Abstract
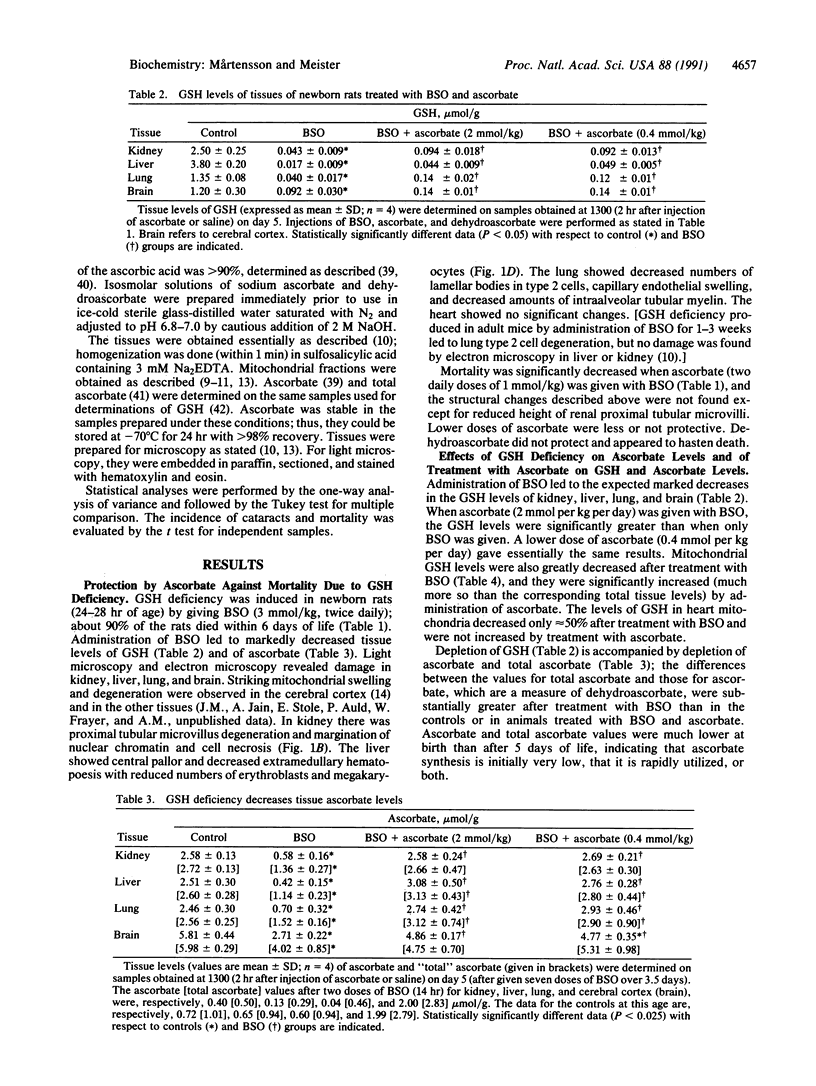
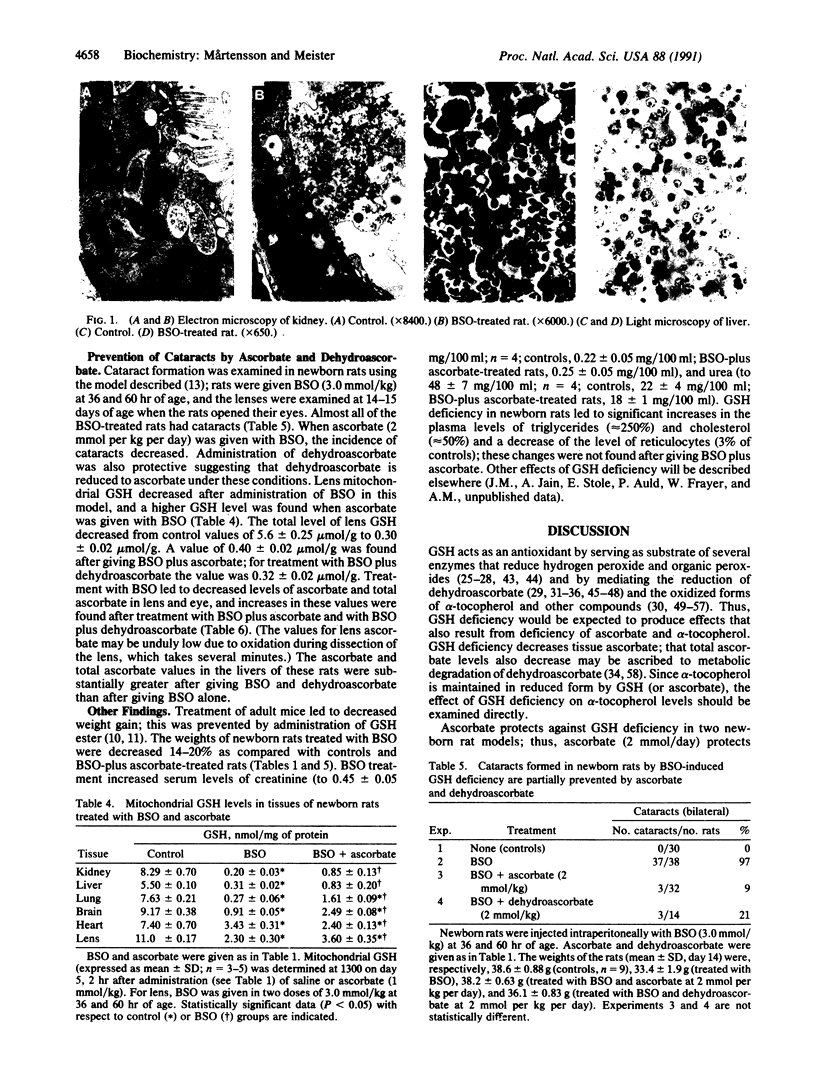
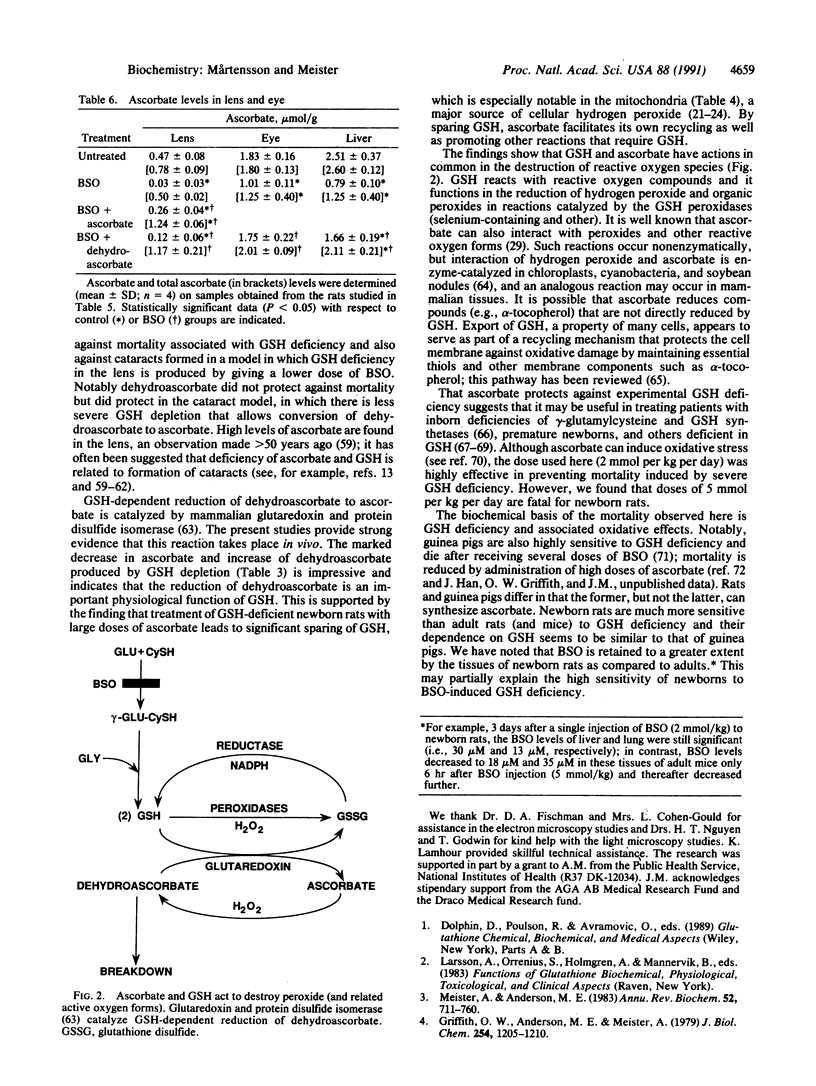
Glutathione deficiency in newborn rats, produced by administration of L-buthionine-(S,R)-sulfoximine, a transition-state inactivator of gamma-glutamylcysteine synthetase, decreases ascorbate levels of kidney, liver, brain, and lung. These tissues, especially their mitochondria, undergo severe damage and the animals die within a few days. When glutathione levels are markedly decreased, ascorbate levels decrease leading to formation of dehydroascorbate, which is degraded. Ascorbate has high antioxidant activity, but it (and other antioxidants such as alpha-tocopherol) must be maintained in reduced forms. These studies show in vivo that an important function of glutathione is to maintain tissue ascorbate. Administration of large doses of ascorbate (but not of dehydroascorbate) to buthionine sulfoximine-treated newborn rats decreases mortality, leads to normal levels of ascorbate, and spares glutathione. Newborn rats given lower doses of buthionine sulfoximine develop cataracts that, as shown previously, can be prevented by giving glutathione monoester; as found here, such cataracts can be partially prevented by administration of high doses of ascorbate or dehydroascorbate. Ascorbate spares glutathione indicating that these compounds have similar antioxidant actions. Ascorbate may have reductive functions that are not efficiently performed by glutathione. Although glutathione normally functions to maintain ascorbate, alpha-tocopherol, and other cellular components in reduced states, ascorbate can serve as an essential antioxidant in the presence of severe glutathione deficiency.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Glutathione monoesters. Anal Biochem. 1989 Nov 15;183(1):16–20. doi: 10.1016/0003-2697(89)90164-4. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Powrie F., Puri R. N., Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys. 1985 Jun;239(2):538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- BELLOWS J. G., SHOCH D. E. Alloxan diabetes and the lens. Am J Ophthalmol. 1950 Oct;33(10):1555–1564. doi: 10.1016/0002-9394(50)91216-5. [DOI] [PubMed] [Google Scholar]
- Bigley R., Riddle M., Layman D., Stankova L. Human cell dehydroascorbate reductase. Kinetic and functional properties. Biochim Biophys Acta. 1981 May 14;659(1):15–22. doi: 10.1016/0005-2744(81)90266-7. [DOI] [PubMed] [Google Scholar]
- Bigley R., Stankova L., Roos D., Loos J. Glutathione-dependent dehydroascorbate reduction: a determinant of dehydroascorbate uptake by human polymorphonuclear leukocytes. Enzyme. 1980;25(3):200–204. doi: 10.1159/000459248. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk R. F., Trumble M. J., Lawrence R. A. Rat hepatic cytosolic glutathione-dependent enzyme protection against lipid peroxidation in the NADPH-microsomal lipid peroxidation system. Biochim Biophys Acta. 1980 Apr 18;618(1):35–41. doi: 10.1016/0005-2760(80)90051-x. [DOI] [PubMed] [Google Scholar]
- CHRISTINE I., THOMSON G., IGGO B., BROWNIE A. C., STEWART C. P. The reduction of dehydroascorbic acid by human erythrocytes. Clin Chim Acta. 1956 Nov-Dec;1(6):557–569. doi: 10.1016/0009-8981(56)90043-2. [DOI] [PubMed] [Google Scholar]
- Calvin H. I., Medvedovsky C., Worgul B. V. Near-total glutathione depletion and age-specific cataracts induced by buthionine sulfoximine in mice. Science. 1986 Aug 1;233(4763):553–555. doi: 10.1126/science.3726547. [DOI] [PubMed] [Google Scholar]
- Campbell G. D., Jr, Steinberg M. H., Bower J. D. Letter: Ascorbic acid-induced hemolysis in G-6-PD deficiency. Ann Intern Med. 1975 Jun;82(6):810–810. doi: 10.7326/0003-4819-82-6-810_1. [DOI] [PubMed] [Google Scholar]
- Crook E. M., Hopkins F. G. Further observations on the system ascorbic acid-glutathione-ascorbic acid-oxidase. Biochem J. 1938 Aug;32(8):1356–1363. doi: 10.1042/bj0321356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook E. M. The system dehydroascorbic acid-glutathione. Biochem J. 1941 Mar;35(3):226–236. doi: 10.1042/bj0350226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D. A., Russell S. A., Hanus F. J., Pascoe G. A., Evans H. J. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3811–3815. doi: 10.1073/pnas.83.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doba T., Burton G. W., Ingold K. U. Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim Biophys Acta. 1985 Jul 9;835(2):298–303. doi: 10.1016/0005-2760(85)90285-1. [DOI] [PubMed] [Google Scholar]
- Graham K. S., Reddy C. C., Scholz R. W. Reduced glutathione effects on alpha-tocopherol concentration of rat liver microsomes undergoing NADPH-dependent lipid peroxidation. Lipids. 1989 Nov;24(11):909–914. doi: 10.1007/BF02544533. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W., Bridges R. J., Meister A. Transport of gamma-glutamyl amino acids: role of glutathione and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6319–6322. doi: 10.1073/pnas.76.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982 Nov 25;257(22):13704–13712. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- HENDRY J. M., EASSON L. H., OWEN J. A. THE UPTAKE AND REDUCTION OF DEHYDROASCORBIC ACID BY HUMAN LEUCOCYTES. Clin Chim Acta. 1964 May;9:498–499. doi: 10.1016/0009-8981(64)90089-0. [DOI] [PubMed] [Google Scholar]
- Hopkins F. G., Morgan E. J. Some relations between ascorbic acid and glutathione. Biochem J. 1936 Aug;30(8):1446–1462. doi: 10.1042/bj0301446. [DOI] [PMC free article] [PubMed] [Google Scholar]
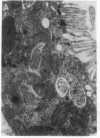
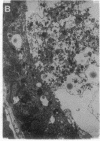
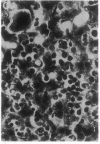
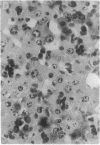
- Jain A., Mårtensson J., Stole E., Auld P. A., Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1913–1917. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen G. L., Meister A. Radioprotection of human lymphoid cells by exogenously supplied glutathione is mediated by gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4714–4717. doi: 10.1073/pnas.80.15.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGAWA Y., MANO Y., SHIMAZONO N. Biodegradation of dehydro-L-ascorbic acid; 2,3-diketo-aldonic acid decarboxylase from rat liver. Biochim Biophys Acta. 1960 Sep 23;43:348–349. doi: 10.1016/0006-3002(60)90451-0. [DOI] [PubMed] [Google Scholar]
- KINSEY V. E., MERRIAM F. C. Studies on the crystalline lens. II. Synthesis of glutathione in the normal and cataractous rabbit lens. AMA Arch Ophthalmol. 1950 Sep;44(3):370–380. [PubMed] [Google Scholar]
- Leedle R. A., Aust S. D. The effect of glutathione on the vitamin E requirement for inhibition of liver microsomal lipid peroxidation. Lipids. 1990 May;25(5):241–245. doi: 10.1007/BF02544382. [DOI] [PubMed] [Google Scholar]
- Loschen G., Flohé L., Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971 Nov 1;18(2):261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- MILLS G. C. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957 Nov;229(1):189–197. [PubMed] [Google Scholar]
- Mannervik B. Glutathione peroxidase. Methods Enzymol. 1985;113:490–495. doi: 10.1016/s0076-6879(85)13063-6. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Bolin T. Sulfur amino acid metabolism in chronic relapsing pancreatitis. Am J Gastroenterol. 1986 Dec;81(12):1179–1184. [PubMed] [Google Scholar]
- Mårtensson J., Denneberg T., Lindell A., Textorius O. Sulfur amino acid metabolism in cystinuria: a biochemical and clinical study of patients. Kidney Int. 1990 Jan;37(1):143–149. doi: 10.1038/ki.1990.20. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Frayer W., Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Lai J. C., Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7185–7189. doi: 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Larsson J., Nordström H. Amino acid metabolism during the anabolic phase of severely burned patients: with special reference to sulphur amino acids. Eur J Clin Invest. 1987 Apr;17(2):130–135. doi: 10.1111/j.1365-2362.1987.tb02392.x. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci U S A. 1989 Jan;86(2):471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Steinherz R., Jain A., Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki E. Interaction of ascorbate and alpha-tocopherol. Ann N Y Acad Sci. 1987;498:186–199. doi: 10.1111/j.1749-6632.1987.tb23761.x. [DOI] [PubMed] [Google Scholar]
- Nishigori H., Hayashi R., Lee J. W., Maruyama K., Iwatsuru M. Preventive effect of ascorbic acid against glucocorticoid-induced cataract formation of developing chick embryos. Exp Eye Res. 1985 Mar;40(3):445–451. doi: 10.1016/0014-4835(85)90157-5. [DOI] [PubMed] [Google Scholar]
- Omaye S. T., Turnbull J. D., Sauberlich H. E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- Reddy C. C., Scholz R. W., Thomas C. E., Massaro E. J. Vitamin E dependent reduced glutathione inhibition of rat liver microsomal lipid peroxidation. Life Sci. 1982 Aug 9;31(6):571–576. doi: 10.1016/0024-3205(82)90486-6. [DOI] [PubMed] [Google Scholar]
- Rose R. C. Renal metabolism of the oxidized form of ascorbic acid (dehydro-L-ascorbic acid). Am J Physiol. 1989 Jan;256(1 Pt 2):F52–F56. doi: 10.1152/ajprenal.1989.256.1.F52. [DOI] [PubMed] [Google Scholar]
- Scholich H., Murphy M. E., Sies H. Antioxidant activity of dihydrolipoate against microsomal lipid peroxidation and its dependence on alpha-tocopherol. Biochim Biophys Acta. 1989 Feb 20;1001(3):256–261. doi: 10.1016/0005-2760(89)90108-2. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. Observations on the function of peroxidase systems and the chemistry of the adrenal cortex: Description of a new carbohydrate derivative. Biochem J. 1928;22(6):1387–1409. doi: 10.1042/bj0221387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F., Maiorino M., Valente M., Ferri L., Gregolin C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta. 1982 Feb 15;710(2):197–211. doi: 10.1016/0005-2760(82)90150-3. [DOI] [PubMed] [Google Scholar]
- Wefers H., Sies H. The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur J Biochem. 1988 Jun 1;174(2):353–357. doi: 10.1111/j.1432-1033.1988.tb14105.x. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Anderson M. E., Puri R. N., Jensen G. L., Meister A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4732–4735. doi: 10.1073/pnas.81.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells W. W., Xu D. P., Yang Y. F., Rocque P. A. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem. 1990 Sep 15;265(26):15361–15364. [PubMed] [Google Scholar]