Abstract
Plasmodium vivax is now the predominant cause of malaria in the Asia-Pacific, South America and Horn of Africa. Laboratory studies of this species are constrained by the inability to maintain the parasite in continuous ex vivo culture, but genomic approaches provide an alternative and complementary avenue to investigate the parasite’s biology and epidemiology. To date, molecular studies of P. vivax have relied on the Salvador-I reference genome sequence, derived from a monkey-adapted strain from South America. However, the Salvador-I reference remains highly fragmented with over 2500 unassembled scaffolds. Using high-depth Illumina sequence data, we assembled and annotated a new reference sequence, PvP01, sourced directly from a patient from Papua Indonesia. Draft assemblies of isolates from China (PvC01) and Thailand (PvT01) were also prepared for comparative purposes. The quality of the PvP01 assembly is improved greatly over Salvador-I, with fragmentation reduced to 226 scaffolds. Detailed manual curation has ensured highly comprehensive annotation, with functions attributed to 58% core genes in PvP01 versus 38% in Salvador-I. The assemblies of PvP01, PvC01 and PvT01 are larger than that of Salvador-I (28-30 versus 27 Mb), owing to improved assembly of the subtelomeres. An extensive repertoire of over 1200 Plasmodium interspersed repeat ( pir) genes were identified in PvP01 compared to 346 in Salvador-I, suggesting a vital role in parasite survival or development. The manually curated PvP01 reference and PvC01 and PvT01 draft assemblies are important new resources to study vivax malaria. PvP01 is maintained at GeneDB and ongoing curation will ensure continual improvements in assembly and annotation quality.
Keywords: Plasmodium, vivax, genome, reference, subtelomere, pir, vir
Introduction
Infection with Plasmodium vivax is associated with significant direct and indirect morbidity that impacts on the poorest communities of malarious countries, with an estimated annual global cost of $1-2.7 billion 1– 3. Accumulating reports of drug-resistant infection and life-threatening disease underscore the urgency to reduce the burden of P. vivax and ensure its ultimate elimination 4– 8. Efforts to contain P. vivax are constrained by a limited understanding of the parasite’s basic biology, in part owing to the inability to maintain this species in continuous ex vivo culture. Genetic studies provide an alternative approach to gain novel insights into the parasite from which epidemiological tools and therapeutic approaches can be developed for clinical application 9– 17. The rapidly declining costs of massively parallel sequencing technologies have made it feasible to undertake whole genome sequencing of hundreds of Plasmodium isolates, with recent population genomic studies of P. vivax revealing novel antimalarial drug resistance and vaccine candidates amongst other biological features of the parasite 16, 17. However, in order to achieve a comprehensive understanding of the structure and composition of the P. vivax genome, and to improve read mapping efforts to characterise genetic polymorphisms, a high quality reference genome(s) representative of naturally occurring patient isolates is essential.
The sequences of 5 monkey-adapted strains including the Salvador-I reference 14 and drafts of Brazil-I, India-VII, North Korea and Mauritania-I 13 have provided important resources for the vivax research community to investigate the core genome of P. vivax. However, over 60% of the genes in the published Salvador-I reference 14 (prior to curation by the authors) had unknown function, limiting insight into underlying biological mechanisms. Furthermore, assembly of the subtelomeric regions is highly fragmented in these strains, with Salvador-I comprising >2500 scaffolds. A subsequent draft assembly of a Cambodian patient isolate (C127) revealed 792 genes not present in Salvador-I, including 366 new pir ( Plasmodium interspersed repeat) genes 11. The pir genes are a highly variable multigene family present in all Plasmodium genomes investigated to date 18. The function of pir-encoded proteins (PIRs) remains poorly understood, although recent studies suggest roles in mechanisms associated with virulence. In vitro studies of P. vivax have demonstrated PIR encoded protein mediated cytoadherence to endothelial cells 19, 20 and a P. chabaudi mouse malaria model demonstrated red blood cell-binding properties consistent with roles in invasion and/or rosette formation 21. A further P. chabaudi study demonstrated that changes in the expression of the pir gene repertoire following mosquito passage may attenuate virulence 22. The sequence diversity amongst the pir genes in P. vivax suggests that different subfamilies may have different functions 14. The published Salvador-I reference sequence revealed 346 pir genes, including 80 fragments and/or pseudogenes, 10 subfamilies and 84 unassigned genes 14. In the most recent computational classification, Lopez et al. re-classified the Salvador-I pir genes, excluding members of 3 major subfamilies (A, D and H) but including previously unassigned genes, and re-defining 39 genes as encoding PIRs rather than hypothetical proteins 23. However, given the limited number of PIRs in Salvador-I, further characterisation is required using a reference(s) with a more complete set of genes.
To address the need of the vivax research community for a P. vivax reference with more comprehensive assembly and annotation, we used Illumina genomic data to establish a reference from a Papua Indonesian patient isolate (PvP01). Since P. vivax exhibits marked regional variation in phenotypes such as duration of the dormant liver-stage, drug resistance and disease severity, we compared PvP01 to C127 and the 5 monkey-adapted strains, and generated draft assemblies of patient isolates from Thailand (PvT01) and central China (PvC01). Our sampling focuses on the Asia-Pacific region, where a large burden of P. vivax infection lies 24. The Indonesian reference provides representation of the island of Papua - the epicentre of multidrug resistance emergence in P. vivax 8. The draft references from Thailand and Central China provide respective representation of the Mekong region, and the temperate north where long latency phenotypes prevail 25.
Methods
Samples
Three P. vivax field isolates that were judged to be clonal infections following preliminary genomic analysis within the framework of a separate study 17 were selected for assembly. The isolates were sourced from a patient presenting at hospital in northern Australia in December 2012 with a recent travel history to Mimika Regency, Papua Indonesia (strain PvP01), and patients presenting with symptomatic infection to local clinics in Nan Province, Thailand in May 2011 (strain PvT01) and Anhui Province, China, in September 2010 (strain PvC01). Patient blood samples were leukodepleted 26, and DNA extracted using the QIAamp blood midi kit (Qiagen). All samples were collected with written informed consent from the patients within the framework of previous studies.
Ethical approval
Ethical approval was provided by the Human Research Ethics Committee of NT Department of Health and Families and Menzies School of Health Research, Darwin, Australia (HREC-09/83), the Mahidol University Faculty of Medical Technology Ethics Committee, Bangkok, Thailand (MUTM 2011-043-03), and the Institutional Review Board of Jiangsu Institute of Parasitic Diseases, Wuxi, China (IRB00004221).
Sequencing, assembly and annotation
Library preparation and sequencing was performed at the Wellcome Trust Sanger Institute. Genomic DNA was sheared into 300–500 base pair (bp) fragments using ultrasonication (Covaris). Amplification-free Illumina libraries were prepared 27 and 75 bp, 100 bp and 250 bp paired end reads were generated on the Illumina GAII, Hi-Seq 2000 v3 and MiSeq platforms respectively, following the manufacturer’s standard cluster generation and sequencing protocols 28. Mate-pair libraries with 2–3 kilobase (kb) inserts were additionally prepared for PvP01 and PvT01, using the Illumina mate-pair library preparation kit (v2), and sequenced on the Illumina HiSeq 2500 platform. Prior to assembly, contaminating host–derived sequences were excluded by mapping against the human reference genome (GRCh37: ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/technical/reference/) using BWA 29 (version 0.7.4). Assemblies were prepared using velvet (version 1.2.07, parameters: -exp_cov auto -ins_length 450 -ins_length_sd 30 -cov_cutoff 8, and using for a kmer of 71) and MaSuRCA 30, 31 (version 2.0.3.1, default parameters). Post-assembly genome improvements were undertaken using a range of automated configuration tools including ABACAS (version 2), IMAGE (version 2, iterating k-mers from 71 down 31, 7 iterations), Gapfiller (version 1–11, 14 iteration, parameter n=31) and iCORN (version 2, 7 iterations). PAGIT (version 1) and REAPR (version 1.0.17) were employed to detect assembly errors 32– 38. This was followed by visual inspection using ACT 39 to identify any further assembly anomalies. Annotation was undertaken initially using the automated algorithms, RATT (version 1) and Augustus (version 2.7, trained on 500 manually curated gene models) 38, 40, 41 and further improved by detailed manual inspection performed by an experienced genome curator. PvT01 and PvC01 were annotated using Companion, a new automated annotation tool 42. RNA-Seq data from asexual blood stage preparations of 4 P. vivax patient isolates from Cambodia (unpublished report, Jessica Hostetler, Lia Chappell, Chanaki Amaratunga, Seila Suon, Thomas D. Otto, Rick Fairhurst and Julian C. Rayner; Accession number ERP017542) was used as supporting evidence to aid the improvement of gene models in PvP01 by manual curation.
For comparative analyses, genome assemblies and gene annotations were sourced for 6 additional P. vivax strains; Salvador-I, C127, Brazil-I, India-VII, Mauritania-I and North Korea 9, 13, 14. The published version of Salvador-I 14 presented in PlasmoDB release 9 was selected for comparison of gene annotations as the additional improvements in release 10 reflected curations performed by the authors. Companion was also used to update the annotation of four previously published genomes (Brazil-I, India-VII, Mauritania-I and North Korea).
OrthoMCL and pir analysis
Comparisons of predicted protein-coding genes between the 9 P. vivax assemblies and P. falciparum 3D7 (Pf3D7) (geneDB.org) were undertaken using OrthoMCL version 1.4 43 using the default parameter settings. We determined core genes as 1-1 orthologous between P. vivax P01 and Pf3D7, in total 4465.
Cluster analysis based on structural and sequence homology was undertaken to compare the subfamily organization of the pirs in the partial (Salvador-I) versus more complete (PvP01) reference. All PIR encoded protein sequences in Salvador-I and PvP01 with length greater than 150 amino acids and not flagged as pseudogenes were included in the analysis. Low complexity regions were excluded using the SEG program 44. The relatedness between sequences was assessed using BLASTp (parameters -F F -e 1e-6), and the results were visualized as a network constructed in Gephi 45. After provisional assessment of cluster resolution at different thresholds, a cut-off of 25% of the global similarity was selected for distinguishing different clusters (subfamilies). To aid comparison against the new PIRs identified in PvP01, the Salvador-I PIRs were colour-coded according to the subfamily classification proposed by Lopez et al 23.
Further investigation of the diversity and relatedness amongst the PIRs was undertaken using the PIR sets from PvP01, PvT01, PvC01, Salvador-I and Brazil-I. Exclusion of proteins with less than 150 amino acids, filtering of low complexity sequences and relatedness analysis using BLASTp were performed as described above. A network was constructed from the BLAST output using tribeMCL with an inflation of 1.5 46. To aid visualization, clusters with less than 15 PIRs were excluded.
Dataset validation
The PvP01 assembly was generated as a new reference sequence and is thus a higher quality, more accurately annotated assembly than PvC01 and PvT01, which were both created as draft assemblies for comparative purposes. The PvP01 assembly quality is greatly improved over the previous Salvador-I reference genome, with fragmentation reduced to <250 scaffolds amongst other features ( Table 1). At 29 megabases (Mb), the assembly is notably larger than Salvador-I (27 Mb), mainly due to newly assembled subtelomeric sequences. A complete mitochondrial sequence (5 kb) and partial apicoplast sequence (29.6 kb) are also available. As in P. falciparum 47, the apicoplast reference will facilitate efforts to identify geographic surveillance markers for P. vivax.
Table 1. Features of the new P. vivax assemblies against Salvador-I.
| Genome features | PvP01 a | PvC01 | PvT01 | Salvador-I b |
|---|---|---|---|---|
| Nuclear genome | ||||
| Assembly size (Mb) | 29.0 | 30.2 | 28.9 | 26.8 |
| Coverage (fold) | 212 | 56 | 89 | 10 |
| G + C content (%) | 39.8 | 39.2 | 39.7 | 42.3 |
| No. scaffolds
assigned to chrom. |
14 | 14 | 14 | 30 |
| No. unassigned
scaffolds |
226 | 529 | 359 | 2745 |
| No. genes c | 6,642 | 6,690 | 6,464 | 5,433 |
| No. pir genes | 1,212 | 1,061 | 867 | 346 |
| Mitochondrial genome d | ||||
| Assembly size (bp) | 5,989 | - | - | 5,990 |
| G + C content (%) | 30.5 | - | - | 30.5 |
| Apicoplast genome | ||||
| Assembly size (kb) | 29.6 | 27.6 e | 6.6 f | 5.1 g |
| G + C content (%) | 13.3 | 12.7 | 19.7 | 17.1 |
| No. genes | 30 | 3 | 0 | 0 |
a Genome version 1.09.2016
b Published reference sequence 14
c Including pseudogenes and partial genes, excluding non-coding RNA genes.
d Mitochdondrial genome is not present in PvT01 and PvC01
e scaffold PvC01_00_191
f scaffold PvT01_00_162
g Partial apicoplast sequence of Salavador-I reference assembly has been published (scaffolds AAKM01000417, AAKM01000371)
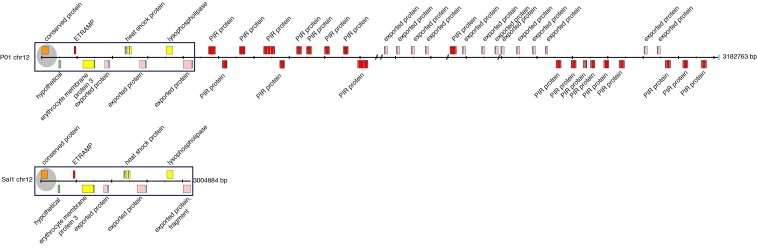
Whilst the assembly quality in the core region is high in Salvador-I 14, PvP01 displays improved gene models and has more complete subtelomeres. Figure 1 provides a schematic of the right-hand end of chromosome 12 from PvP01 and Salvador-I, illustrating the generally greater extension into the subtelomeric regions of chromosomes in PvP01. Furthermore, owing to detailed manual curation and continuous maintenance within the GeneDB framework, the level of gene annotation in the core genome of PvP01 greatly exceeds that of the other available P. vivax assemblies. The asexual stage P. vivax RNA-Seq data enabled correction of the structure of 377 genes. Of the 4577 core P. vivax genes with 1:1 orthologues in P. falciparum, 3318 genes were transcribed with RPKM (reads per kilobase of transcript per million mapped reads) values greater than 15, and contained a total of 4887 splice sites. Of these splice sites, a total of 4845 (99.1%) were confirmed by ≥ 10 reads, highlighting the high quality of the structural annotation. Whereas the published Salvador-I reference includes functions attributed to a total of 1783 (38.0%) core genes 14, we have been able to expand this to 2848 (58.6%) in PvP01, as of the latest GeneDB release (1st September 2016). Ongoing curation on PvP01 will yield further improvements to the annotation statistics, and progress is highlighted in Table 2, which summarizes annotation changes over a 12 month period between GeneDB releases in 2015 and 2016. To date, a total of 1209 genes have been identified in PvP01 that were either completely absent from Salvador-I or have arisen by splitting gene structures that were falsely joined previously ( Table 1). Although the majority of newly identified genes belong to subtelomeric gene families, we confirmed the recently identified EBP2 (erythrocyte binding protein 2, PVP01_0102300) and RBP2e (reticulocyte binding protein 2e, PVP01_0700500) genes 11. These genes are members of families encoding proteins implicated in host cell recognition during red blood cell (RBC) invasion, and present potential vaccine targets 48– 51.
Figure 1. Organization of the subtelomeric regions of chromosome 12 of the PvP01 and Salvador-I P. vivax references illustrating the higher assembly quality of PvP01.
The order and orientation of the genes in the 3’ subtelomeric region of chromosomes 12 of PvP01 (top) and Salvador-I (bottom) are shown. Exons are shown in coloured boxes, with introns illustrated by linking lines. Gaps in PvP01 are indicated with a forward slash (“/”). The blue box indicates the start of the telomeric heptamer repeats. The shaded (grey) areas mark the start of the conserved core of the chromosome that shares synteny with other Plasmodium species (e.g. P. falciparum). The black box shows the syntenic area of PvP01 and Salvador-I. The last gene in this syntenic area is fragmented in Salvador-I.
Table 2. Annotation changes in P. vivax P01 from 1 st of September 2015 until 27 th of September 2016.
| Annotation event type | PvP01 a |
|---|---|
| Assigned or updated product | 408 |
| Product updated from “conserved
Plasmodium protein, unknown function” |
107 |
| Updated GO term | 597 |
| Linked to publication | 291 |
| All unique genes with new functional
annotations, e.g. EC number, gene name |
608 |
| All unique genes with new structural
annotations |
50 |
a Genome version 1.09.2016
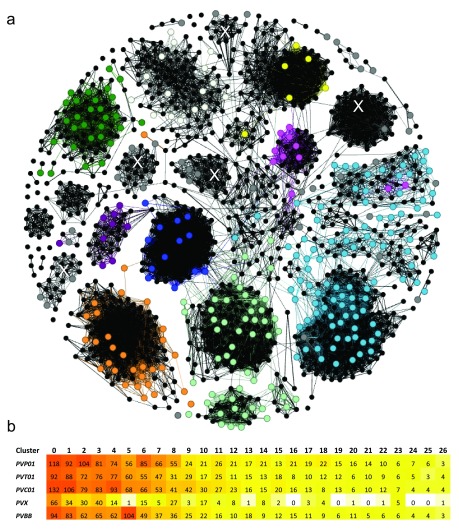
As summarised in Table 3, the comparatively high assembly quality in the subtelomeres of PvP01 greatly expanded the repertoire of genes belonging to multigene families in these chromosome regions. Notably, more than 1200 pir genes were identified in PvP01 versus 346 in Salvador-I. To generate a snapshot of the diversity and structural organization of this expanded gene family in P. vivax, we conducted cluster analysis of the PIRs in PvP01 with comparison to previous homology classifications performed by Lopez et al on the partial set of PIRs from Salvador-I 23. As illustrated in the network diagram in Figure 2a, the main subfamily clusters defined in earlier classifications are expanded but, on addition of the new PvP01 PIRs, the clusters remained moderately stable with no pooling between or sub-structure within subfamilies. However, the new PvP01 PIRs reveal several large subfamilies containing just 1–4 Salvador-I genes that were previously unclassified ( Figure 2a). Additional investigation with the PvC01, PvT01 and Brazil-I assemblies using tribeMCL (also used in Lopez et al) confirmed the stability of the new subfamilies identified in PvP01 across a geographically divergent collection of isolates ( Figure 2b). The analysis conducted here provides a broad overview of the diversity and relatedness amongst the expanded P. vivax pir gene sets, however further investigation beyond the scope of this study will be required to provide detailed characterisation of this family and its contribution to virulence and pathophysiology.
Figure 2. Cluster analysis illustrating the relatedness between the PIR proteins in PvP01 versus Salvador-I (a), and the stability of the major clusters in several other P. vivax assemblies (b).
Panel a) presents a network illustrating the relatedness between the 1063 PIR proteins of PvP01 and 341 PIRs of Salvador-I (Sal-I) with length greater than 150 amino acids. The PvP01 PIRs are illustrated by black dots (nodes). The Sal-I PIRs are illustrated by coloured dots with colour-coding according to the subfamily classification of Lopez et al 23 as follows; purple = A, pink = B, pale green = C, red = D, pale blue = E, orange = G, green = H, blue = I, white = J, yellow = K , and grey = unassigned genes. Two nodes (PIRs) are connected if they have a global similarity of at least 25%. With the exception of a few proteins, the majority of Sal-I PIRs demonstrate clustering consistent with the classification of Lopez et al. Five new, interconnected clusters comprising previously unassigned Sal-I PIRs are denoted with a white “X”. In Panel b, a heat map summarises the number of PIRs assigned to the 27 major clusters (minimum 15 PIRs in total) in five geographically divergent P. vivax strains; PvP01 (Papua Indonesia), PvT01 (Thailand), PvC01 (Central China), Sal-I (El Salvador) and Brazil-I (Brazil). With the exception of Sal-I, which displayed fewer genes than the other isolates in several of the major clusters, the isolates demonstrated similar numbers of genes in most clusters.
Table 3. Number of most abundant genes in the subtelomeres in the genomes of Salvador-I, PvP01, PvT01 and PvC01.
| Description | Sal-I a | PvP01 b | PvC01 | PvT01 | |
|---|---|---|---|---|---|
| Multigene family | PIR protein c | 346 | 1212 | 1061 | 867 |
| tryptophan-rich protein d | 34 | 40 | 40 | 40 | |
| lysophospholipase e | 11 | 10 | 9 | 8 | |
| STP1 protein f | 9 | 10 | 11 | 3 | |
| early transcribed membrane protein (ETRAMP) | 10 | 9 | 9 | 9 | |
| Plasmodium exported protein (PHIST), unknown function g | 64 | 84 | 22 | 23 | |
| reticulocyte binding protein (RBP) | 9 h | 9 h | 9 | 8 | |
| Other genes | Plasmodium exported proteins of unknown function i | 23 | 447 | 266 | 261 |
| Total | n/a | 497 | 1812 | 1427 | 1219 |
Numbers include pseudogenes and partial genes
a Published reference sequence 14
b Genome version 1.09.2016
c Other names include VIR protein and Pv-fam-c protein
d Other names include Pv-fam-a, trag and tryptophan-rich antigen
e Other names include PST-A protein
f Other names include PvSTP1
g Other names include Phist protein (Pf-fam-b) and RAD protein (Pv-fam-e)
h Includes RBP2e (PVP01_0700500) that was not present in the Salvador-I assembly. RBP1b (PVP01_0701100) is complete in PvP01. In Salvador-I RBP1b consists of two partial genes (PVX_098582, PVX_125738)
i Other names include Pv-fam-d protein and Pv-fam-c protein
The PvP01 reference is an important new resource for the vivax research community. It will support studies of the complex subtelomeric regions and provide insights into the mechanisms by which the gene families in this region contribute to virulence-associated functions. It will also allow investigation of an array of other biological functions that will expand with continual improvements in annotation in the core genome. PvP01, PvC01 and PvT01 add new geographic locations to the collection of P. vivax assemblies, facilitating biological studies of the diversity of this phenotypically divergent species.
Data availability
The raw sequence data for PvP01, PvT01 and PvC01 can be retrieved from the European Nucleotide Archive; sample accession numbers PvP01 ERS017708, ERS312161 3kb ERS328510, PvT01 ERS055881, ERS312160 3kb ERS328509 and PvC01 ERS407449. The assemblies can be found under the study PRJEB14589. The individual accession numbers are PvP01 (chromosomes: currently in submission to EBI, files on ftp, contigs: FLZR01000001-FLZR01000226), PvT01 (chromosomes LT615239-LT615252, contigs: FLYH01000001-FLYH01000360) and PvC01 (chromsomes LT615256-LT615269, contigs: FLYI01000001-FLYI01000530). PvP01 is maintained in GeneDB: http://www.genedb.org/Homepage/PvivaxP01 and updates are synchronized to PlasmoDB.
This section will be updated with accession numbers for PvP01 chromosomes onces available.
Acknowledgments
We would like to thank the patients who contributed samples and the health workers who assisted with the sample collections as well as Julian Rayner, Rick Fairhurst, Chanaki Amaratunga, Lia Chappell and Seila Suon for use of unpublished P. vivax RNA-Seq data. We would also like to thank staff from the Illumina Bespoke Sequencing Team at the Wellcome Trust Sanger Institute for their contribution.
Funding Statement
This work was supported by Wellcome Trust [098051], [099198], [091625].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Carlton JM, Sina BJ, Adams JH: Why is Plasmodium vivax a neglected tropical disease? PLoS Negl Trop Dis. 2011;5(6):e1160. 10.1371/journal.pntd.0001160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mendis K, Sina BJ, Marchesini P, et al. : The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64(1–2 Suppl):97–106. [DOI] [PubMed] [Google Scholar]
- 3. Price RN, Tjitra E, Guerra CA, et al. : Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77(6 Suppl):79–87. [PMC free article] [PubMed] [Google Scholar]
- 4. Karyana M, Burdarm L, Yeung S, et al. : Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar J. 2008;7:148. 10.1186/1475-2875-7-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nosten F, McGready R, Simpson JA, et al. : Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354(9178):546–9. 10.1016/S0140-6736(98)09247-2 [DOI] [PubMed] [Google Scholar]
- 6. Poespoprodjo JR, Fobia W, Kenangalem E, et al. : Vivax malaria: a major cause of morbidity in early infancy. Clin Infect Dis. 2009;48(12):1704–12. 10.1086/599041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poespoprodjo JR, Fobia W, Kenangalem E, et al. : Adverse pregnancy outcomes in an area where multidrug-resistant Plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis. 2008;46(9):1374–81. 10.1086/586743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Price RN, von Seidlein L, Valecha N, et al. : Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(10):982–91. 10.1016/S1473-3099(14)70855-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan ER, Menard D, David PH, et al. : Whole genome sequencing of field isolates provides robust characterization of genetic diversity in Plasmodium vivax. PLoS Negl Trop Dis. 2012;6(9):e1811. 10.1371/journal.pntd.0001811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dharia NV, Bright AT, Westenberger SJ, et al. : Whole-genome sequencing and microarray analysis of ex vivo Plasmodium vivax reveal selective pressure on putative drug resistance genes. Proc Natl Acad Sci U S A. 2010;107(46):20045–50. 10.1073/pnas.1003776107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hester J, Chan ER, Menard D, et al. : De novo assembly of a field isolate genome reveals novel Plasmodium vivax erythrocyte invasion genes. PLoS Negl Trop Dis. 2013;7(12):e2569. 10.1371/journal.pntd.0002569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menard D, Chan ER, Benedet C, et al. : Whole genome sequencing of field isolates reveals a common duplication of the Duffy binding protein gene in Malagasy Plasmodium vivax strains. PLoS Negl Trop Dis. 2013;7(11):e2489. 10.1371/journal.pntd.0002489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neafsey DE, Galinsky K, Jiang RH, et al. : The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nat Genet. 2012;44(9):1046–50. 10.1038/ng.2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carlton JM, Adams JH, Silva JC, et al. : Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455(7214):757–63. 10.1038/nature07327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bright AT, Manary MJ, Tewhey R, et al. : A high resolution case study of a patient with recurrent Plasmodium vivax infections shows that relapses were caused by meiotic siblings. PLoS Negl Trop Dis. 2014;8(6):e2882. 10.1371/journal.pntd.0002882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hupalo DN, Luo Z, Melnikov A, et al. : Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat Genet. 2016;48(8):953–8. 10.1038/ng.3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pearson RD, Amato R, Auburn S, et al. : Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nat Genet. 2016;48(8):959–64. 10.1038/ng.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cunningham D, Lawton J, Jarra W, et al. : The pir multigene family of Plasmodium: antigenic variation and beyond. Mol Biochem Parasitol. 2010;170(2):65–73. 10.1016/j.molbiopara.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 19. Bernabeu M, Lopez FJ, Ferrer M, et al. : Functional analysis of Plasmodium vivax VIR proteins reveals different subcellular localizations and cytoadherence to the ICAM-1 endothelial receptor. Cell Microbiol. 2012;14(3):386–400. 10.1111/j.1462-5822.2011.01726.x [DOI] [PubMed] [Google Scholar]
- 20. Carvalho BO, Lopes SC, Nogueira PA, et al. : On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis. 2010;202(4):638–47. 10.1086/654815 [DOI] [PubMed] [Google Scholar]
- 21. Yam XY, Brugat T, Siau A, et al. : Characterization of the Plasmodium Interspersed Repeats (PIR) proteins of Plasmodium chabaudi indicates functional diversity. Sci Rep. 2016;6:23449. 10.1038/srep23449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spence PJ, Jarra W, Lévy P, et al. : Vector transmission regulates immune control of Plasmodium virulence. Nature. 2013;498(7453):228–31. 10.1038/nature12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopez FJ, Bernabeu M, Fernandez-Becerra C, et al. : A new computational approach redefines the subtelomeric vir superfamily of Plasmodium vivax. BMC Genomics. 2013:14:8. 10.1186/1471-2164-14-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guerra CA, Howes RE, Patil AP, et al. : The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4(8):e774. 10.1371/journal.pntd.0000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. White NJ: Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. 10.1186/1475-2875-10-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Auburn S, Marfurt J, Maslen G, et al. : Effective preparation of Plasmodium vivax field isolates for high-throughput whole genome sequencing. PLoS One. 2013;8(1):e53160. 10.1371/journal.pone.0053160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kozarewa I, Ning Z, Quail MA, et al. : Amplification-free Illumina sequencing-library preparation facilitates improved mapping and assembly of (G+C)-biased genomes. Nat Methods. 2009;6(4):291–5. 10.1038/nmeth.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bentley DR, Balasubramanian S, Swerdlow HP, et al. : Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456(7218):53–9. 10.1038/nature07517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li H, Durbin R: Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zerbino DR, Birney E: Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–9. 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zimin AV, Marçais G, Puiu D, et al. : The MaSuRCA genome assembler. Bioinformatics. 2013;29(21):2669–77. 10.1093/bioinformatics/btt476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Assefa S, Keane TM, Otto TD, et al. : ABACAS: algorithm-based automatic contiguation of assembled sequences. Bioinformatics. 2009;25(15):1968–9. 10.1093/bioinformatics/btp347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hunt M, Kikuchi T, Sanders M, et al. : REAPR: a universal tool for genome assembly evaluation. Genome Biol. 2013;14(5):R47. 10.1186/gb-2013-14-5-r47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Otto TD, Sanders M, Berriman M, et al. : Iterative Correction of Reference Nucleotides (iCORN) using second generation sequencing technology. Bioinformatics. 2010;26(14):1704–7. 10.1093/bioinformatics/btq269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swain MT, Tsai IJ, Assefa SA, et al. : A post-assembly genome-improvement toolkit (PAGIT) to obtain annotated genomes from contigs. Nat Protoc. 2012;7(7):1260–84. 10.1038/nprot.2012.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsai IJ, Otto TD, Berriman M: Improving draft assemblies by iterative mapping and assembly of short reads to eliminate gaps. Genome Biol. 2010;11(4):R41. 10.1186/gb-2010-11-4-r41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boetzer M, Pirovano W: Toward almost closed genomes with GapFiller. Genome Biol. 2012;13(6):R56. 10.1186/gb-2012-13-6-r56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Otto TD: From sequence mapping to genome assemblies. Methods Mol Biol. 2015;1201:19–50. 10.1007/978-1-4939-1438-8_2 [DOI] [PubMed] [Google Scholar]
- 39. Carver T, Berriman M, Tivey A, et al. : Artemis and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics. 2008;24(23):2672–6. 10.1093/bioinformatics/btn529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Otto TD, Dillon GP, Degrave WS, et al. : RATT: Rapid Annotation Transfer Tool. Nucleic Acids Res. 2011;39(9):e57. 10.1093/nar/gkq1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stanke M, Steinkamp R, Waack S, et al. : AUGUSTUS: a web server for gene finding in eukaryotes. Nucleic Acids Res. 2004;32(Web Server issue):W309–12. 10.1093/nar/gkh379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steinbiss S, Silva-Franco F, Brunk B, et al. : Companion: a web server for annotation and analysis of parasite genomes. Nucleic Acids Res. 2016;44(W1):W29–34. 10.1093/nar/gkw292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li L, Stoeckert CJ, Jr, Roos DS: OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13(9):2178–89. 10.1101/gr.1224503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wootton JC, Federhen S: Analysis of compositionally biased regions in sequence databases. Methods Enzymol. 1996;266:554–71. 10.1016/S0076-6879(96)66035-2 [DOI] [PubMed] [Google Scholar]
- 45. Bastian M, Heymann S, Jacomy M: Gephi: An Open Source Software for Exploring and Manipulating Networks. In: International AAAI Conference on Weblogs and Social Media2009. Reference Source [Google Scholar]
- 46. Enright AJ, Van Dongen S, Ouzounis CA: An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002;30(7):1575–84. 10.1093/nar/30.7.1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Preston MD, Campino S, Assefa SA, et al. : A barcode of organellar genome polymorphisms identifies the geographic origin of Plasmodium falciparum strains. Nat Commun. 2014;5:4052. 10.1038/ncomms5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fang XD, Kaslow DC, Adams JH, et al. : Cloning of the Plasmodium vivax Duffy receptor. Mol Biochem Parasitol. 1991;44(1):125–32. 10.1016/0166-6851(91)90228-X [DOI] [PubMed] [Google Scholar]
- 49. Galinski MR, Medina CC, Ingravallo P, et al. : A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell. 1992;69(7):1213–26. 10.1016/0092-8674(92)90642-P [DOI] [PubMed] [Google Scholar]
- 50. França CT, He WQ, Gruszczyk J, et al. : Plasmodium vivax Reticulocyte Binding Proteins Are Key Targets of Naturally Acquired Immunity in Young Papua New Guinean Children. PLoS Negl Trop Dis. 2016;10(9):e0005014. 10.1371/journal.pntd.0005014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ntumngia FB, Thomson-Luque R, Torres Lde M, et al. : A Novel Erythrocyte Binding Protein of Plasmodium vivax Suggests an Alternate Invasion Pathway into Duffy-Positive Reticulocytes. MBio. 2016;7(4): pii: e01261-16. 10.1128/mBio.01261-16 [DOI] [PMC free article] [PubMed] [Google Scholar]