e.g., Matrigel), and is not dependent on recombinant sources of ECM proteins (e.g., recombinant human vitronectin, laminin), thus providing lower cost and potentially improved reproducibility.[24] Further studies will be required to more deeply explore the potential of these coatings for culture and expansion of pluripotent stem cells.
Figure 8.
Embryonic stem cell attachment at 4 and 36 h on coatings patterned with 1.1 mm spots of cRGDfK or the mutant peptide as a control. Scale bars on the image represent 500 µm.
3. Conclusions
The copolymer coating designed herein shows promise as a chemically defined surface for stem cell culture. The solubility of the copolymer in ethanol and the low temperature crosslinking expands its applicability beyond silicon, glass, and gold to plastic substrates. The azlactone ring provides an efficient route to functionalize the coating with peptides via thioester or amide linkages. Characterization of the peptide functionalized coating by XPS, PM-IRAAS, and cell culture assays show that the amide linkages can present peptides for extended timeframes, while shorter-term presentation is possible using more labile thioester linkages. The coating promoted initial hMSC adhesion and spreading in a peptide density-dependent manner and supports multiple human stem cell types. Furthermore, hMSCs can be successfully passaged using chemically defined and protease-free solution on large area plastic surfaces. This coating platform can potentially elucidate cell–material interactions in vitro and may have far-reaching effects on stem cell culture methods.
4. Experimental Section
Materials
PEGMEMA (Mn ≈300 g mol−1), GMA, 2-cyano-2-propyl benzodithioate, 2,2′-azobis(2-methylpropionitrile), anisole, acetone, sulfuric acid (H2SO4), hydrogen peroxide 30% in water (H2O2), and ethanol (EtOH) were purchased from Sigma Aldrich Co. VDM was a gift from Dr. Steve Heilmann from the 3M Corporation (Milwaukee, WI). Silicon wafers (〈100〉, p-type) were purchased from University Wafer (Boston, MA). Bone marrow–derived hMSCs were purchased from Lonza. αMEM (1×) was from CellGro (Mannassas, VA). Trypsin (0.05%) and penicillin/streptomycin were from Hyclone (Logan, UT). VDM and GMA were purified by vacuum distillation. Peptides cRGDfC, cRADfC, cRGDfK, and cRADfK were purchased from Peptides International and had purities over 90% as characterized by HPLC. All other materials were used as received.
Polymer Synthesis
Copolymers P(VDM-GMA) were synthesized by ATRP with 200:1 monomer to initiator ratio. Briefly, VDM (9.0 mmol, 2.5 g) and GMA (0.5 mmol, 0.28 g) were added to a Schlenk flask with copper (I) bromide (0.05 mmol, 21.4 mg), N,N,N ′,N″,N″-pentamethyldiethylenetriamine (PMDETA, 0.05 mmol, 26 mg) and 3 mL toluene. The flask was degassed by three freeze–pump–thaw cycles. The flask was then heated to 60 °C in an oil bath, and the initiator ethyl-2-bromoisobyrate (0.05 mmol, 29.3 mg) was injected to start the reaction. The reaction was allowed to proceed for 4.5 h after which the copolymer was precipitated in hexane and dried. H1 NMR showed the composition to be 11.9% GMA and 88.1% VDM. Copolymers of P(PEGMEMA-r-GMA-r-VDM) were synthesized by RAFT polymerization. In a typical synthesis, PEGMEMA (8.3 mmol, 2.49 g), GMA (0.7 mmol, 99.5 mg), and VDM (1 mmol, 139.2 mg) were added to a 25 mL Schlenk flask. The solvent anisole (13.6 mL) was added at a 1:5 monomer to solvent ratio. The chain transfer agent (CTA), 2-cyano-2-propyl benzodithioate (0.01 mmol, 2.2 mg) was added at a total monomer to chain transfer agent ratio of 1000:1. Finally, the initiator 2,2′-azobis(2-methylpropionitrile) (0.01 mmol, 1.6 mg) was added at an initiator to CTA of 1:1. The mixture was degassed with three freeze–pump–thaw cycles. Polymerization was allowed to proceed at 60 °C for 18 h, after which the mixture was precipitated in n-hexanes and redissolved in tetrahydrofuran three times. The resulting light pink copolymer was stored in tetrahydrofuran at −20 °C. P(PEGMEMA-r-GMA-r-VDM) was analyzed using gel permeation chromatography (GPC) and proton nuclear magnetic resonance spectroscopy (1H-NMR), data are tabulated in Table S1 (Supporting Information) (composition 1 was used for all studies in this paper).
Substrate Preparation
Glass microscope slides purchased from Fisher Scientific were cut into thirds. Silicon and glass substrates were then sequentially cleaned by sonication in DI water, ethanol for 5 min each and then dried in a stream of air. Following, substrates were placed in a piranha solution (3:1 H2SO4:H2O2) at 95 °C for 30 min, washed with DI water and ethanol, and used within 24 h of cleaning. Caution! Piranha reacts violently in contact with organic matter. Polycarbonate substrates (GraceBio, HybriSlip 22 mm × 22 mm), sterile treated tissue culture dishes (Fisher Scientific), and gold substrates (EMF, 50 Å Ti, 1000 Å Au) were rinsed with DI water and ethanol and used directly.
Coating Formation and Crosslinking
Terpolymers of P(PEGMEMA-GMA-VDM) were diluted in 100% ethanol (Decon Labs) and spin-coated onto the prepared substrates using a CEE 100 spin coater (Brewer Science). To achieve 30 nm thicknesses, a concentration of 12 mg mL−1 of copolymer was used with a spin speed of 4000 rpm and acceleration of 2000 rotations s−2 for 60 s. Coatings were promptly annealed for 45 min at 160 °C to crosslink the coating or at 110 °C for 3 h or 85 °C for 24 h all under vacuum.
Coating Stability
A large 6 in. silicon wafer was coated with polymer (thicknesses of 30 nm) and crosslinked at 160 °C. The wafer was cut into ≈1 in. square pieces and put into DI water for 38 d while at 37 °C. Periodic replacement of the aqueous solution was done every 3–4 d. Dry coating thickness was determined at designated time points by ellipsometry using a Rudolph Auto EL null ellipsometer. Measurements were made at designated time points after drying the coatings under vacuum overnight using an angle of incidence of 70° and FilmEllipse software version 1.1 (Scientific Company Intl.). Three samples were removed for analysis per time point.
Contact Angle
Measurements were made on 30 nm coatings crosslinked onto gold, glass, silicon, and polycarbonate substrates using a Dataphysics OCA 15 Plus instrument with an automatic liquid dispenser at ambient temperature. Static water contact angles were measured with a 5 µL droplet of deionized water in five different locations on the coatings. The advancing and receding contact angles were measured and the data are reported as the average with standard deviation. The same was done for bare uncoated substrates for comparison.
Elastomeric Stencil Formation
Elastomeric stencils were created using a soft lithography process already reported in the literature.[25] Briefly, a master mold was created from SU-8 100 (Microchem, Newton, MA) spin-coated onto a silicon wafer and the pattern was transferred using conventional photolithography techniques. To prepare the polydimethylsiloxane (PDMS) (Slygard 184, Dow Corning, Midland, MI), a 1:10 ratio of base to curing agent (w/w) was mixed and degassing for 1 h. The mixture was cast over the master mold and cured for 6 h at 85 °C. The resulting PDMS stencil was cleaned by solvent extraction in hexanes overnight.[26]
Peptide Immobilization
Coatings were washed with PBS at pH 7.4 prior to peptide coupling. The cRGDfC peptides were coupled at either 0.084, 1, 0.84, or 8.4 × 10−3 m in PBS at pH 7.4 for 1 h. The cRGDfK peptide was coupled at the same concentrations only in a 0.25 m sodium phosphate buffer at pH 9.5 with 1.5 m sodium sulfate. For PM-IRRAS this pH was adjusted up from pH 7.4 to pH 9.5 with HCl. For patterning, elastomeric stencil with 1.1 mm diameter spots were placed atop the coating on the desired substrate. The spots were then filled with 1.3 µL of peptide solution, and covered to prevent evaporation for 1 h. After, the solution was aspirated and replaced with deionized water (1.3 µL) three times. Then the elastomeric stencil was removed, and the coating soaked in PBS for 1 h. Samples for cell culture were sterilized with 70% ethanol for 20 min, transferred to a new sterile six-well plate, and rinsed three times with sterile PBS to remove ethanol.
PM-IRRAS Analysis
Gold substrates (1000 Å, EMF Corporation, TA134) coated with the terpolymer (1 in. × 1 in.) and crosslinked at 110 °C were placed at incident angle of 83° in a Nicolet Magna-IR 860 Fourier transform IR spectrophotometer equipped with a photoelastic modulator (PEM-90, Hinds Instruments, Hilsoboro, OR), a synchronous sampling demodulator (SSD-100, GWC Technologies, Madison, WI), and a liquid nitrogen cooled mercury–cadmium–telluride detector. The modulation was set at 1600 cm−1 and 500 scans were obtained for each sample with a resolution of 8 cm−1. An aperture of 20 in the OMNIC software settings was used which corresponds to a spot size of 4.75 mm. The differential reflectance IR spectra were then normalized and converted to absorbance spectra using OMNIC software. Three samples (1 in. × 1 in.) were analyzed for each condition.
XPS Analysis
The measurements were performed with a Thermo Scientific Model K-Alpha XPS instrument using monochromatic Al Kα radiation (1486.7 eV). The instrument uses a hemispherical electron energy analyzer equipped with a 128 multichannel detector system. Survey spectra and high-resolution spectra were acquired using analyzer pass energies of 200 and 50 eV, respectively. The X-ray spot size was 400 µm for single point analysis. Depth profiling was done using large Argon clusters with 4000 eV for etching and data were collected every 30 s using the snap capture function, approximate etch rate of 0.05 nm s−1. Data were analyzed using Avantage XPS software package. Peak fitting was performed using Gaussian/Lorentzian peak shapes and a Shirley/Smart type background. For mapping a 0.1 mm X-ray spot size was used and the scan area was 1.5 mm2. At least three points were taken per sample and the averages and standard deviations were reported. All experiments shown were done in triplicates.
Cell Culture
The hMSCs (Lonza, Cat PT2501) were expanded at low density on tissue culture treated polystyrene plates. At passage 6 the cells were harvested using a 0.05% trypsin solution and suspended in 1 mL of αMEM (Cellgrow, Manassas, VA). In a rectangular eight-well plate (Thermofisher Scientific/Nunc, Rochester, NY), 4 mL of αMEM containing 1% penicillin/streptomycin (Hyclone, Logan, UT) and 10% MSC qualified FBS (Invitrogen, Carlsbad, CA) was added. hMSCs were seeded at 5000 cells cm−2 for copolymer coated polycarbonate samples with patterned peptide spots in Figure 5. Larger area coatings (1 in. × 1 in.) were used for Figure 5 where the entire surface was coated with just one peptide. Here, hMSCs were seeded at 7000 cells cm−2. Wells were then gently rocked to evenly distribute the cells. Cells were incubated at 37 °C and 5% CO2 to promote cell attachment for 6–7 (patterned) or 18 h (large area samples). At the end of the attachment period, the medium was aspirated from the wells and gently washed with sterile 1× PBS (pH 7.4) to remove any dead or loosely attached cells after which cells were fixed with 3.7% formaldehyde in PBS buffer for 15 min.
Immunocytochemistry
Staining of the actin cytoskeleton and nuclei was performed as directed by the manufacturer (Catalogue No. FAK100, Millipore, MA). Samples were washed with a solution containing 0.05% Tween 20 in a 1× PBS solution. Cells were permeabilized using 0.1% Triton X-100 (MP Biomedicals, Aurora, OH) in 1× PBS for 5 min. The coatings were rinsed twice with a wash buffer and then blocked to prevent nonSpecific antibody adsorption using 1% bovine serum albumin (BSA) (Fisher Scientific) in 1× PBS for 30 min. The coatings were then incubated in 1× PBS with the TRITC-conjugated phalloidin for 1 h. After rinsing with the wash buffer three times, coatings were dipped face down onto a glass slide with a drop of Prolong Gold antifade mounting media with DAPI (Invitrogen). Cells were imaged on an inverted microscope equipped with FITC, TRITC, and DAPI filter cube sets. For this experiment three replicates of each condition were used.
Peptide–Polymer Stability Assay
Coatings on polycarbonate substrates, patterned with 2 mm diameter spots of cRGDfK or cRGDfC or the nonadhesive cRADfC or cRADfK were sterilized by incubating in 70% ethanol for 20 min, which was followed by three rinses with sterile PBS. Coatings were then incubated at 37 °C in αMEM + 10% FBS or PBS for two weeks. Media was replaced every 3–4 d. After two weeks, the samples were rinsed with sterile PBS and deionized water. hMSCs were seeded at 7000 cells cm−2 and allowed to adhere for 7 h before fixing and staining for actin and nuclei. Here each sample had six replicated spots, and two individual samples were used per condition.
Passage of hMSCs
Plastic polystyrene dishes (round, Fisher Scientific) with area of 150 or 70 cm2 were coated with a 20 nm polymer coating as previously described and crosslinked at 85 °C. The cRGDfK peptide was coupled to approximately half of the dish, using a PDMS strip as a divider. The peptide was coupled at 0.084 × 10−3 m concentration in the 1.5 m sodium sulfate buffer at pH 9.5 for 1 h at room temperature. Then soaked in PBS for 30 min, followed by 70% ethanol for 20 min to sterilize. hMSCs (P6) were seeded at low density 1000 cells cm−2 in αMEM + 10% FBS. Cells reached confluence in 3 d. Versene solution (Life Technologies) was warmed to 37 °C in a water bath. Media above cells was aspirated and rinsed twice with PBS before place 10 mL of Versene solution into the dish for 1.5 min. Versene was then aspirated and fresh αMEM (5 mL three times) was used to remove the cells from the surface. On the same surface, 20% of the cells were reseeded down and imaged after 24 h. Three separate 70 cm2 area dishes were used to confirm the procedure.
Human Embryonic Stem Cell Culture
H1 human embryonic stem cells (WiCell, Madison, WI) were maintained on six-well plates coated with Matrigel (8.7 µg cm−2 ; BD Biosciences) in Essential 8 medium (E8; Invitrogen) with daily media exchange, and passaged using Versene (Invitrogen) every 3–4 d. For coatings on polycarbonate surfaced patterned with cRGDfK and cRADfK, cells (passage 42) were washed with PBS and incubated with TrypLE (Invitrogen) at 37 °C for 5 min to promote singularization. Cell suspensions were diluted with E8 supplemented with 5 × 10−6 m Rho kinase inhibitor (Y-27632; CalBiochem) and pelleted by centrifugation at 200g for 5 min before counting by hemacytometer and seeding at 1.8 × 105 cells cm−2.
Statistical Analysis
Analysis of images was done using Nikon NIS Elements software (Melville, NY). Here, actin and nuclei stained images were thresholded and automated measurements of cell area and number were taken, respectively. For each image, the average cell area was calculated by taking the red channel and dividing through by the number of nuclei from the blue channel. Values reported are the mean plus or minus the standard deviation. Experiments were analyzed using a two-tailed Student’s t-test. Data were considered “statistically significant” if p < 0.05.
Supplementary Material
Acknowledgments
This research was funded by the National Science Foundation (NSF DMR 1306482). The authors acknowledge support from staff and the use of equipment at the Materials Science Center at UW-Madison (NSF DMR-1121288).
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Samantha K. Schmitt, Department of Materials Science and Engineering, University of Wisconsin-Madison, Madison, WI 53706, USA
Angela W. Xie, Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI 53706, USA
Raha M. Ghassemi, Department of Materials Science and Engineering, University of Wisconsin-Madison, Madison, WI 53706, USA
David J. Trebatoski, Department of Materials Science and Engineering, University of Wisconsin-Madison, Madison, WI 53706, USA
Prof. William L. Murphy, Department of Materials Science and Engineering, University of Wisconsin-Madison, Madison, WI 53706, USA Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI 53706, USA; Department of Orthopedics and Rehabilitation, University of Wisconsin-Madison, Madison, WI 53706, USA.
Prof. Padma Gopalan, Department of Materials Science and Engineering, University of Wisconsin-Madison, Madison, WI 53706, USA Department of Chemistry, University of Wisconsin-Madison, Madison, WI 53706, USA.
References
- 1.a) Watt FM, Huck WTS. Nat. Rev. Mol. Cell Biol. 2013;14:467. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]; b) Huang NF, Li S. Ann. Biomed. Eng. 2011;39:1201. doi: 10.1007/s10439-011-0297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madrigal M, Rao KS, Riordan NH. Transl. Med. 2014;12:260. doi: 10.1186/s12967-014-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vroman L. Nature. 1962;196:476. doi: 10.1038/196476a0. [DOI] [PubMed] [Google Scholar]
- 4.a) Liu Y, Tan TTY, Yuan S, Choong C. Mater. Chem. B. 2013;1:157. doi: 10.1039/c2tb00014h. [DOI] [PubMed] [Google Scholar]; b) Koepsel JT, Brown PT, Loveland SG, Li W-J, Murphy WL. Mater. Chem. 2012;22:19474. doi: 10.1039/C2JM32242K. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kilian KA, Mrksich M. Angew. Chem. 2012;124:4975. doi: 10.1002/anie.201108746. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Nat. Mater. 2008;7:816. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Broderick AH, Azarin SM, Buck ME, Palecek SP, Lynn DM. Biomacromolecules. 2011;12:1998. doi: 10.1021/bm200296a. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Kurkuri MD, Driever C, Johnson G, McFarland G, Thissen H, Voelcker NH. Biomacromolecules. 2009;10:1163. doi: 10.1021/bm801417s. [DOI] [PubMed] [Google Scholar]
- 5.a) Tugulu S, Klok HA. Biomacromolecules. 2008;9:906. doi: 10.1021/bm701293g. [DOI] [PubMed] [Google Scholar]; b) Irvine DJ, Mayes AM, Griffith LG. Biomacromolecules. 2001;2:85. doi: 10.1021/bm005584b. [DOI] [PubMed] [Google Scholar]; c) Zhang M, Desai T, Ferrari M. Biomaterials. 1998;19:953. doi: 10.1016/s0142-9612(98)00026-x. [DOI] [PubMed] [Google Scholar]
- 6.Veronese FM. Biomaterials. 2001;22:405. doi: 10.1016/s0142-9612(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 7.a) Hudalla GA, Murphy WL. Langmuir. 2010;26:6449. doi: 10.1021/la1008208. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Koepsel JT, Murphy WL. ChemBioChem. 2012;13:1717. doi: 10.1002/cbic.201200226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem. Rev. 2005;105:1103. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 9.a) Fernández ICS, van der Mei HC, Lochhead MJ, Grainger DW, Busscher HJ. Biomaterials. 2007;28:4105. doi: 10.1016/j.biomaterials.2007.05.023. [DOI] [PubMed] [Google Scholar]; b) Ross AM, Nandivada H, Ryan AL, Lahann J. Polymer. 2012;53:2533. [Google Scholar]
- 10.Schmitt SK, Murphy WL, Gopalan P. Mater. Chem. B. 2013;1:1349. doi: 10.1039/c2tb00253a. [DOI] [PubMed] [Google Scholar]
- 11.a) Zdyrko B, Klep V, Luzinov I. Langmuir. 2003;19:10179. [Google Scholar]; b) Han E, Gopalan P. Langmuir. 2010;26:1311. doi: 10.1021/la902483m. [DOI] [PubMed] [Google Scholar]
- 12.Ostaci R-V, Damiron D, Al Akhrass S, Grohens Y, Drockenmuller E. Polym. Chem. 2011;2:348. [Google Scholar]
- 13.Fredin NJ, Broderick AH, Buck ME, Lynn DM. Biomacromolecules. 2009;10:994. doi: 10.1021/bm900045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman PL, Walker MM, Milbrath DS, Stauffer DM, Rasmussen JK, Krepski LR, Heilmann SM. Chromatogr. A. 1990;512:345. [Google Scholar]
- 15.Jones MW, Richards S-J, Haddleton DM, Gibson MI. Polym. Chem. 2013;4:717. [Google Scholar]
- 16.a) Messman JM, Lokitz BS, Pickel JM, Kilbey SM. Macromolecules. 2009;42:3933. [Google Scholar]; b) Tocce EJ, Broderick AH, Murphy KC, Liliensiek SJ, Murphy CJ, Lynn DM, Nealey PF. Biomed. Mater. Res. Part A. 2012;100A:84. doi: 10.1002/jbm.a.33233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porath J. Biopolymers. 1987;26:S193. doi: 10.1002/bip.360260017. [DOI] [PubMed] [Google Scholar]
- 18.a) Heilmann SM, Rasmussen JK, Krepski LR. Polym. Sci., Part A: Polym. Chem. 2001;39:3655. [Google Scholar]; b) Buck ME, Lynn DM. Polym. Chem. 2012;3:66. doi: 10.1039/C1PY00314C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koepsel JT, Loveland SG, Schwartz MP, Zorn S, Belair DG, Le NN, Murphy WL. Integr. Biol. 2012;4:1508. doi: 10.1039/c2ib20029e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen JV, Ong S-E, Mann M. Mol. Cell. Proteomics. 2004;3:608. doi: 10.1074/mcp.T400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.a) Nienow AW, Rafiq QA, Coopman K, Hewitt CJ. Biochem. Eng. Journal. 2014;85:79. [Google Scholar]; b) Heng B, Cowan C, Basu S. Biol. Proced. Online. 2009;11:161. doi: 10.1007/s12575-009-9001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a) Mas-Moruno C, Rechenmacher F, Kessler H. Anti-Cancer Agents Med. Chem. 2010;10:753. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shuhendler AJ, Prasad P, Leung M, Rauth AM, DaCosta RS, Wu XY. Adv. Healthcare Mater. 2012;1:600. doi: 10.1002/adhm.201200006. [DOI] [PubMed] [Google Scholar]
- 23.Iimura S, Manabe K, Kobayashi S. Org. Lett. 2002;5:101. doi: 10.1021/ol026906m. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JMC, Thomson JA. Nat. Methods. 2011;8:424. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jo BH, Van Lerberghe LM, Motsegood KM, Beebe DJ. Microelectromech. Syst. 2000;9:76. [Google Scholar]
- 26.Thibault C, Séverac C, Mingotaud A-F, Vieu C, Mauzac M. Langmuir. 2007;23:10706. doi: 10.1021/la701841j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.