Abstract
Background:
KYBELLA, ATX-101, is an injectable form of sodium deoxycholic acid. It is currently the only Food and Drug Administration–approved injectable drug for the reduction of submental fat.
Objectives:
A literature review and discussion of the treatment of submental fat.
Results:
KYBELLA is a well-tolerated alternative for the treatment of submental fat.
Conclusions:
KYBELLA is a safe and efficacious, first in class, injectable drug for the reduction of submental fat.
Submental fat represents an aesthetic problem in both the younger and older male and female patients. It can occur regardless of body mass. Although localized fat deposits are in many parts of the body, because of the location, submental fat is particularly noticeable.1 Until now, treatments of submental fat have been limited to invasive, surgical procedures such as liposuction or fat excision and even complete neck reconstruction. Because surgery is associated with the risks of anesthesia, infection, bleeding, bruising, and scarring, as well as the possibility of poor outcome, discomfort, and the prolonged “downtime” for the patient, there is a large demand for nonsurgical alternatives.2
There has been significant growth in nonsurgical aesthetic procedures in the area of fat reduction.1–5 Physicians and patients are increasingly seeking out noninvasive procedures as rejuvenation options in general. The submental area represents an ideal anatomical area for an injectable treatment for fat reduction. Observations had been made that sodium deoxycholate injection can be used to dissolve fat in other locations of the face and body with attendant tissue tightening.1,2,5–9
KYBELLA (sodium deoxycholate), ATX-101, an adipolytic medication, represents the only Food and Drug Administration–approved, nonsurgical treatment for unwanted submental fat deposition.1 When injected into subcutaneous fat, KYBELLA causes focal adipolysis. After the cell destruction, a macrophage infiltrate has been observed, which serves a 2-fold purpose. First, the macrophages clear the cell membrane fragments and free lipids, and second, through fibroblast recruitment, neocollagenesis is stimulated.2,10 Once destroyed, the adipocytes cannot store or accumulate fat, resulting in an improvement in the appearance of the submental area through both reduction of localized subcutaneous fat deposits in patients along with tissue tightening.
BACKGROUND AND MECHANISM OF ACTION
Physiologically, deoxycholic acid (DC) is an auxiliary bile acid produced by intestinal bacteria after primary bile acids are discharged from the liver.2 Chemically, DC is a detergent that unsettles the integrity of the biological membrane and eventually breaks it down. In high doses, it has been observed that injection of DC leads to solubilization and cell eradication.2,11 Subcutaneous injection of DC causes necrosis of the tissue as a result of its cyotoxic, detergent effects on the cellular layer.10
CLINICAL DATA
In the Refine 1 ATX-101 trial, a randomized, double blind, placebo controlled study, 500 patients were injected with either saline or DC. Results were evaluated both by a blinded evaluator and patient self-assessment.1 A smaller cohort was evaluated by magnetic resonance imaging. Injections of DC (2 mg/cm2) were administered to the submental fat present in the preplatysmal plane using a 30-gauge needle. The submental area was assessed using a validated scale designed by Kythera for the trial, and patients assessed by the clinician as 2 or 3 on the submental fullness scale were considered for inclusion in the trial. Patients also had to demonstrate the absence of significant tissue laxity, be between the ages of 18 and 65, have a stable body weight, and have a body mass index less than or equal to 40 kg/m2. Submental fat was measured by using a caliper. The patients also had to be dissatisfied with the appearance of their submental area as assessed by using a separate scale and not have had any recent radiofrequency treatment, toxin injection, or surgery in the area.1 Treatments were administered in 0.2 mL doses spaced 1 cm apart using a grid once every 4 weeks over the course of 24 weeks, depending on patient response. A patient could receive less than or equal to 100 mg per treatment. Data showed that approximately 80% of patients demonstrated greater than 1 grade improvement in submental fat in approximately 12 weeks after their last treatment,10 which persisted 24 weeks after treatment. In this trial, 70% ATX-101 and 18.6% placebo had more than 2 grade responder rates. The side effect profile was mostly limited to the customary side effects seen with injections, bruising, swelling, and anesthesia, lasting on average 4 days. Of special interest was temporary mandibular nerve paresis that occurred in 4.3% of ATX-101–treated subjects. This adverse event had a median duration of 31 days and resolved without sequelae.1 At the end of the trial, 82.4% of patients reported significantly improved satisfaction with their appearance.2
In the 20 clinical trials performed worldwide, 68.2% of subjects treated with KYBELLA were responders compared with 20.5% of placebo-treated subjects based on validated physician and patient measurements. Seventy-nine percent of patients treated with KYBELLA reported satisfaction with their appearance in association with their face and chin. The AE profile was very similar to the Refine 1 trial.1,2,12
CLINICIAN INJECTION GUIDELINES
Patient Selection
First and foremost, to insure the success of the treatment, it is imperative to select an ideal candidate. This is true for all cosmetic procedures of course but particularly important for this procedure. As an investigator in 2 ATX-101 trials, ATX-101-11-26 and ATX-101-11-23, one open label and the second double blind, investigators learned that patients responded both with a large amount of submandibular fat and a moderate amount of fat.3,7,13 Although a significant amount of tissue laxity eliminated patient candidates, patients with moderate tissue laxity responded with significant tissue tightening in a number of cases. Our site treated both men and women and patients of every ethnicity and skin color.
Patients need to be advised that multiple treatments will be required and that they will experience a significant amount of swelling with the first treatment especially. Final results require patience as the swelling takes up to a week to resolve, and the tissue tightening takes place over months. Swelling in subsequent treatments is dependent of the amount of fat remaining from the previous treatment. Obviously, having a stable body weight and good skin elasticity adds to the overall success of the procedure. As seen in Figures 1 and 2, the results of ATX-101 are most effective in patients with moderate submental fat and a low level of skin laxity.
Fig. 1.
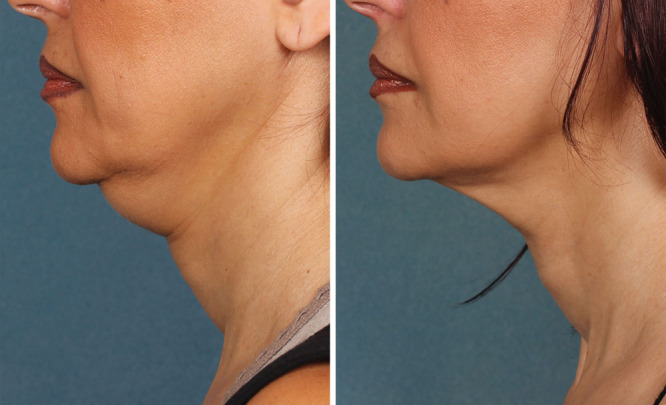
Site results of 4 consecutive treatments consisting of 4 mL of KYBELLA once every 30 days. Before treatment (A) and after treatment (B). Photograph is used with patient consent.
Fig. 2.
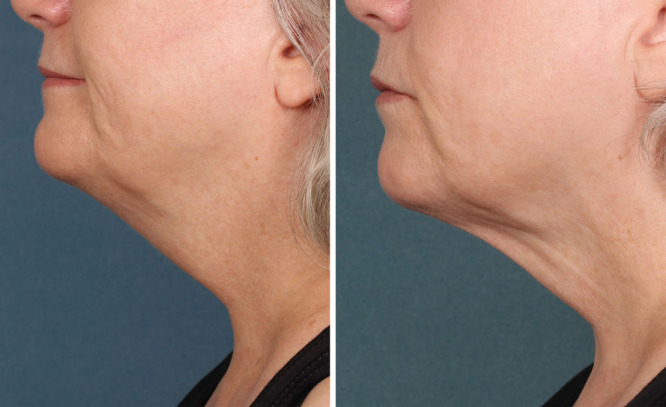
Site results of 6 KYBELLA treatments, 4 mL treatments spaced 30 days apart. Before treatment (A) and after treatment (B). Photograph is used with patient consent.
Ideal Parameters of the Submental Profile
The ideal submental profile is characterized by a visible mandibular border, a subhyoid indentation, a noticeable thyroid cartilage lump, a visible anterior sternocleidomastoid, a sternocleidomastoid-submental angle of 90°, and a cervicomental angle of about 105° to 120°. In comparison, submental fullness is characterized by a rounded sternocleidomastoid-submental angle and an indistinct inferior mandibular border.2,13,14 A series of injections with KYBELLA has proven to be an efficacious, nonsurgical way to rebuild the characteristics of the ideal submental profile.
In Figure 3, we see the key anatomic structures in the cervicomental region. These are also the general boundaries for KYBELLA injections. These key structures and landmarks include the inferior border of the mandible, the digastric muscle, the hyoid bone (at the bend in the cervicomental angle), the thyroid notch (a nontreatment area), the anterior border of the sternocleidomastoid muscle, and the thyroid gland.9 It is helpful to sketch the borders of these structures with some type of a marking pen before planning your injections.
Fig. 3.
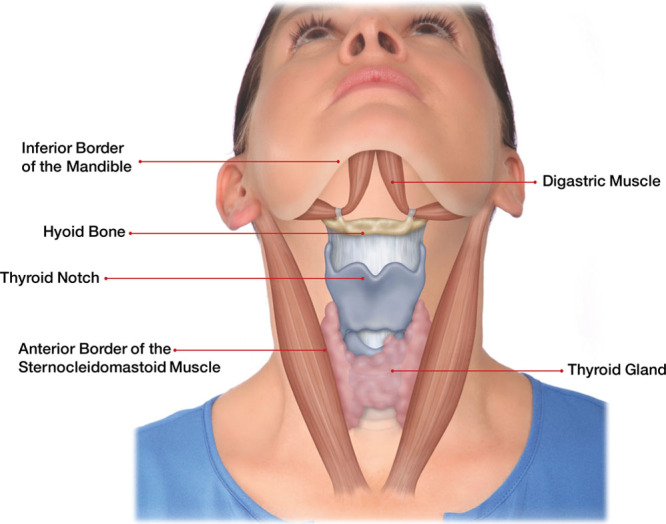
Landmarks for injections with KYBELLA. Reprinted with permission from Allergan plc.
The platysma originates from the fascia overlying the pectoralis and deltoid muscles and inserts in multiple points above the angle of the mandible. It is to be noted that the postplatysmal fat, as seen in Figure 4, is not the target tissue for KYBELLA injections.
Fig. 4.
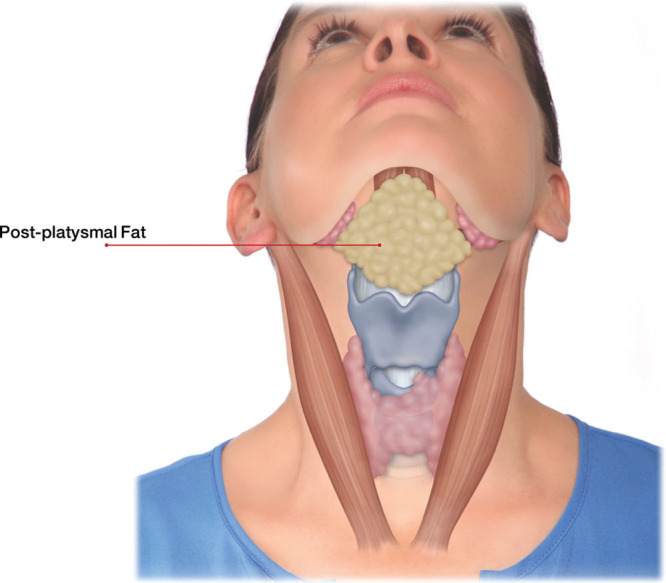
Postplatysmal fat. Reprinted with permission from Allergan plc.
Figure 5 shows the anterior view of the preplatysmal fat. This is the target tissue for KYBELLA injection treatment. Only preplatysmal fat in the submental area should be treated with KYBELLA. To evaluate a patient’s submental fat, first, pinch and palpate the submental area to ensure that there is sufficient submental fat. Assess skin laxity, and lastly, ask the patient to tense the platysmal muscles or “grimace.”2,3,9 This allows you to assess the subcutaneous fat between the dermis and platysma within the target treatment area.
Fig. 5.
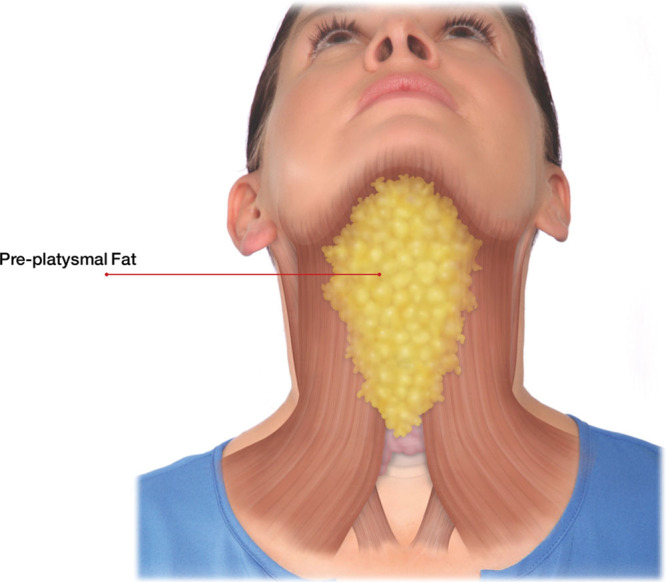
Anterior view of the preplatysmal fat. Reprinted with permission from Allergan plc.
To avoid nerve injury, it is important to review the location of the marginal mandibular nerve relative to the arteries and veins in the area. Posterior to the facial artery, the marginal mandibular nerve (shown in yellow in Fig. 6) runs along the inferior border of the mandible. Relative to the mandibular border, the marginal mandibular nerve is about 1 to 2 cm below it in most cases. But it has been described in the literature up to 4 cm below it in some cases. Anterior to the facial artery, the marginal mandibular nerve passes above the mandibular border.15
Fig. 6.
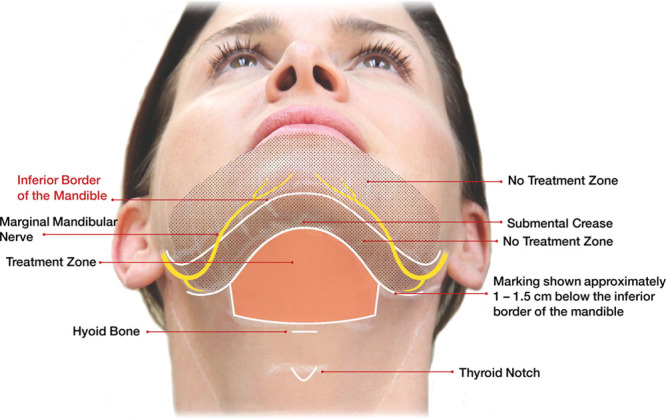
Treatment area. Reprinted with permission from Allergan plc.
To properly inject KYBELLA, it is advised to pinch the preplatysmal fat between 2 fingers to isolate it from the underlying structures and then inject KYBELLA perpendicular to the skin until the needle is midway into the underlying preplatysmal subcutaneous fat layer. Confirm that there is no resistance felt after passing through the dermis to avoid structures such as the platysma, glands, etc. If resistance is sensed, withdraw the needle and begin again. Inject 0.2 mL of KYBELLA next to each grid marking (grids are provided to physicians to assist with injections) within the treatment area.9 Do not inject KYBELLA 1 to 1.5 cm below the inferior mandibular border (from the angle of the mandible to the mentum), as seen in Figure 6; this is considered the “no treatment zone.” It is extremely helpful to have a staff member at your side to assist with the syringes, so that the injection points are addressed in a stepwise fashion and ice or a Zimmer flow can be done simultaneously to prevent or reduce patient discomfort. Immediately post treatment, ice or a Zimmer chiller is applied for approximately 15 minutes. Occasionally, a patient will request a neck garment to be applied as they leave the office. If not, patients are advised as post care to apply ice for the next hour and sleep with their head elevated for the next several nights. The most common treatment reactions are pain, swelling, bruising, and numbness. Pain can be addressed with 800 mg ibuprofen unless medically contraindicated, swelling with ice and elevation, and a few days of rest accelerates the recovery too.
SUMMARY
KYBELLA, ATX-101, has been shown to be extremely effective as a solitary noninvasive injectable treatment at objectively reducing submental fat through a series of injections with minimal discomfort and side effects. Tissue tightening has been observed in the submental area after a series of KYBELLA injections. Most patients describe a subjective improvement in the size of their submental fat and satisfaction with the aesthetic appearance of their neck.
CONCLUSIONS
KYBELLA, ATX-101, is an exciting addition to our noninvasive tool box in the area of focal fat reduction. It is the only Food and Drug Administration–approved nonsurgical treatment indicated for the submental area. The only other alternatives are invasive surgical procedures, which in general have a different risk and benefit profile than ATX-101.
There is a dramatic upswing in the numbers of people seeking cosmetic medical procedures supporting the direct connection between appearance and improved self-esteem.12
Whether or not Kybella supplants liposuction in the future as the noninvasive treatment of choice for submental fat, it represents a definite advance in our ability to treat a troubling aesthetic issue for a large number of people.
Footnotes
Presented at the The Cosmetic Bootcamp meeting, Aspen, Colo., July 8-11, 2016.
Disclosure: The author has no financial interest to declare in relation to the content of this article. The Article Processing Charge for this proceeding was paid for by Allergan plc, as part of an unrestricted educational grant to support the entire Cosmetic Boot Camp 2016 Supplement. Allergan plc had no involvement in the production, selection, or review of this proceeding supplement.
Cosmetic Bootcamp: PRS Global Open proudly publishes the proceedings from The Cosmetic Bootcamp July 2016 meeting that was held in the St Regis Resort in Aspen, Colorado on July 8-11th, 2016.
REFERENCES
- 1.Jones DH, Carruthers J, Joseph JH, et al. REFINE-1, a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial with ATX-101, an injectable drug for submental fat reduction. Dermatol Surg. 2016;42:38–49. doi: 10.1097/DSS.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 2.Duncan D, Rotunda AM. Injectable therapies for localized fat loss: state of the art, clinics in plastic surgery. Clin Plast Surg. 2011;38:489–501. doi: 10.1016/j.cps.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Beer K, Donofrio L, Gross TM, et al. Clinically meaningful reduction in submental fat during and after treatment with ATX-101 in the US/CAN phase 3 trials, REFINE-1, and REFINE-2. Presented at 73rd Annual Meeting of the American Academy of Dermatology; March 20–24, 2015; San Francisco, CA. [Google Scholar]
- 4.Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26(1S):S4–S9. doi: 10.1016/j.asj.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Ascher B, Hoffmann K, Walker P, et al. Efficacy, patient-reported outcomes and safety profile of ATX-101 (deoxycholic acid), an injectable drug for the reduction of unwanted submental fat: results from a phase III, randomized, placebo-controlled study. J Eur Acad Dermatol Venereol. 2014;28:1707–1715. doi: 10.1111/jdv.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rzany B, Griffiths T, Walker P, et al. Reduction of unwanted submental fat with ATX-101 (deoxycholic acid), an adipocytolytic injectable treatment: results from a phase III, randomized, placebo-controlled study. Br J Dermatol. 2014;170:445–453. doi: 10.1111/bjd.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann K, Rubin MG, Goodman G, et al. Reductions in submental fat achieved with ATX-101 (deoxycholic acid) are maintained over time. Presented at 13th Aesthetic & Anti-Aging Medicine World Congress; March 26–28, 2015; Monte Carlo, Monaco. [Google Scholar]
- 8.Wollina U, Goldman A. ATX-101 for reduction of submental fat. Expert Opin Pharmacother. 2015;16:755–762. doi: 10.1517/14656566.2015.1019465. [DOI] [PubMed] [Google Scholar]
- 9.Kythera Biopharmaceuticals, Inc.: KYBELLA [Package Insert] Agoura Hills, CA: Kythera Biopharmaceuticals, Inc.; 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206333Orig1s0008)lbl.pdf. Accessed March 15, 2016. [Google Scholar]
- 10.Saedi N, Rotunda AM, Dover JS. Injectable fat-reducing therapies: fat reduction. In: Orringer JS, Murad A, Dover JS, editors. Body Shaping, Skin Fat and Cellulite: Procedures in Cosmetic Dermatology Series. Vol. 91 2014. [Google Scholar]
- 11.Walker P, Fellmann J, Lizzul PF. A phase I safety and pharmacokinetic study of ATX-101: injectable, synthetic deoxycholic acid for submental contouring. J Drugs Dermatol. 2015;14:279–287. [PubMed] [Google Scholar]
- 12.Schlessinger J, Weiss SR, Jewell M, et al. Perceptions and practices in submental fat treatment: a survey of physicians and patients. Skinmed. 2013;11:27–31. [PubMed] [Google Scholar]
- 13.Farrior E, Eisler L, Wright HV. Techniques for rejuvenation of the neck platysma. Facial Plast Surg Clin North Am. 2014;22:243–252. doi: 10.1016/j.fsc.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Rohrich RJ, Rios JL, Smith PD, et al. Neck rejuvenation revisited. Plast Reconstr Surg. 2006;118:1251–1263. doi: 10.1097/01.prs.0000209406.80690.9f. [DOI] [PubMed] [Google Scholar]
- 15.Batra AP, Mahajan A, Gupta K. Marginal mandibular branch of the facial nerve: an anatomical study. Indian J Plast Surg. 2010;43:60–64. doi: 10.4103/0970-0358.63968. [DOI] [PMC free article] [PubMed] [Google Scholar]


