Abstract
In this review, we advance a new concept in developing vaccines and/or drugs to target specific proteins expressed during the early stage of Bacillus anthracis (anthrax) infection and address existing challenges to this concept. Three proteins (immune inhibitor A, GPR-like spore protease, and alanine racemase) initially identified by proteomics in our laboratory were found to have differential expressions during anthrax spore germination and early outgrowth. Other studies of different bacillus strains indicate that these three proteins are involved in either germination or cytotoxicity of spores, suggesting that they may serve as potential targets for the design of anti-anthrax vaccines and drugs.
Key words: proteomics, Bacillus anthracis, vaccines, drugs
Introduction
Bacillus anthracis (anthrax) spores were distributed through the postal system as biological weapons in the US in the autumn of 2001, resulting in cases of cutaneous and inhalational anthrax. It has been reported that the mortality associated with inhalational anthrax was 45% (1). There are three clinical forms of anthrax: cutaneous, inhalational and gastrointestinal (2). Ciprofloxacin has become a treatment of choice for all clinical forms of anthrax owing to potential resistance to penicillins and tetracyclines, and demonstrated efficacy in a primate model (1). Anthrax possesses a penicillinase gene and a constitutive cephalosporinase. Additionally, it is reported that the former Soviet Union engineered penicillin and tetracycline resistant anthrax spores (3). The infection cycle of Bacillus anthracis (anthrax) is progressive. The initial stage of infection occurs when pathogenic spores interact with macrophages at the site of host entry (4). Once inhaled, spores germinate while associated with alveolar macrophages traveling en route to regional lymph nodes. During systemic anthrax infection, late stage events include vegetative growth in the blood to very high titers and synthesis of the anthrax toxins, causing disease symptoms and ultimately death 5., 6.. Contemporary anthrax therapy focuses on three-component toxins: protective antigen (PA), lethal factor (LF), and edema factor (EF), which are produced by vegetative cells during the late stage of anthrax infection (7). Targeting PA has been variably effective, and the ability of existing PA-targeting methods to protect humans against inhalational anthrax that might be released by biological weapons is an open question. Targeting the early stages of anthrax infection (macrophage interaction, germination, and early outgrowth) may be more effective than targeting PA, LF, and/or EF in counteracting anthrax in that the upstream events that lead to their production could be prevented. Several factors favor developing interventions that target the early stages of anthrax infection. First, the interaction between spores and macrophages is an upstream event in the life cycle of anthrax. The interruption of upstream events (macrophage interaction, germination and early outgrowth) precludes downstream events including the production of PA, LF, or EF. Second, PA, LF, and EF are encoded by a plasmid in anthrax. Therefore, by replacing this plasmid with others that encode virulent toxins (e.g., tetanus toxin, cobra toxin, etc.), bioterrorists could potentially introduce mutant anthrax spores that produce toxins other than PA/LF/EF. Third, vegetative cells may develop antibiotic resistance over time or, bioterrorists might incorporate antibiotic-resistance genes into such cells. Lastly, in contrast to toxins and antibiotic resistance that are prone to change, macrophage interaction and germination factors are highly conserved and less likely to be altered (8). Thus, blocking upstream events with vaccines and/or inhibitory drugs may prevent the use of anthrax spores as bioweapons. The objective of this article is to draw your attention that while the vast majority of anti-anthrax works center around PA/LF/EF, proteins expressed during the early anthrax infection are potential targets for the development of anti-anthrax vaccines or drugs.
Bacillus anthracis (anthrax)
Bacillus anthracis, the causative agent of anthrax, is an endospore-forming, gram-positive, aerobic, and rod-shaped bacterium (9). The dormant spores of anthrax are formed during sporulation, a process triggered by starvation or harsh environment. They have high resistance to heat, radiation, desiccation, pH extremes and toxic chemicals (10). As shown in Figure 1, the dormant spores have a unique and complicated structural anatomy. The mature spore is made up of different zones, an exosporium on the outside with multi-layered spore coats below, a cortex, and a central core. Between the center core and the coat is an inner membrane that is similar to a regular cell membrane. The spore also has an outer membrane covering the coat that may correspond to the regular bacterial cell wall peptidoglycan. Anthrax can infect the host through intradermal inoculation, ingestion, or inhalation of the spore. In vitro and in vivo studies have demonstrated that the dormant spores monitor the environment until conditions are favorable for growth. The spores then germinate and transform through outgrowth (11). The germination process consists of an irreversible series of degradative reactions that result in the breaking of spore dormancy. Although still poorly understood, it appears to be essential for the development of the diseases caused by anthrax. Once inhaled by animals or humans, anthrax spores interact with alveolar macrophages, which have been known to play a central role in innate and acquired immune responses. They act as guard cells to help clear invading bacteria from the lung alveoli 5., 6.. Although understanding of cellular and molecular interactions between anthrax spores and alveolar macrophages at the early stages of infection is incomplete, it is known that the majority of inhaled spores are rapidly and efficiently phagocytosed by alveolar macrophages via recruitment of F-actin (12). Germination of the anthrax spores occurs once they have been phagocytosed by macrophages. This is followed by extracellular multiplication, together with the production of a capsule and toxins (including PA, LF, and EF). Thus the events occurring at the early stage of anthrax infection, including spore/macrophage interaction, germination, and early outgrowth, may be promising immunologic and pharmacologic targets against this organism. Arrest of the anthrax life cycle at its onset would necessarily preclude all downstream events including the lethal manifestations of bacteremia and toxemia.
Fig. 1.
The structure of dormant anthrax spore (Sterne strain). Spores were fixed in modified Karnovsky’s fixative overnight at 4°C, which contained 2% paraformaldehyde, 2.5% glutaraldehyde, 0.06% CaCl2, in 0.1 M cacodylate buffer, pH 7.4. Spores were then washed in 0.1 M cacodylate buffer, postfixed in 0.25% ruthenium tetroxide in 0.1 M cacodylate for 45 min in the dark at the room temperature. The specimens were then rinsed in buffer, dehydrated in graded ethanol solutions, and embedded in an Epon-epoxy resin mixture. Ultrathin 60–80 nm sections were examined under a Hitachi 7000 transmission electron microscope. Images were captured at 75 KV after staining with uranyl acetate/lead citrate. E, exosporium; OC, outer coat membrane; C, coat; IC, inner coat membrane; CX, cortex. Bar, 0.2 μm. (Ref. 5., 18., 11., and Huang, C.M., unpublished data).
Proteomics to screen highly specific anthrax proteins
Currently, there are no anti-anthrax drugs that are marketed for commercial use. The US anthrax vaccine that was licensed in 1970 is made from the filtrate of a non-encapsulated attenuated strain (13). This vaccine has unknown efficacy, and often contains impurities that can trigger local pain and edema as well as other undesirable side effects, such as headaches and malaise (14). It has thus not been readily accepted for public use. The emerging technology of proteomics may be useful in eliminating antigen impurities. In addition, proteomics may also help to detect unexpected changes in protein expression that may be missed by conventional biochemical techniques (15). Specifically two-dimensional (2D) electrophoresis gels can be used to separate thousands of proteins quantitatively over a wide dynamic range. Matrix-assisted laser-desorption-ionization-time-of-flight mass spectrometry (MALDI-TOF MS; PerSeptive Biosystems Inc., Framingham, USA) and quadrupole-time-of-flight (Q-TOF) MS/MS (Micromass, Manchester, UK) with high sensitivity have been commonly used to identify and sequence specific proteins by generating peptides or even amino acid mass “fingerprints” of proteins, which are then searched against a comprehensive computer database (16). Identification of spore proteins represents a crucial step towards the development of anti-anthrax vaccines and drugs. Many anthrax proteins including membrane proteins, spore coat proteins, and exosporium glycoproteins have been identified via 2D electrophoresis or a variety of mass spectrometric methods, including the multidimensional chromatography tandem mass spectrometry and ion trap time-of-flight hybrid mass spectrometer (qIT-TOF MS; ref. 17., 18., 19., 20., 21., 22.).
To identify proteins involved in the early stage of anthrax infection, we constructed 2D protein profiles associated with anthrax germination and early outgrowth (11). By screening 587 paired protein spots that were isolated from dormant and germinating anthrax spores, respectively, we identified 10 spore proteins with statistically significant germination associated increases and decreases. In this review, we will describe two proteases (immune inhibitor A and GPR-like spore protease) and alanine racemase that were differentially altered during the germination and early outgrowth of anthrax spores. Based on information in the literature and findings in our laboratory, we propose that these three proteins (immune inhibitor A, GPR-like spore protease, and alanine racemase) may play vital roles during the early stages of anthrax infection. Developing vaccines and/or inhibitory drugs targeting these three proteins may be an effective strategy to prevent or halt the development of the disease that this organism produces.
Immune Inhibitor A
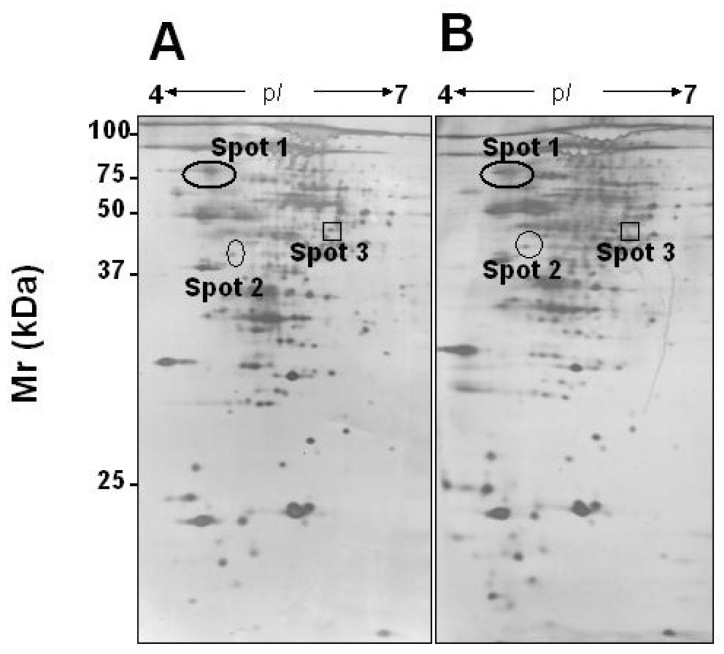
Over 500 proteins from the dormant Sterne spore strain were reproducibly displayed across an isoelectric focusing (IEF) range of 4 to 7. A total of 50 protein spots (Figure 2A), corresponding to 36 different anthrax proteins, were successfully identified or sequenced by MALDI-TOF MS or Q-TOF 2 MS/MS (11). GeneMark predicted six hypothetical proteins derived from the anthrax genome and these were coded as unknown proteins. Following a protein sequence database search, one (spot 1) of the six hypothetical proteins was shown to share 96% homology with immune inhibitor A (gi|9858110) of Bacillus thuringiensis 23., 24.. The level of immune inhibitor A was increased nearly two folds during germination and early outgrowth of anthrax (Figure 2). To identify this protein spot, the spot was cut from the silver-stained gels, in-gel digested with the trypsin enzyme, and then analyzed on a PerSeptive Voyager-DE MALDI-TOF MS. Peptide fingerprint mass spectra generated from MALDI-TOF MS were interpreted and matched to SWISS-PROT database entries using Mascot (http://www.matrixscience.com). Sixteen peaks within the peptide mass fingerprint of spot 1 matched those of a protein with accession number NP_843763 in the SWISS-PROT database (Figure 3). The protein named as a hypothetical protein predicted by GeneMark is in fact a predicted protein from the anthrax genome (25). By using a Q-TOF 2 MS/MS mass spectrometer, we have also determined the internal sequence of this predicated protein at amino acids between 450 and 463 with m/z value at 1465.77 (Figure 4). All 14 amino acid residues (GIGLATYLDQSVTK) from spot 1 were thus determined. More interestingly, the entire amino acid sequence of spot 1 of anthrax shares a high identity with immune inhibitor A of Bacillus thuringiensis (Figure 4). Both of these proteins contain a HEXXH motif, which is defined as a zinc-binding domain of a metalloprotease (23), indicating that spot 1 may be a zinc dependent metalloprotease. Immune inhibitor A is a secreted virulence protease from Bacillus thuringiensis and it specifically degrades two antibacterial proteins (cecropins and attacins) produced by an insect host (26), suggesting that it may contribute to the overall virulence of Bacillus thuringiensis. Bacillus thuringiensis is highly resistant to the insect immune system due to its production of two factors, inhibitor A and inhibitor B, which selectively block the humoral defense system developed by insects against E. coli and Bacillus cereus (24). Immune inhibitor A is known to be a metalloprotease with similarity to the thermolysin of Bacillus thermoproteolyticus, the elastase of Pseudomonas aeruginosa, and the protease E-15 from Serratia (23). It has been shown, using the transcriptional immune inhibitor A’-lacZ fusion construct, that expression of this protein occurs at the onset of sporulation (27). Immune inhibitor A has also been shown to be present as an active 73-kDa polypeptide on the exosporium of Bacillus cereus (28). These results demonstrate for the first time that immune inhibitor A is expressed in anthrax (Fig. 2, Fig. 3). Anthrax harbors the pXO1 plasmid, which has been known to encode anthrax toxins including PA/LF/EF. The pXO1 plasmid sequence of anthrax is highly conserved among closely related bacterial species including Bacillus thuringiensis (29), suggesting that anthrax may possess many toxins, which are highly similar to other bacillus species.
Fig. 2.
Comparative proteomic profiles of dormant and germinating/early outgrowth anthrax (Sterne strain). Proteins (300 μg) collected at 0 (A: dormant spores) and 10 min (B: germinating/early outgrowth anthrax) after incubation were separated by 2D gels via pH 4–7 IEF and 12.5% SDS-PAGE (PolyAcrylamide Gel Electrophoresis), and then silver-stained. Numbered protein spots indicated up- (circles) or down- (square) regulated expression compared to that at time 0. Via MALDI-TOF MS and Q-TOF 2 MS/MS (8), spots 1, 2 and 3 have been identified as immune inhibitor A, GPR-like spore protease, and alanine racemase, respectively.
Fig. 3.
Proteomics identified immune inhibitor A of anthrax. Fingerprint mass spectra of MALDI-TOF MS (A) and the predicted peptide fragments corresponding to observed m/z values (B) for spot 1 (Fig. 2) are shown (11). The tryptic autodigestive peak at m/z value 2164.04 (asterisk) served as an internal calibration standard.
Fig. 4.
Q-TOF 2 MS/MS peptide sequencing of immune inhibitor A (11). Designations for fragment ions from a peptide (A). An internal peptide of immune inhibitor A with m/z value at 1465 analyzed by MALDI-TOF MS (Fig. 3) was sequenced by Q-TOF 2 MS/MS as GIGLATYLDQSVTK (B).
GPR-like Spore Protease
The level of spot 2 was increased during spore germination and early outgrowth (Figure 2). Mass spectrometry matched this spot with protein accession number NP_846769 in the SWISS-PROT database. Since this protein shared 91% sequence identity with the spore protease of Bacillus cereus and 67% identity with the small, acid soluble proteins (SASP) degradation spore germinate protease (GPR) precursor of Bacillus subtilis (30), we assigned it GPR-like spore protease. In the center of the dormant spore is the spore core that contains most of the spore’s enzymes, ribosomes and DNA (30). Enzymes that are present in the dormant spore core are inactive, probably due to very low water content in the core. It has been shown that one of the mechanisms to protect the dormant spore against external damaging effects is saturation of the spore chromosome with low molecular weight proteins termed SASP (31). In the early stage of spore germination, SASP are degraded by GPR. Amino acids are then released for protein synthesis, allowing DNA transcription and also releasing energy for metabolism during the very early stages of germination and outgrowth. Studies in our laboratory have demonstrated that the expression of GPR-like spore protease is significantly increased during germination and early outgrowth (Figure 2). The three-dimensional structure and molecular mechanism of GPR have recently been extensively investigated (10). GPR-like spore protease could be an ideal target for interventions to influence the early stage of anthrax life cycle.
Alanine Racemase
Alanine racemase (spot 3, Figure 2) was found to be strongly absorbed into the exosporium. Alanine and inosine are the two major chemical triggers of spore germination (32). Alanine racemase converts L-ananine to D-alanine, a germination inhibitor and essential component of the peptidoglycan found within the cell wall and spore cortex 11., 32.. Its activity is generally higher in spores than in vegetative cells 11., 33.. The expression level of alanine racemase was decreased during spore germination and early outgrowth 11., 32., supporting the hypothesis that inhibition of this enzyme may be one of the prerequisites for germination of anthrax spores. Expression of alanine racemase in exosporium may allow inactivation of this enzyme by specific antibodies or inhibitory drugs without the requirement of penetrating a physical barrier. Inhibition of alanine racemase may therefore minimize the survival potential of anthrax by triggering premature germination of spores in a suboptimal environment. It is thus possible to decontaminate an anthrax-spore-covered area by sprinkling inhibitors targeting this enzyme in conjunction with specific amino acids and nucleoside combinations that trigger the gerA-like sensor operons (18) to induce death of anthrax through pre-mature spore germination.
Challenges
An approach using 2D gels in conjunction with mass spectrometry has been employed in an attempt to look for candidate vaccines and/or drugs that specifically target proteins expressed at the early stage of anthrax infection. The amount of protein in a 2D gel may be altered by numerous events including changes in extractability from dormant and germinated/early outgrowth spores. Nonetheless, proteins characterized by 2D gels have a high probability of acting as targets, given the magnitude of changes that are occurring during germination. This makes it likely that they play a critical role in germination, outgrowth, maintenance, and stabilization of spores. Further studies to determine the functions and distribution of anthrax proteins that have been identified are essential to the design of appropriate anti-anthrax vaccines and/or drugs. Immune inhibitor A has also been shown to be present on the exosporium of Bacillus cereus (28), but its location in Bacillus anthracis is unknown. If immune inhibitor A is located within the spores, antibodies or anti-anthrax drugs may be unable to gain access to this protein. The same challenge is also present for GPR-like spore protease, which has a high sequence homology with GPR, the protease expressed within the spore core of Bacillus cereus. Since the spore core is protected by an inner membrane and other physical layers outside of the core, it will be necessary to design an intervention that can penetrate all of these barriers to reach the spore core and react with the GPR-like spore protease.
There are other challenges in the development of vaccines/drugs targeting the early stages of anthrax infection. For example, once spores are inhaled, portions of immune inhibitor A and alanine racemase may remain intracellular before macrophages are lysed by anthrax toxins and/or immune inhibitor A. Alternatively, discharge of immune inhibitor A and alanine racemase from dormant spores may occur within alveolar macrophages where they are beyond the reach of circulating antibodies or drugs. It has been reported that multiple signal pathways may be required for the breakdown of dormant anthrax spores (34). Many molecules including immune inhibitor A, GPR-like spore protease, and alanine racemase may coordinate to trigger the early-outgrowth of anthrax spores. Additionally, immune inhibitor A may contribute to the cytotoxicity of anthrax spores by working together with PA/LF/EF. If that is the case, then multivalent vaccines or drugs that combine PA/LF/EF and immune inhibitor A may be a novel method of developing an effective anti-anthrax remedy.
Conclusion
The development of vaccines and/or inhibitory drugs targeting specific proteins expressed during the early stage of anthrax infection may be an effective strategy to halt the attack and spread of the disease (Figure 5). Blocking upstream processes (macrophage interaction, germination, and early outgrowth) that occur early during an infection would prevent downstream events including the production of its three natural toxins, PA, LF and EF. The three anthrax proteins (immune inhibitor A, GPR-like spore protease, and alanine racemase) highlighted in this review have differential expressions during spore germination and outgrowth. Designing anti-anthrax vaccines and/or drugs primarily targeting these three proteins rather than PA, LF and EF may provide a safer and more effective approach to counteract anthrax.
Fig. 5.
A cartoon showing the early stages of the anthrax life cycle. Three proteins (immune inhibitor A, ●; GPR-like spore protease, ▲; and alanine racemase, ■) are thought to be involved in the spore germination/early outgrowth and macrophage interaction. It has been reported that GPR-like spore protease and alanine racemase play an important role in triggering the spore germination 11., 30., 33.. Immune inhibitor A may be discharged from dormant spores during the early stage of anthrax infection and facilitate anthrax invasion into host cells and escape the host immune system. These proteins may be excellent potential targets for vaccines or inhibitory drugs.
Acknowledgements
We thank S. Barnes, L. Wilson, and M. Kirk for their assistance with MALDI-TOF MS and Q-TOF 2 MS/MS analysis; J. Frank for editorial assistance, and L. Millican for her assistance with transmission electron microscope.
This work was supported by National Institutes of Health Grants (1-R21-AI58002-01, R01-CA79820, R01-AI50150, P30-AR050948, 1-R43-AI-47558-01A2 and R01 CA86172-01), a Dermatology Foundation Grant, a VA Grant 18-103-02, an Office of Naval Research Grant N00014-01-1-0945, a Department of Defense Grant APN 07363, a SERCEB grant 5 U54 AI057157-02, and the UAB Comprehensive Cancer Center of USA.
References
- 1.Inglesby T.V. Anthrax as a biological weapon. Updated recommendations for management. In: Henderson D.A., editor. Bioterrorism: Guidelines for Medical and Public Health Management. American Medical Association Press; Chicago, USA: 2002. pp. 63–97. [Google Scholar]
- 2.Bell D.M. Conference summary: clinical issues in the prophylaxis, diagnosis, and treatment of anthrax. Emerg. Infect. Dis. 2002;8:222–225. doi: 10.3201/eid0802.01-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alibek K., Handelman S. Biohazard: The Chilling True Story of the Largest Covert Biological Weapons Program in the World. Random House; New York, USA: 1999. [Google Scholar]
- 4.Agrawal A., Pulendran B. Anthrax lethal toxin: a weapon of multisystem destruction. Cell. Mol. Life Sci. 2004;61:2859–2865. doi: 10.1007/s00018-004-4251-4. [DOI] [PubMed] [Google Scholar]
- 5.Welkos S. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology. 2001;147:1677–1685. doi: 10.1099/00221287-147-6-1677. [DOI] [PubMed] [Google Scholar]
- 6.Dixon T.C. Early Bacillus anthracis macrophage interactions: intracellular survival survival and escape. Cell Microbiol. 2000;2:453–463. doi: 10.1046/j.1462-5822.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaur R. Effect of nasal immunization with protective antigen of Bacillus anthracis on protective immune response against anthrax toxin. Vaccine. 2002;20:2836–2839. doi: 10.1016/s0264-410x(02)00207-4. [DOI] [PubMed] [Google Scholar]
- 8.Foster S.J., Johnstone K. Pulling the trigger: the mechanism of bacterial spore germination. Mol. Microbiol. 1990;4:137–141. doi: 10.1111/j.1365-2958.1990.tb02023.x. [DOI] [PubMed] [Google Scholar]
- 9.Wenner K.A., Kenner J.R. Anthrax. Dermatol. Clin. 2004;22:247–256. doi: 10.1016/j.det.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Jedrzejas M.J. Three-dimensional structure and molecular mechanism of novel enzymes of spore forming bacteria. Med. Sci. Monit. 2002;8:RA183–RA190. [PubMed] [Google Scholar]
- 11.Huang C.M. Identification of Bacillus anthracis proteins associated with germination and early outgrowth by proteomic profiling of anthrax spores. Proteomics. 2004;4:2653–2661. doi: 10.1002/pmic.200400831. [DOI] [PubMed] [Google Scholar]
- 12.Guidi-Rontani C. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol. 2002;10:405–409. doi: 10.1016/s0966-842x(02)02422-8. [DOI] [PubMed] [Google Scholar]
- 13.Inglesby T.V. Anthrax as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999;281:1735–1745. doi: 10.1001/jama.281.18.1735. [DOI] [PubMed] [Google Scholar]
- 14.Whiting G.C. Characterisation of adsorbed anthrax vaccine by two-dimensional gel electrophoresis. Vaccine. 2004;22:4245–4251. doi: 10.1016/j.vaccine.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y.H. Proteomic analysis of lipopolysaccharide-induced apoptosis in PC12 cells. Proteomics. 2002;2:1220–1228. doi: 10.1002/1615-9861(200209)2:9<1220::AID-PROT1220>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Sechi S. Mass spectrometric approaches to quantitative proteomics. Contrib. Nephrol. 2004;141:59–78. doi: 10.1159/000074590. [DOI] [PubMed] [Google Scholar]
- 17.Chitlaru T. Identification of chromosomally encoded membranal polypeptides of Bacillus anthracis by a proteomic analysis: prevalence of proteins containing S-layer homology domains. Proteomics. 2004;4:677–691. doi: 10.1002/pmic.200300575. [DOI] [PubMed] [Google Scholar]
- 18.Liu H. Formation and composition of the Bacillus anthracis endospore. J. Bacteriol. 2004;186:164–178. doi: 10.1128/JB.186.1.164-178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ariel N. Genome-based bioinformatic selection of chromosomal Bacillus anthracis putative vaccine candidates coupled with proteomic identification of surface-associated antigens. Infect Immun. 2003;71:4563–4579. doi: 10.1128/IAI.71.8.4563-4579.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai E.M. Proteomic analysis of the spore coats of Bacillus subtilis and Bacillus anthracis. J. Bacteriol. 2003;185:1443–1454. doi: 10.1128/JB.185.4.1443-1454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daubenspeck J.M. Novel oligosaccharide side chains of the collagen-like region of BclA, the major glycoprotein of the Bacillus anthracis exosporium. J. Biol. Chem. 2004;279:30945–30953. doi: 10.1074/jbc.M401613200. [DOI] [PubMed] [Google Scholar]
- 22.Warscheid B. MALDI analysis of Bacilli in spore mixtures by applying a quadrupole ion trap time-of-flight tandem mass spectrometer. Anal. Chem. 2003;75:5608–5617. doi: 10.1021/ac0344081. [DOI] [PubMed] [Google Scholar]
- 23.Lovgren A. Molecular characterization of immune inhibitor A, a secreted virulence protease from Bacillus thuringiensis. Mol. Microbiol. 1990;4:2137–2146. doi: 10.1111/j.1365-2958.1990.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 24.Dalhammar G., Steiner H. Characterization of inhibitor A, a protease from Bacillus thuringiensis which degrades attacins and cecropins, two classes of antibacterial proteins in insects. Eur. J. Biochem. 1984;139:247–252. doi: 10.1111/j.1432-1033.1984.tb08000.x. [DOI] [PubMed] [Google Scholar]
- 25.Read T.D. Comparative genome sequencing for discovery of novel polymorphisms in Bacillus anthracis. Science. 2002;296:2028–2033. doi: 10.1126/science.1071837. [DOI] [PubMed] [Google Scholar]
- 26.Edlund T. Evidence for two immune inhibitors from Bacillus thuringiensis interfering with the humoral defense system of saturniid pupae. Infect Immun. 1976;14:934–941. doi: 10.1128/iai.14.4.934-941.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandvalet C. Identification of genes involved in the activation of the Bacillus thuringiensis inhA metalloprotease gene at the onset of sporulation. Microbiology. 2001;147:1805–1813. doi: 10.1099/00221287-147-7-1805. [DOI] [PubMed] [Google Scholar]
- 28.Charlton S. Characterization of the exosporium of Bacillus cereus. J. Appl. Microbiol. 1999;87:241–245. doi: 10.1046/j.1365-2672.1999.00878.x. [DOI] [PubMed] [Google Scholar]
- 29.Pannucci J. Bacillus anthracis pXO1 plasmid sequence conservation among closely related bacterial species. J. Bacteriol. 2002;184:134–141. doi: 10.1128/JB.184.1.134-141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosman J., Setlow P. Effects of carboxy terminal modifications and pH on binding of a Bacillus subtilis small, acid-soluble spore protein to DNA. J. Bacteriol. 2003;185:6095–6103. doi: 10.1128/JB.185.20.6095-6103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J.K. The effect of acid shock on sporulating Bacillus subtilis cells. J. Appl. Microbiol. 2003;94:184–190. doi: 10.1046/j.1365-2672.2003.01816.x. [DOI] [PubMed] [Google Scholar]
- 32.Redmond C. Identification of proteins in the exosporium of Bacillus anthracis. Microbiology. 2004;150:355–363. doi: 10.1099/mic.0.26681-0. [DOI] [PubMed] [Google Scholar]
- 33.Kanda-Nambu K. Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis. Amino Acids. 2000;18:375–387. doi: 10.1007/s007260070076. [DOI] [PubMed] [Google Scholar]
- 34.Ireland J.A., Hanna P.C. Amino acid- and purine ribonucleoside-induced germination of Bacillus anthracis DeltaSterne endospores: gerS mediates responses to aromatic ring structures. J. Bacteriol. 2002;184:1296–1303. doi: 10.1128/JB.184.5.1296-1303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]