Abstract
Chemosensory proteins (CSPs) are identifiable by four spatially conserved Cysteine residues in their primary structure or by two disulfide bridges in their tertiary structure according to the previously identified olfactory specific-D related proteins. A genomics- and bioinformatics-based approach is taken in the present study to identify the putative CSPs in the malaria-carrying mosquito, Anopheles gambiae. The results show that five out of the nine annotated candidates are the most possible Anopheles CSPs of A. gambiae. This study lays the foundation for further functional identification of Anopheles CSPs, though all of these candidates need additional experimental verification.
Key words: chemosensory protein, proteomics, bioinformatics, olfaction, African malaria mosquito, Anopheles gambiae
Introduction
Anopheles gambiae (A. gambiae) is the principal vector of malaria that afflicts more than 500 million people and causes more than 1 million deaths each year. Analysis of the whole genome of A. gambiae revealed strong evidence for about 14,000 protein-coding transcripts, which need further annotation and experimental verification (1).
Olfaction plays a key role in host selection of agricultural pests and disease vectors. Recent advancement in understanding the molecular mechanism of olfaction is the result of multidisciplinary research efforts by using a variety of model organisms including insects. The reception of pheromones and general odorants is mediated by specific neurons located in specialized cuticular sensilla in insects (2). Chemosensory neurons extend their dendrites to a lymphatic cavity, where the soluble and low molecular weight proteins that are supposed to transfer the hydrophobic odorants across the fluid barrier to the receptive dendritic membrane are contained. Molecular cloning and biochemical surveys of insect antennae have identified two abundant but unrelated families of small soluble proteins with proposed odorant transport function, that is, odorant-binding proteins (OBPs) and olfactory specific-D (OS-D) related proteins 3., 4.. OS-D related proteins (average 13 kDa) were first identified by subtractive hybridisation experiments using antennae of Drosophila melanogaster 5., 6.. Many OS-D homologues were subsequently identified based on sequence similarity in different insect orders (Table 1).
Table 1.
Previously Identified Insect Chemosensory Proteins*
| Order | Species | Protein namea | Length(a.a) | Accession No.b | References |
|---|---|---|---|---|---|
| Hymenoptera | Apis mellifera | ASP3c | 130 | AF481963 | 7., 8. |
| Lepidoptera | Cactoblastis cactorum | CLP-1 | 130 | U95046 | 9 |
| Manduca sexta | SAP1 | 105 | AF117574 | 10 | |
| SAP3 | 126 | AF117585 | 10 | ||
| SAP2 | 127 | AF117592 | 10 | ||
| SAP5 | 231 | AF117594 | 10 | ||
| SAP4 | 127 | AF117599 | 10 | ||
| Bombyx mori | BmorCSP2 | 120 | AF509238 | 11 | |
| BmorCSP1 | 127 | AF509239 | 11 | ||
| Mamestra brassicae | CSP-MbraA1 | 112 | AF211177 | 12 | |
| CSP-MbraA2 | 112 | AF211178 | 12 | ||
| CSP-MbraA3 | 112 | AF211179 | 12 | ||
| CSP-MbraA4 | 112 | AF211180 | 12 | ||
| CSP-MbraA5 | 112 | AF211181 | 12 | ||
| CSP-MbraB1 | 108 | AF211182 | 12 | ||
| CSP-MbraB2 | 108 | AF211183 | 12 | ||
| CSP-MbraA6 | 128 | AF255918 | 13 | ||
| CSP-MbraB3 | 108 | AF255919 | 13 | ||
| CSP-MbraB4 | 108 | AF255920 | 13 | ||
| Heliothis virescens | HvirCSP2 | 126 | AY101511 | 14 | |
| HvirCSP1 | 114 | AY101512 | 14 | ||
| HvirCSP3 | 106 | AY101513 | 14 | ||
| Mamestra brassicae | SAP | 111 | AY026760 | unpublished | |
| Helicoverpa armigera | CSP-Harm | 127 | AF368375 | unpublished | |
| Helicoverpa zea | CSP-Hzea | 128 | AF448448 | unpublished | |
| Diptera | D. melanogaster | A10 | 155 | U05244 | 15 |
| RH70879p | 124 | BT001865 | unpublished | ||
| PEBmeIII | 158 | U08281 | 16 | ||
| Anopheles gambiae | SAP-1 | 127 | AF437891 | 17 | |
| Orthoptera | Schistocerca gregaria | CSP-sg1 | 109 | AF070961 | 18 |
| CSP-sg2 | 109 | AF070962 | 18 | ||
| CSP-sg3 | 103 | AF070963 | 18 | ||
| CSP-sg4 | 109 | AF070964 | 18 | ||
| CSP-sg5 | 109 | AF070965 | 18 | ||
| Locusta migratoria | OS-D1 | 103 | AJ251075 | 19 | |
| OS-D2 | 120 | AJ251076 | 19 | ||
| OS-D3 | 125 | AJ251077 | 19 | ||
| OS-D4 | 125 | AJ251078 | 19 | ||
| OS-D5 | 125 | AJ251079 | 19 | ||
| Phasmatodea | Eurycantha calcarata | CSP-ec1 | 107 | AF139196 | 20 |
| CSP-ec2 | 102 | AF139197 | 20 | ||
| CSP-ec3 | 107 | AF139198 | 20 | ||
| Dictyoptera | Periplaneta americana | p10 | 130 | AF030340 | 21., 22. |
GenBank (04/2003);
Registered names in GenBank;
GenBank accession numbers.
OS-D related proteins differ from OBPs in several aspects: they share no sequence similarity with OBPs and contain only four of the six spatially conserved Cysteine residues that are characterised by OBPs (4). Although two of the protein families are both represented by multiple genes within a given species, OS-D related proteins are more conserved than OBPs across evolution or between different phyla, with 40%-50% of identical residues even between most distant species. They have been identified in a variety of tissues, while most OBPs appear to be restricted to olfactory tissues. They are common in Orthopteroid (phasmid and grasshopper) and holometabolous (Lepidoptera and Diptera) insects (Table 1) and thus may be present throughout the Neoptera, while OBPs are only known within the holometabolous and hemipteroid lineages (4). There is no strong evidence for the physiological role of OS-Ds so far, and they may be involved in chemical communication and perception. To contrast with OBPs that are found in olfactory sensilla, the OS-D related proteins were designated as Chemosensory Proteins (CSPs; ref. 18., 23.).
Compared with the twenty-nine putative A. gambiae OBPs characterized for similarity to OBPs of Drosophila and other insects (24), no bioinformatics-based annotation has been carried out to identify the CSP candidates of A. gambiae. The NMR (Nuclear Magnetic Resonance) solution structure of chemosensory protein Csp2 (1K19_A) from moth Mamestra brassicae has been established (23), which is the best elucidated insect CSP and would be used as a model for homology modelling. We created an algorithm for identifying the conserved domains present in Anopheles putative CSPs through Perl programming.
Results
Conserved domain of insect CSP candidates
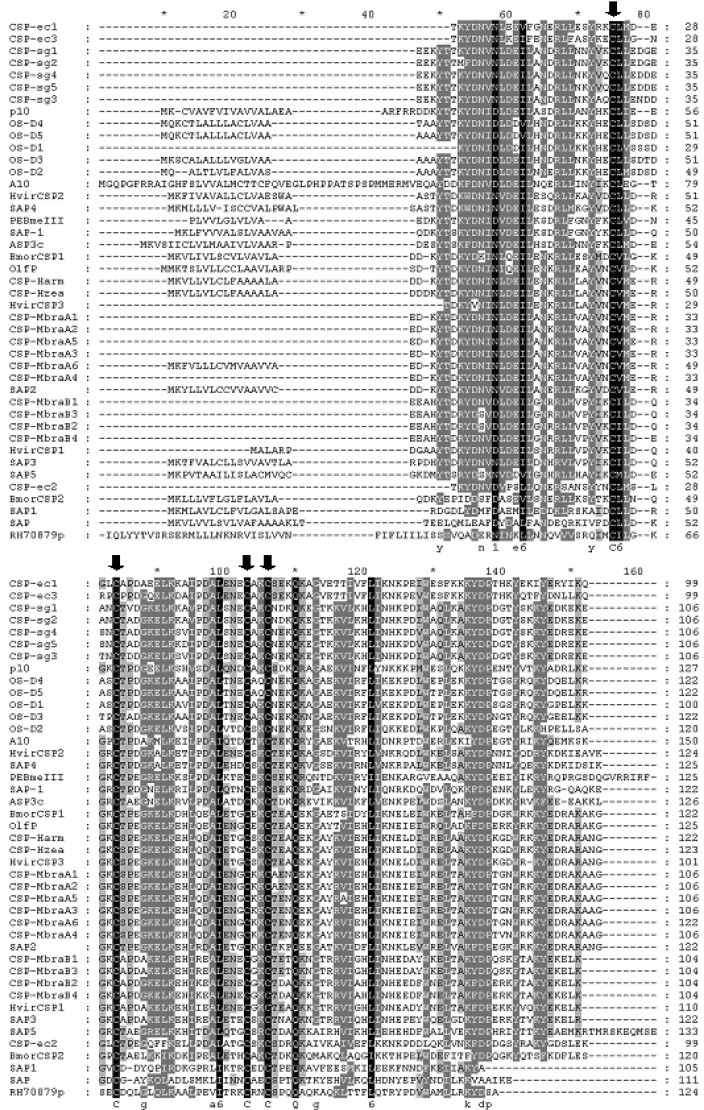
Exhaustive queries with all previously identified insect CSP sequences retrieved from GenBank (April 2003) and ClustalX multialignment resulted in an absolutely conserved structure for insect CSPs, that is, Cx(6,8)Cx(18)Cx(2)Cx(3) (Figure 1). We used a program developed by the authors through Perl programming to search the local database that contains the Fasta files of Anopheles gDNA (genomic DNA) and cDNA (complementary DNA) sequences downloaded from Ensembl Mosquito Genome Server for the identified pattern in their primary structure. Totally eight sequences were hit, including agCP10968, agCP11079, agCP11481, agCP11484, agCP11532, agCP11545, agCP6514, and agCP12965. The hits were in turn corroborated by BLAST searching in GenBank, and the prediction was made for their biochemical properties and secondary structure.
Fig. 1.
Multiple alignment showing the absolutely conserved domain in insect CSPs: Cx(6,8)Cx(18)Cx(2)Cx(3). All acronyms in the figure refer to the protein names listed in Table 1.
CSP candidates corroborated by BLAST
The hits obtained through pattern searching were corroborated by BLAST in GenBank (http://ncbi.nlm.nih.gov/). Six sequences (agCP10968, agCP 11079, agCP11481, agCP11484, agCP11532, and agCP11545) were found closely related to the previously identified CSPs, whereas two sequences (agCP6514 and agCP12965) matched no other proteins, so the CSP candidacy of agCP6514 and agCP12965 could not be excluded. The most interesting discovery was that a novel Anopheles CSP candidate (agCP11435) had been identified by BLAST searching, though the Expect (E) values are not very high (Table 2).
Table 2.
Anopheles CSP Candidates Found by CSPMOT and BLAST (E*-values<0.0001)
| Peptide ID | Previously identified insect CSPs | ||||||
|---|---|---|---|---|---|---|---|
| agCP10968 | SAP-1 | ASP3c | SAP2 | CSP-Harm | HvirCSP2 | OS-D3 | CSP-sg1 |
| (6e-18) | (7e-18) | (6e-17) | (1e-16) | (2e-16) | (2e-16) | (2e-16) | |
| OS-D1 | CSP-sg4 | CSP-sg2 | CSP-MbraA6 | OS-D4 | CSP-Hzea | OS-D5 | |
| (2e-16) | (3e-16) | (3e-16) | (3e-16) | (4e-16) | (5e-16) | (3e-16) | |
| SAP4 | PEBmeIII | CSP-sg5 | OS-D2 | A10 | CSP-ec3 | CSP-MbraA3 | |
| (7e-16) | (9e-16) | (9e-16) | (2e-15) | (2e-15) | (2e-15) | (2e-15) | |
| CSP-MbraA1 | CSP-MbraA2 | CSP-sg3 | p10 | CSP-MbraA5 | SAP3 | ||
| (2e-15) | (3e-15) | (3e-15) | (3e-15) | (7e-15) | (8e-15) | ||
| agCP11079 | SAP-1 | PEBmeIII | ASP3c | p10 | HvirCSP2 | SAP4 | A10 |
| (1e-45) | (1e-34) | (2e-34) | (4e-28) | (3e-27) | (1e-26) | (2e-26) | |
| OS-D2 | CSP-MbraA6 | OS-D3 | SAP3 | BmorCSP1 | SAP5 | CSP-sg1 | |
| (6e-26) | (2e-23) | (4e-23) | (7e-23) | (8e-23) | (9e-23) | (1e-22) | |
| CLP-1 | CSP-MbraA3 | CSP-sg4 | HvirCSP1 | CSP-sg2 | OS-D4 | OS-D5 | |
| (2e-22) | (3e-22) | (4e-22) | (5e-22) | (6e-22) | (7e-22) | (7e-22) | |
| CSP-sg4 | OS-D1 | CSP-MbraA2 | CSP-sg3 | SAP2 | CSP-MbraA3 | agCP11435 | |
| (1e-21) | (1e-21) | (2e-21) | (3e-21) | (4e-21) | (4e-21) | (5e-08) | |
| agCP11481 | ASP3c | PEBmeIII | A10 | SAP4 | HvirCSP2 | SAP5 | SAP3 |
| (4e-28) | (3e-27) | (3e-26) | (3e-25) | (4e-25) | (4e-24) | (2e-23) | |
| CSP-sg2 | SAP-1 | CSP-sg1 | OS-D2 | CSP-MbraA6 | CLP-1 | CSP-sg5 | |
| (1e-22) | (1e-22) | (2e-22) | (3e-22) | (7e-22) | (1e-21) | (2e-21) | |
| CSP-sg4 | CSP-MbraA3 | CSP-sg3 | CSP-MbraA2 | OS-D3 | HvirCSP1 | CSP-MbraA1 | |
| (2e-21) | (9e-21) | (1e-20) | (2e-20) | (3e-20) | (4e-20) | (6e-20) | |
| CSP-MbraA3 | CSP-MbraB1 | SAP2 | CSP-MbraA4 | CSP-MbraA5 | BmorCSP1 | agCP11435 | |
| (7e-20) | (8e-20) | (1e-19) | (2e-19) | (2e-19) | (3e-19) | (1e-06) | |
| agCP11484 | SAP-1 | ASP3c | PEBmeIII | HvirCSP2 | p10 | CSP-MbraA6 | OS-D2 |
| (3e-55) | (1e-31) | (5e-32) | (6e-29) | (4e-28) | (3e-27) | (9e-27) | |
| SAP4 | BmorCSP1 | CLP-1 | SAP2 | A10 | SAP3 | SAP5 | |
| (1e-26) | (2e-26) | (2e-26) | (1e-25) | (6e-25) | (4e-24) | (6e-24) | |
| CSP-Hzea | CSP-MbraA2 | HvirCSP1 | CSP-MbraA1 | CSP-MbraA3 | CSP-Harm | CSP-MbraA4 | |
| (3e-23) | (5e-23) | (5e-23) | (7e-23) | (7e-23) | (9e-23) | (2e-22) | |
| CSP-MbraA5 | CSP-sg1 | CSP-sg4 | CSP-sg2 | OS-D3 | OS-D1 | CSP-ec1 | |
| (2e-22) | (4e-22) | (8e-22) | (1e-21) | (2e-21) | (3e-21) | (3e-21) | |
| CSP-sg5 | agCP11435 | ||||||
| (3e-21) | (2e-08) | ||||||
| agCP11532 | RH70879 | ASP3c | SAP5 | CSP-sg4 | CSP-MbraA6 | CSP-sg5 | CSP-sg2 |
| (5e-30) | (4e-10) | (6e-08) | (6e-08) | (1e-07) | (1e-07) | (2e-07) | |
| HvirCSP2 | CSP-sg1 | SAP2 | CSP-ec3 | BmorCSP2 | CSP-sg3 | OS-D3 | |
| (2e-07) | (3e-07) | (4e-07) | (7e-07) | (7e-07) | (9e-07) | (1e-06) | |
| CLP-1 | CSP-MbraA3 | CSP-MbraA1 | CSP-MbraA2 | CSP-MbraA4 | SAP4 | CSP-MbraA5 | |
| (1e-06) | (1e-06) | (1e-06) | (1e-06) | (1e-06) | (2e-06) | (2e-06) | |
| OS-D2 | OS-D1 | A10 | CSP-Hzea | HvirCSP3 | CSP-ec1 | ||
| (3e-06) | (5e-06) | (1e-05) | (1e-05) | (1e-05) | (3e-05) | ||
| agCP11545 | SAP-1 | PEBmeIII | ASP3c | p10 | HvirCSP2 | A10 | OS-D2 |
| (3e-40) | (2e-33) | (2e-32) | (1e-30) | (6e-30) | (1e-28) | (1e-27) | |
| SAP4 | CSP-MbraA6 | CSP-sg1 | BmorCSP1 | SAP3 | CSP-sg4 | CSP-sg2 | |
| (3e-26) | (1e-25) | (3e-24) | (3e-24) | (3e-24) | (6e-24) | (6e-24) | |
| OS-D3 | SAP5 | CSP-sg5 | CSP-sg3 | HvirCSP1 | OS-D4 | OS-D1 | |
| (1e-23) | (2e-23) | (3e-23) | (3e-23) | (4e-23) | (5e-23) | (7e-23) | |
| CSP-MbraA3 | CSP-MbraA5 | CLP-1 | CSP-ec1 | CSP-MbraA2 | CSP-Hzea | agCP11435 | |
| (2e-22) | (2e-22) | (2e-22) | (5e-22) | (7e-22) | (1e-21) | (7e-09) | |
| agCP6514 | no matchesa | ||||||
| agCP12965 | no matchesa | ||||||
| agCP11435b A10 | OS-D1 | OS-D3 | OS-D5 | OS-D4 | SAP-1 | SAP4 | |
| (2e-12) | (5e-10) | (7e-10) | (1e-09) | (2e-09) | (2e-08) | (3e-08) | |
| PEBmeIII | OS-D2 | HvirCSP2 | ASP3c | CSP-sg5 | CSP-ec2 | CSP-sg4 | |
| (3e-08) | (4e-08) | (8e-08) | (1e-07) | (3e-07) | (5e-07) | (5e-07) | |
| CSP-sg3 | CSP-sg2 | CSP-sg1 | CSP-ec1 | CSP-ec3 | p10 | CSP-MbraA6 | |
| (6e-07) | (9e-07) | (9e-07) | (2e-06) | (2e-06) | (5e-05) | (5e-05) | |
| CSP-Hzea | CSP-Harm | CSP-MbraA3 | CSP-MbraA1 | ||||
| (1e-04) | (1e-04) | (1e-04) | (1e-04) | ||||
No significant hits obtained by BLAST;
BLAST identified;
The E value is a parameter that describes the number of hits one can “expect” to see just by chance when searching a database of a particular size. All acronyms of insect CSPs refer to protein names listed in Table 1.
Biochemical properties, prediction of secondary structure and ORFs of Anopheles CSP candidates
The cDNA sequences of the Anopheles Genome Project stored in GenBank and in the Ensembl Mosquito Genome Browser (ftp://ftp.ensembl.org/pub/current_mosquito/) had been annotated jointly by the privately funded Celera and EBI (http://www.ensembl.org/Anopheles_gambiae/). All the entries are the results from preliminary prediction. Even the positions of start codon for most of the annotated transcripts were not determined in the database. We have predicted the complete coding sequences (CDSs) and open reading frames (ORFs) for the Anopheles CSP candidates identified through pattern searching and BLAST, based on the genomic DNA sequences of the candidates. The redefined ORFs were listed in Table 3, along with the corresponding Celera IDs, GenBank IDs (#EAA), chromosomal locations, and scaffold numbers (#AAAB). Positions of the signal peptides, isoelectric points (pI) and hydrophobicity were also presented. The information contained in the table shows that all of the CSP candidates except agCP12965 and agCP6154 are located on one scaffold (AAAB01008964) of the chromosome 3 R. This stimulating discovery may indicate that these olfactory genes were duplicated at some point of Anopheles evolution. Meanwhile, all of the CSP candidates have small molecular weights (<15 kDa in most cases) and are hydrophilic (<35% hydrophobic amino acids in most cases). Most of the candidates have a signal peptide, though no signal peptides have been found in two of the sequences, that is, agCP10968 and agCP12965. In fact, we have used several different tools to predict the ORFs of agCP10968, unfortunately no signal peptides have been found, which indicates that a sequencing error may have occurred in the Anopheles Genome Project.
Table 3.
Chromosomal Location, New ORFs, Signal Peptides, and Biochemical Properties of Anopheles CSP Candidates
| Celera_ID | GB_ID | Chrom | Scaffold No. | Original length (a.a.) | New ORF length (a.a.) | Signal peptide | MW (kDa) | pI | Hydrophobic a.a. (%) |
|---|---|---|---|---|---|---|---|---|---|
| agCP10968 | EAA12703 | 3R | AAAB01008964 | 127 | 109 | none | 12.3 | 9.5 | 25.7 |
| agCP11079 | EAA12353 | 3R | AAAB01008964 | 143 | 127 | 1–17 | 14.8 | 5.4 | 33.1 |
| agCP11481 | EAA12591 | 3R | AAAB01008964 | 137 | 123 | 1–19 | 14.3 | 9.4 | 29.3 |
| agCP11484 | EAA12322 | 3R | AAAB01008964 | 149 | 127 | 1–17 | 14.7 | 8.6 | 33.1 |
| agCP11532 | EAA12601 | 3R | AAAB01008964 | 150 | 117 | 1–33 | 12.9 | 9.8 | 41.0 |
| agCP11545 | EAA12338 | 3R | AAAB01008964 | 141 | 126 | 1–17 | 14.6 | 8.6 | 31.0 |
| agCP11435 | EAA12702 | 3R | AAAB01008964 | 102 | 137 | 1–16 | 15.7 | 5.0 | 35.0 |
| agCP12965 | EAA05664 | 3L | AAAB01008834 | 173 | 137 | none | 14.1 | 3.5 | 23.4 |
| agCP6514 | EAA10937 | 2L | AAAB01008960 | 132 | 117 | 1–19 | 13.0 | 8.6 | 25.6 |
Table 4 summarized the secondary structure prediction of Anopheles CSP candidates. The predictions showed that most of the candidates could be classified as all-alpha structure, with a high probability to form a globular domain. However, agCP10968 and agCP6514 did not appear to be globular.
Table 4.
Secondary Structure of Anopheles CSP Candidates*
| Peptide ID | Predicted secondory structure (%) |
Globularity | |||
|---|---|---|---|---|---|
| Helix | Sheet | Loop | Class | ||
| agCP10968 | 25.70 | 15.50 | 58.80 | mixed | appears not to be globular |
| agCP11079 | 72.40 | 0.00 | 27.60 | all-alpha | may be globular, but it is not as compact as a domain |
| agCP11481 | 71.50 | 0.00 | 28.50 | all-alpha | may be globular, but it is not as compact as a domain |
| agCP11484 | 71.70 | 1.60 | 26.80 | all-alpha | appears as compact, as a globular domain |
| agCP11532 | 72.70 | 0.00 | 27.40 | all-alpha | appears as compact, as a globular domain |
| agCP11545 | 68.20 | 2.40 | 29.40 | all-alpha | may be globular, but it is not as compact as a domain |
| agCP11435 | 75.90 | 0.00 | 24.10 | all-alpha | may be globular, but it is not as compact as a domain |
| agCP12965 | 13.10 | 16.80 | 70.10 | mixed | appears as compact, as a globular domain |
| agCP6514 | 10.30 | 0.00 | 89.70 | mixed | appears not to be globular |
Prediction server—http://www.sbg.bio.ic.ac.uk/3dpssm.
Homology modelling
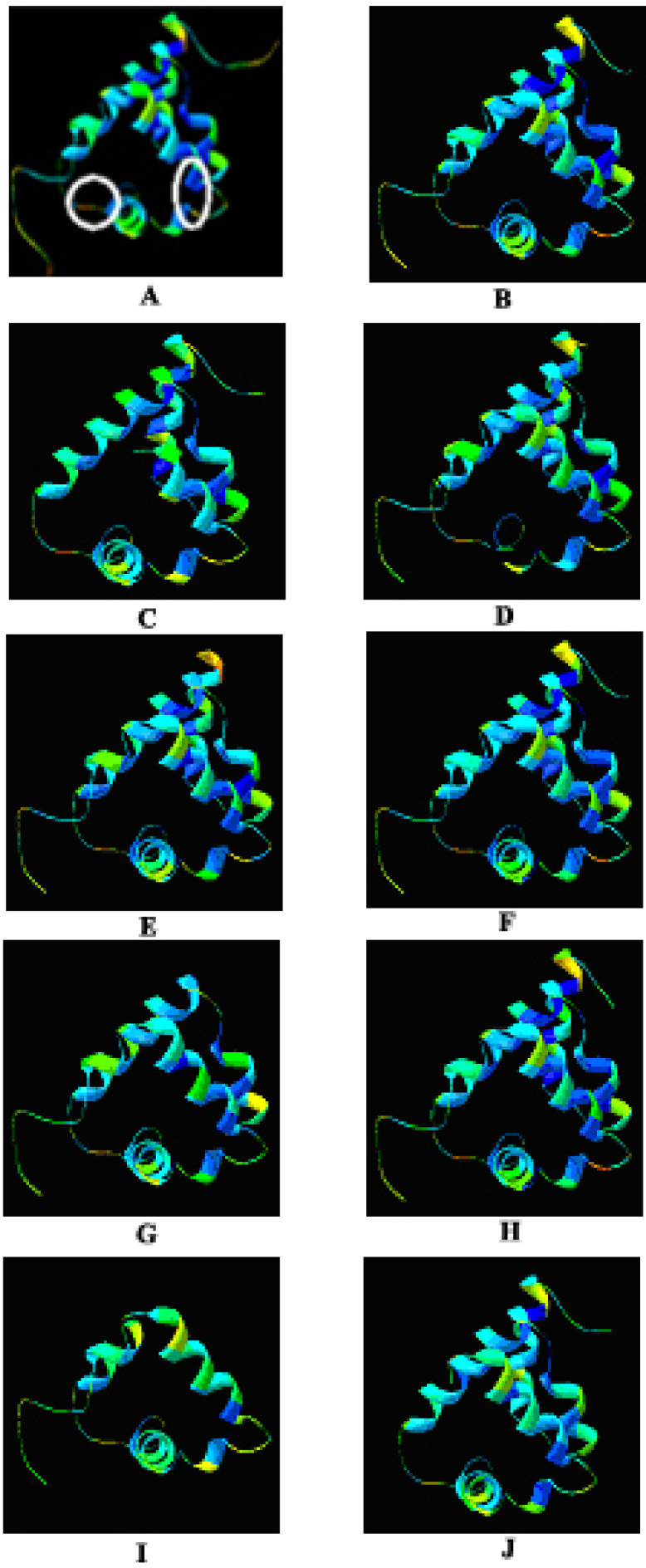
The NMR solution structure of chemosensory protein Csp2 of Mamestra brassicae (1K19_A) was retrieved from Protein Data Bank (PDB ID: 1K19). The 3D structure of this model molecule is shown in Figure 2A, which is characterized by two disulfide bonds (CysI-CysII, CysIII-CysIV), instead of three disulfide bonds (CysI-CysIII, CysII-CysV and CysIV-CysVI) characterized by OBPs. The model has a typical hydrophobic core that is supposed to act as a pocket for ligand binding. The pocket is formed by hydrophobic amino acids, surrounded by hydrophilic amino acids.
Fig. 2.
Homology modelling of Anopheles CSP candidates with 1K19_A_MbraCSP (PDB ID: 1K19) as the model. A. 3D-structure of 1K19_A_MbraCSP showing the position of two disulfide bridges; B-J: predicted 3D-structure of Anopheles CSPs, including agCP11079, agCP10968, agCP11435, agCP11481, agCP11484, agCP11532, agCP 11545, agCP12695, and agCP6514. The figures are generated in Swiss-PdbViewer.
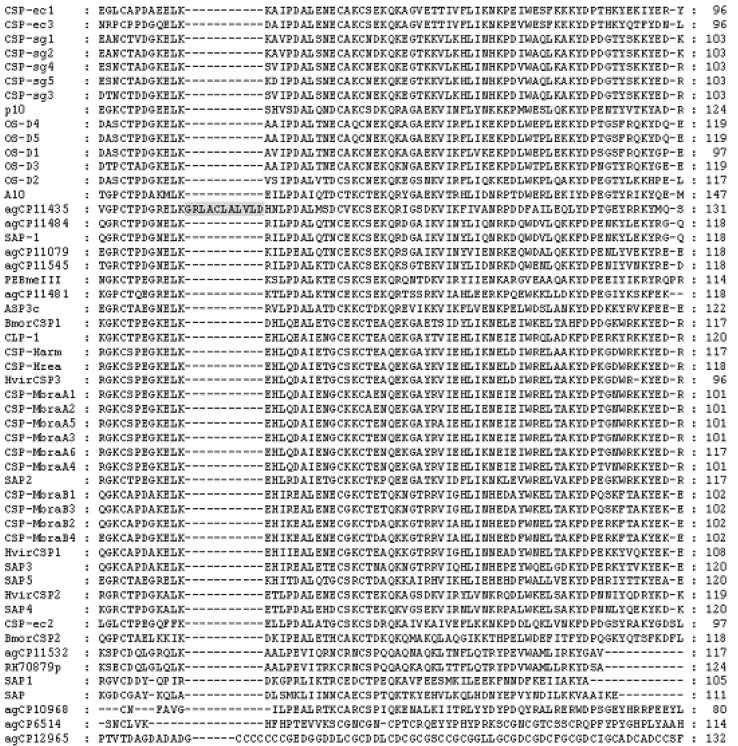
Homology modelling of the Anopheles CSP candidates was made in Swiss-PdbViewer (Figure 2). The figures showed that most candidates folded similarly as 1K19_A. However, a structurally weak linkage occurred between the two disulfide bridges of agCP11435 (Figure 2D), though a hydrophobic pocket was formed in the core of its structure. Further sequence analysis was carried out, which showed that the structural abnormality might be caused by a surplus sequence insertion (GRLACLALVL; Figure 3).
Fig. 3.
Partial alignment of candidate Anopheles CSPs with the previously identified CSPs shows the surplus sequence of agCP11435 that causes its 3D-structural abnormality. All acronyms of the gene names refer to the protein names listed in Table 1.
Discussion
Sequence similarity in CSP primary structure is significantly higher than that in OBPs. OBP sequences of the same species could be less similar than those of different species, whereas CSP sequences are more conserved at species level as well as between phylogenetically distant groups. In the present study, the sequence similarity between agCP11484 and other CSP candidates is: 77%, 76%, 47%, 39%, 21%, 15%, 13%, and 8%, respectively. Moreover, the positions of two disulfide bridges were highly conserved. The structural conservation should be extremely important in forming a strong hydrophobic core that function as an odorant-binding site.
Thirty-eight OBP candidates have been annotated in D. melanogaster, whereas only 29 OBP candidates have been conceptually identified in A. gambiae, based on sequencing and genome analysis 10., 24., 25., 26.. In the present study, we located nine CSP candidates in Anopheles genome, among which five candidates, i.e. agCP11079, agCP11481, agCP11484, agCP11532, and agCP11545, are the most possible Anopheles CSPs, considering the sequence similarity in their primary structure, the biochemical properties such as hydrophobicity and molecular weight (<15 kDa), in particular the secondary structure and 3-D structure. It would be very interesting to discuss the number of insect OBPs and CSPs (29 OBPs to 9 CSPs for Anopheles), because the numbers may be closely related to the functions. It has been shown that the previously identified OBPs are spatially distributed in insect olfactory sensilla, while CSPs are distributed in different tissues, including non-olfactory tissues. Pheromone-binding proteins (PBPs) are typical insect OBPs, which are highly specific. However, CSPs are less specific, which indicate that one CSP may be able to bind more than one odorant. We postulated that more OBPs might be needed to bind different odorants specifically, although any further hypothesis on the physiological function of CSPs can only be made when reliable experimental evidence has been presented, such as identification of the specific ligands that show CSPs function as chemosensory proteins.
It must be noted that agCP11435 may be a good model for discussion on its structural relations to its functions. We hope agCP11435 is a CSP candidate, but its structural peculiarity may provide some space for exploring its binding behaviour. It is expected that a minor insertion into the structural core may lead to destruction of the fundamental functions of a protein.
No strong evidence has been provided for the physiological role of CSPs so far, though researchers believed they might be involved in chemical communication and perception. We checked the spatial expression pattern of the putative Anopheles CSP candidates (data unpublished) and found they were distributed not only in mosquito antennae (olfactory tissues), but also in non-olfactory tissues such as heads stripped off antennae and maxillary palps, legs and bodies. The preliminary results indicated that CSPs might have other functions than olfaction. Further studies will be focused on functional research, such as ligand identification and crystallization of CSP recombinants.
Materials and Methods
Defining conserved domains in insect CSPs
The amino acid sequences of previously identified CSPs were downloaded from GenBank. All the sequences were then aligned in ClustalX 8.1 (27) using Multiple Alignment Mode with the default gappenalty parameters. The multiple alignment was manually checked, and the absolutely conserved Cysteine residues in the alignment were defined as CSP motifs.
Pattern search, selection of Anopheles CSP candidates in the whole genome sequences of A. gambiae
The Fasta files of gDNA, cDNA and the according translated peptide sequences of A. gambiae were downloaded from the Ensembl Mosquito Genome Browser (ftp://ftp.ensembl.org/pub/current_mosquito/). A pattern-searching program was created through standard PERL programming and named as CSPMOT by the authors. This program is run in Windows Commander, and can scan a local database for a pattern defined by the users. In this case, the CSP motif was used as a pattern to match every sequence stored in a local database that contains the Fasta files downloaded from the public databases. When a sequence matches the pattern, the program will display the sequence name, the position of the first residue where the pattern matches, and the pattern match alignment.
BLAST
BLAST was performed at NCBI (http://ncbi.nlm.nih.gov/BLAST/; April 2003). The Anopheles CSP candidates identified by the program described above were used as queries to Blast GenBank. The E values smaller than 0.0001 were accepted. The E value is a parameter that describes the number of hits one can “expect” to see just by chance when searching a database of a particular size.
Annotations
GENSCAN (http://genes.mit.edu/GENSCAN.html; ref. 28., 29.) and ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) were used to predict the full-length genes, based on gDNA and cDNA sequences of Anopheles CSP candidates. The annotated CDSs were then used for calculation of hydrophobicity, pI and molecular weight of the CSP candidates, by using the comprehensive biosoftware Vector NTI (InforMax Inc., Bethesda, USA). The positions of signal peptides were determined online (http://www.cbs.dtu.dk/services/SignalP2.0/; ref. 30., 31.), and finally the secondary structure, in particular the globularity, was predicted (http://maple.bioc.columbia.edu/predictprotein/; ref. 32., 33., 34.).
Homology modelling
Csp2 of Mamestra brassicae (PDB ID: 1K19; ref. 35) is an elucidated insect chemosensory protein, which was used as a model to predict the 3-D structure of Anopheles CSP candidates in Swiss-Pdb Viewer (http://us.expasy.org/spdbv/; ref. 36). Swiss-Pdb Viewer is a program that allows analysis of several proteins at the same time. It could be used to deduce 3-D structure of proteins and compare the active sites of different molecules.
Acknowledgements
The authors thank Rothamsted International for its support of Dr. Zhengxi Li’s post-doctoral fellowship in Biological Chemistry Division, Rothamsted Research, UK. Special thanks should be given to Division of Science and Technology, China Agricultural University for its financial support to the completion of the manuscript.
References
- 1.Holt R.A. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- 2.Singh R.N., Nayak S.V. Fine structure and primary sensory projections of sensilla on the maxillary palp of Drosophila melanogaster Meigen (Diptera: Drosophilidae) Int. J. Insect Morphol. Embryol. 1985;14:291–306. [Google Scholar]
- 3.Pelosi P., Maida R. Odorant-binding proteins in insects. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 1995;111:503–514. doi: 10.1016/0305-0491(95)00019-5. [DOI] [PubMed] [Google Scholar]
- 4.Vogt R.G. Odorant-binding proteins diversity and distribution among the insect orders, as indicated by LAP, an OBP-related protein of the true bug Lygus lineolaris (Hemiptera, Heteroptera) Chem. Senses. 1999;24:481–495. doi: 10.1093/chemse/24.5.481. [DOI] [PubMed] [Google Scholar]
- 5.McKenna M.P. Putative Drosophila pheromone-binding proteins expressed in a subregion of the olfactory system. J. Biol. Chem. 1994;269:16340–16347. [PubMed] [Google Scholar]
- 6.Pikielny C.W. Members of a family of Drosophila putative odorant-binding proteins are expressed in different subsets of olfactory hairs. Neuron. 1994;12:35–49. doi: 10.1016/0896-6273(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 7.Danty E. Separation, characterization and sexual heterogeneity of multiple putative odorant binding proteins in the honeybee Apis mellifera L. (Hymenoptera: Apidea) Chem. Senses. 1998;23:83–91. doi: 10.1093/chemse/23.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Briand L. Characterization of a chemosensory protein (ASP3c) from honeybee (Apis mellifera L.) as a brood pheromone carrier. Eur. J. Biochem. 2002;269:4586–4596. doi: 10.1046/j.1432-1033.2002.03156.x. [DOI] [PubMed] [Google Scholar]
- 9.Maleszka R., Stange G. Molecular cloning by a novel approach, of a cDNA encoding a putative olfactory protein in the labial palps of the moth Cactoblastis cactorum. Gene. 1997;202:39–43. doi: 10.1016/s0378-1119(97)00448-4. [DOI] [PubMed] [Google Scholar]
- 10.Robertson H.M. Diversity of odorant binding proteins revealed by an expressed sequence tag project on male Manduca sexta moth antennae. Insect Mol. Biol. 1999;8:501–518. doi: 10.1046/j.1365-2583.1999.00146.x. [DOI] [PubMed] [Google Scholar]
- 11.Picimbon J.F. Purification and molecular cloning of chemosensory proteins from Bombyx mori. Arch. Insect Biochem. Physiol. 2000;44:120–129. doi: 10.1002/1520-6327(200007)44:3<120::AID-ARCH3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Nagnan-Le Meillour P. Chemosensory proteins from the proboscis of Mamestra brassicae. Chem. Senses. 2000;25:541–553. doi: 10.1093/chemse/25.5.541. [DOI] [PubMed] [Google Scholar]
- 13.Jacquin-Joly E. Functional and expression pattern analysis of chemosensory proteins expressed in antennae and pheromonal gland of Mamestra brassicae. Chem. Senses. 2001;26:833–844. doi: 10.1093/chemse/26.7.833. [DOI] [PubMed] [Google Scholar]
- 14.Picimbon J.F. Identity and expression pattern of chemosensory proteins in Heliothis virescens (Lepidoptera, Noctuidae) Insect Biochem. Mol. Biol. 2001;31:1173–1181. doi: 10.1016/s0965-1748(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 15.Pikielny C.W. Members of a family of Drosophila putative odorant-binding proteins are expressed in different subsets of olfactory hairs. Neuron. 1994;12:35–49. doi: 10.1016/0896-6273(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 16.Dyanov H.M., Dzitoeva S.G. Method for attachment of microscopic preparations on glass for in situ hybridization, PRINS and in situ PCR studies. BioTechniques. 1995;18:822–824. [PubMed] [Google Scholar]
- 17.Biessmann H. Isolation of cDNA clones encoding putative odourant binding proteins from the antennae of the malaria-transmitting mosquito, Anopheles gambiae. Insect Mol. Biol. 2002;11:123–132. doi: 10.1046/j.1365-2583.2002.00316.x. [DOI] [PubMed] [Google Scholar]
- 18.Angeli S. Purification, structural characterization, cloning and immunocytochemical localization of chemoreception proteins from Schistocerca gregaria. Eur. J. Biochem. 1999;262:745–754. doi: 10.1046/j.1432-1327.1999.00438.x. [DOI] [PubMed] [Google Scholar]
- 19.Picimbon J.F. Chemosensory proteins of Locusta migratoria (Orthoptera, Acrididae) Insect Biochem. Mol. Biol. 2000;30:233–241. doi: 10.1016/s0965-1748(99)00121-6. [DOI] [PubMed] [Google Scholar]
- 20.Marchese S. Soluble proteins from chemosensory organs of Eurycantha calcarata (Insecta, Phasmatodea) Insect Biochem. Mol. Biol. 2000;30:1091–1098. doi: 10.1016/s0965-1748(00)00084-9. [DOI] [PubMed] [Google Scholar]
- 21.Normura A. Purification and localization of p10, a novel protein that increases in nymphal regenerating legs of Periplaneta americana (American cockroach) Int. J. Dev. Biol. 1992;36:391–398. [PubMed] [Google Scholar]
- 22.Kitabayashi A.N. Molecular cloning of cDNA for p10, a novel protein that increases in nymphal regenerating legs of Periplaneta americana (American cockroach) Insect Biochem. Mol. Biol. 1998;28:785–790. doi: 10.1016/s0965-1748(98)00058-7. [DOI] [PubMed] [Google Scholar]
- 23.Campanacci V. Chemosensory protein from the moth Mamestra brassicae. Expression and secondary structure from 1H and 15N NMR. Eur. J. Biochem. 2001;268:4731–4739. doi: 10.1046/j.1432-1327.2001.02398.x. [DOI] [PubMed] [Google Scholar]
- 24.Vogt R.G. A comparative study of odorant-binding protein genes: differential expression of the PBP1-GOBP2 gene cluster in Manduca sexta (Lepidoptera) and the organization of OBP genes in Drosophila melanogaster (Diptera) J. Exp. Biol. 2002;205:719–744. doi: 10.1242/jeb.205.6.719. [DOI] [PubMed] [Google Scholar]
- 25.Galindo K., Smith D.P. A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics. 2001;159:1059–1072. doi: 10.1093/genetics/159.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham L.A., Davies P.L. The odorant binding proteins of Drosophila melanogaster: annotation and characterization of a divergent gene family. Gene. 2002;292:43–55. doi: 10.1016/s0378-1119(02)00672-8. [DOI] [PubMed] [Google Scholar]
- 27.Thompson J.D. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burge C., Karlin S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 29.Burge C.B., Karlin S. Finding the genes in genomic DNA. Curr. Opin. Struct. Biol. 1998;8:346–354. doi: 10.1016/s0959-440x(98)80069-9. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen H. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Engineering. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen H., Krogh A. Proceedings of the Sixth International Conference on Intelligent Systems for Molecular Biology (ISMB 6) AAAI Press; California, USA: 1998. Prediction of signal peptides and signal anchors by a hidden Markov model; pp. 122–130. [PubMed] [Google Scholar]
- 32.Rost B. Predicting one-dimensional protein structure by profile-based neural networks. Methods Enzymol. 1996;266:525–539. doi: 10.1016/s0076-6879(96)66033-9. [DOI] [PubMed] [Google Scholar]
- 33.Rost B., Sander C. Prediction of protein secondary structure at better than 70% accuracy. J. Mol. Biol. 1993;232:584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- 34.Rost B., Sander C. Conservation and prediction of solvent accessibility in protein families. Proteins. 1994;20:216–226. doi: 10.1002/prot.340200303. [DOI] [PubMed] [Google Scholar]
- 35.Campanacci V. Recombinant chemosensory protein (CSP2) from the moth Mamestra brassicae: crystallization and preliminary crystallographic study. Acta Crystallogr. D. Biol. Crystallogr. 2001;57:137–139. doi: 10.1107/s0907444900013822. [DOI] [PubMed] [Google Scholar]
- 36.Guex N., Peitsch M.C. SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modelling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]