ABSTRACT
This study investigates the preparation of ligustrazine hydrochloride carboxymethyl chitosan and collagen microspheres. This experiment investigates effects of the ratio of carboxymethyl chitosan and collagen blend, water to oil ratio, stirring speed, and other factors on the microsphere properties. The experiment had the following conditions: a 1:2 proportion of carboxymethyl chitosan and collagen, a 1:2 proportion of drugs and materials, a 5:1 proportion of oil phase and water phase, 0.5% of span80, a 600r/min stirring speed, 3 ml of a cross-linking agent, 3 h of cross-linking curing, 1.25 ± 0.05 mm diameter LTH microcapsules, a 54.08% envelop rate, and a 14.16% carrier rate. The microspheres release rate reached 66% within 1 h, then steadily released within 5 h in vitro. The experimental results showed that the ligustrazine hydrochloride microsphere production process was stable and exhibited a good release effect compared with other ligustrazine hydrochloride tablets and pills.
KEYWORDS: carboxymethyl chitosan, collagen, ligustrazine hydrochloride, micro spheres
Introduction
Chitosan (CS) is a natural polymer extracted from crustacean shells. Recent studies have found that Chitosan has many excellent properties: it is compatibile with biological tissues, it is non-irritatable, it is non-toxic, it has non-immunogenic, non-mutagenic, biodegradable, and biocompatible properties, and it has a non-pyrogenic response.1 Consequently, Chitosan is a very promising drug carrier material. Experimental studies have concluded that Chitosan dissolves in acid and is insoluble in neutral and alkaline mediums, which are the defects of Chitosan, resulting in the application in medicine is limited. Carboxymethyl chitosan is an important derivative of CS, it retains all the advantages of CS, Water-soluble greatly improved compared with CS. Carboxymethyl chitosan is an amphoteric polymer electrolyte because the molecule contains a large number of amino and carboxyl groups, which makes it an excellent pharmaceutical carrier material; it shows great potential for the development of new pharmaceutical excipients.
The toxicity and biocompatibility in vivo degradation of Collagen are significantly superior to those of gelatin. Collagen composes the main structural proteins of the extracellular matrix in humans and vertebrates. It is composed of connective tissue, skin tissue, tendons, and bones. Collagen is constituted by a peptide bond with a triple helix arrangement structure.2 Because of its unique structure, Collagen has many useful features, such as high tensile strength, low antigenic activity, biodegradability, low cytotoxicity, low irritation, and it promotes cell growth.3 Thus, Collagen is an excellent biomedical material and drug carrier.
Tetramethylpyrazine (TCM) drugs have been widely used in the pharmaceutical industry. Studies have shown that tetramethylpyrazine has anti-platelet aggregation, expands small arteries, clears microcirculation, and has other effects. It has great potential in treating cardiovascular and cerebrovascular diseases. As they are currently marketed, Tetramethylpyrazine injections have many disadvantages; they are inconvenient to use, painful, can cause allergic reactions, and rely on microspheres as a drug carrier. In contrast, oral or intravenous administration, such as a polymer carrier system, can mask the pungent odor of drugs, increase drug efficiency, and prolong the drug release dosage; therefore, it has obvious advantages compared with general pharmaceutical preparations. This paper mainly studies the preparation of Ligustrazine hydrochloride carboxymethyl chitosan and collagen microspheres.
Experiment
Materials and methods
Carboxymethyl Chitosan and Collagen were obtained from the Beijing Wellcome Source Biotechnology Co., Ltd.; the Ligustrazine hydrochloride reference substance was obtained from the National Institutes for Food and Drug Control, Ligustrazine materials, Beijing Sunshine lied Ltd.; liquid paraffin was obtained from the West Long Chemical Co., Ltd.; span80 was obtained from the Tianjin Guangfu Fine Chemical Research Institute; glutaraldehyde was obtained from the Shantou West Long Chemical Co., Ltd.; soybean oil was obtained from China Oils & Foodstuffs Co., Ltd..
The SEM was obtained from Dutch FEI Company; the NEXUS-470 Fourier transform infrared spectrometer was obtained from Nicolet, USA; the ZRS-8G Intelligent Dissolution Tester was obtained from Tianjin University Radio Factory; Waters e2695 Venu sil (XBPC18, 4.6 × 250 mm).
Blank microspheres
The blank carboxymethyl chitosan-collagen microspheres were prepared by emulsion–chemical crosslinking. The sodium carboxymethyl chitosan and collagen solution made by a certain ratio as the dispersed phase, soybean oil is a dispersion medium, oil phase: aqueous phase = 5:1, span-80(1%V/V) is an emulsifier, pre-emulsified oil phase at 40°C 20 min, then the aqueous phase is slowly dropped to the oil phase, emulsion is stirred for some time at a constant speed, with ice bath cooling, add 3 ml glutaraldehyde, the reaction of crosslinking need 3 h, then discard the upper oil phase, filter, and then the oil attached to the microspheres surface is absorbed by filter paper, the microspheres are dried in a vacuum oven for a while which can be used as blank microspheres.
Preparation of ligustrazine hydrochloride carboxymethyl chitosan and collagen microspheres
The preparation method was the same as that of the blank microcapsules, the Ligustrazine to microencapsulated material ratio was 1:2, the carboxymethyl chitosan to collagen ratio was 1:2; the volume ratio of oil phase to water phase was 5:1, the span-80 dosage was 0.5%; the stirring speed was 600 r/min.
Analysis method
Microsphere drug loading capability and encapsulation efficiency were determined by HPLC. HPLC analysis was performed using a Waters e2695liquid chromatographic (Waters company, USA) HPLC system. Venu sil (XBPC18, 4.6 × 250 mm) was used to separate the samples. The detector was set to 295 nm and the injection volume was 10 µl. A 0.1% solution that had a phosphoric acid to methanol to acetonitrile ratio of 60:35:5 was adopted as the mobile phase. The flow rate was 1.0 ml/min. All HPLC was performed at (25 ± 1) °C. The Ligustrazine hydrochloride retention time was 5.63 min. The working calibration curve based on Ligustrazine hydrochloride standard solutions showed good linearity over the range of 10∼320 μ2ymL−1. The regression line was Y = 44827X-131289 (R = 0.9992, n = 6), where Y is the peak area of Ligustrazine hydrochloride, and X is the Ligustrazine hydrochloride concentration (μonmL−1).
The envelop rate and the carrier rate
1 g of dried drug-loaded microspheres were weighed. They were fully milled, transferred to a 50 ml volumetric flask and diluted to volume with 90% methanol, and homogenized with a homogenizer. They went through a vortex for 5 min, left out to stand for 3 h, and then followed by another 5 min in the vortex again. 10 ml of the liquid was moved to the centrifuge tube, centrifuged, and then the supernatant was filtered by a 0.8 μm membrane before HPLC analysis.
Characterization methods
Microspheres form
Microsphere morphology was observed by a scanning electron microscope: S4800 Field-Emission Scanning Electron Microscope: 15.0 KV 13.8 mm × 30.0 SE(M), 15.0 KV 13.8 mm × 700 SE(M), 15.0 KV 13.5 mm × 30.0 SE(M).
IR spectrums of drug-loaded microspheres
A certain proportion of the microspheres and potassium bromide was dried and tableted before they were analyzed by infrared spectroscopy.
Determining microsphere swelling properties
The swelling degree (SD) of the drug-loaded microspheres were determined using the gravimetric method:4 at room temperature, the dried microspheres were placed in buffer solutions of different pH levels (1, 3.6, 5, 6.8, 9) and then removed after a certain time. Filter paper was then used to absorb the water attached to the microsphere surfaces. The microspheres were weighed; they reached a swelling equilibrium within 24 h. Where SD = (Wt–W0)/W0, W0 is the dry microsphere mass before swelling (g), and Wt is the dry microsphere mass at t moment (g). The different pH buffers were prepared according to literature.5
In vitro release experiments
According to Chinese Pharmacopoeia, the gastrointestinal tract has a pH range of about 1∼7.8. This paper used an artificial gastric juice (0.l mol·L−1HCI, pH = 1.0), artificial intestinal fluid (pH = 6.8 phosphate), pH = 4.5 buffer solution (prepared in accordance with Pharmacopoeia 2005 edition), and distilled water as the dissolution medium.
600 ml of the dissolution medium was degassed and then heated to 37°C ± 0.5°C by a water bath in accordance with the 2005 edition of the Pharmacopoeia mug slurry method. The distance between the bottom of the rotary paddle and the bottom of the dissolution cup was adjusted to be 25 mm ± 2 mm. It had a 50 r/min revolution speed. 0.5 g of the dried drug-loaded microspheres were put in the dissolution cup, and 5 mL samples were taken at 5, 15, 30, 60, 120, 180, 240, 300, 360 min. At the same time, complementing the dissolution medium 5 mL, the sample was filtered by a 0.8 μm membrane, analysis by the HPLC system. When the pH levels of the dissolution medium reached 1, 4.5, and 6.8, the dissolution curve was analyzed in the same manner.
Results and discussion
Blank microspheres preparation
On the basis of the single factor, 4 factors: the ratio of carboxymethyl chitosan and collagen(A), the volume ratio of oil phase and water phase (B), the dosage of span-80 (C), stirring speed (D) are investigated, select the 3 levels of each factor, according to L9(34) orthogonal table design experiments, determine the process parameters. Table 1 shows the factor level arrangements.
Table 1.
The 3 levels and 4 factors of the experimental design.
| Level | Mcarboxymethyl chitosan :Mcollagen (A) | Voil :Vwater (B) | Span-80 dosage (C) % | Stirring speed (D) r/min |
|---|---|---|---|---|
| 1 | 1:1 | 7:1 | 0.5 | 500 |
| 2 | 1:2 | 6:1 | 1 | 600 |
| 3 | 1:3 | 5:1 | 1.5 | 700 |
Microsphere morphology was observed by an optical microscope. The evaluation index included microspheres roundness, particle size uniformity, adhesion degree, and liquidity. Each metric was weighed on a 25 point scale for a total of 100 potential points. However, this assessment is subjective and arbitrary by nature; therefore, in an effort to reduce error, we selected 5 laboratory workers to score and calculate the average. Preparation of blank microsphere, Table 2 shows the results.
Table 2.
Results and calculations of L9(3)4.
| Factor |
|||||
|---|---|---|---|---|---|
| Test |
A |
B |
C |
D |
Composite score |
| 1 | 1 | 1 | 1 | 1 | 74.6 |
| 2 | 1 | 2 | 2 | 2 | 81.0 |
| 3 | 1 | 3 | 3 | 3 | 82.6 |
| 4 | 2 | 1 | 2 | 3 | 87.0 |
| 5 | 2 | 2 | 3 | 1 | 81.0 |
| 6 | 2 | 3 | 1 | 2 | 91.5 |
| 7 | 3 | 1 | 3 | 2 | 86.8 |
| 8 | 3 | 2 | 1 | 3 | 85.4 |
| 9 | 3 | 3 | 2 | 1 | 75.2 |
| k1 | 238.2 | 248.4 | 251.5 | 230.8 | |
| k2 | 259.5 | 247.4 | 243.2 | 259.3 | |
| k3 | 247.4 | 249.3 | 250.4 | 255.0 | |
| R | 21.3 | 1.9 | 8.3 | 28.5 | |
R analysis shows that in comparison with the influence of 4 different factors to prepare blank microspheres, in a descending order D>A>C>B, the optimum composition is A2B3C1D2, the ratio of carboxymethyl chitosan and collagen is 1:2, the volume ratio of oil phase and water phase is 5:1, the dosage of span-80 is 0.5%, stirring speed is 600 r/min.
Preparation of Ligustrazine hydrochloride carboxymethyl chitosan and collagen microspheres (LTH-CMC-COL)
The ligustrazine hydrochloride, sodium carboxymethyl chitosan, and collagen solution was made by a certain ratio as the aqueous phase in Table 3. Soybean oil was used as a dispersion medium. The oil phase to aqueous phase ratio was 5:1; span-80 (1%V/V) was used as an emulsifier; the pre-emulsified oil phase was 40°C for 20 min. Then, the aqueous phase slowly dropped to the oil phase. The emulsion was stirred for some time at a constant speed and cooled by an ice bath. 3 ml of glutaraldehyde was then added. It was left for 3 hours to allow time for the crosslinking reaction. Then, the upper oil phase was discarded, it was filtered, and the the oil attached to the microsphere surface was absorbed by filter paper. The microspheres were then dried in a vacuum oven.
Table 3.
Properties of drug-loaded microspheres.
| Drugs : Capsule materials (m : m) | Microsphere yield(%) | Envelop rate (%) | Carrier rate (%) |
|---|---|---|---|
| 1:3 | 90.67 | 61.75 | 10.78 |
| 1:2 | 86.20 | 53.43 | 14.24 |
| 1:1 | 75.82 | 40.85 | 15.06 |
The microsphere yield (%) = microspheres weight / (dosage + the content of carboxymethyl chitosan and collagen) ×100%.
The envelop rate (%) = the amount of drug contained in the microspheres / the total dosing × 100%.
The carrier rate (%) = the amount of the drug contained in the microspheres / the microspheres weight × 100%
Considering the microsphere yield, envelop rate, and carrier rate, the Ligustrazine to microencapsulated material ratio should be 1:2.
In accordance with optimal preparation, 3 batches of ligustrazine carboxymethyl chitosan-collagen microspheres were continuously made to investigate process stability optimization. Table 4 shows the test data.
Table 4.
Confirmatory test.
| Test | Microsphere yield (%) | Envelop rate (%) | Carrier rate (%) |
|---|---|---|---|
| 1 | 86.12 | 54.28 | 14.15 |
| 2 | 86.28 | 53.95 | 14.00 |
| 3 | 85.86 | 54.02 | 14.33 |
| average value | 86.09 | 54.08 | 14.16 |
We can see from Table 4 that according to the process parameters, the Ligustrazine to microencapsulated material ratio should be 1:2, the carboxymethyl chitosan to collagen ratio was 1:2, the oil phase volume to water phase volume ratio was 5:1, the span-80 dosage was 0.5%, the stirring speed was 600 r/min, the microsphere yield was 86.09%, the envelop rate reached 54.08%, the carrier rate reached 14.16%, and the preparation was very stable. The microsphere samples were dried, and their morphology was observed by scanning electron microscope (see Fig. 1).
Figure 1.
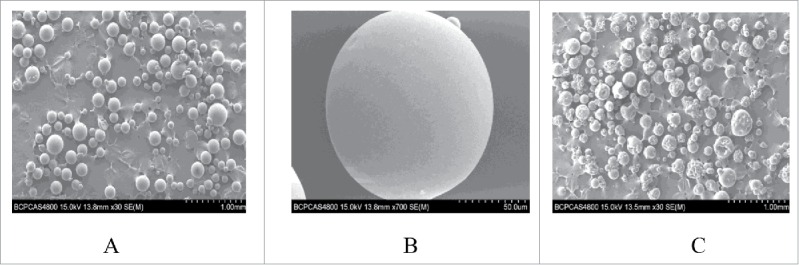
LTH-CMC-COL (A); LTH-CMC-COL enlarged view (B) After 6 months LTH-CMC-COL (C).
Figure 1 shows a different magnification electron microscope view of the microspheres. We can see that the LTH-CMC-COL microspheres made by the emulsion-crosslinking method were more structured in form and had a smooth outer surface. After 6 months, the morphology of these microspheres changed slightly, but the overall shape was still round and uniform.
The prepared microspheres and dried KBr were mixed in a certain proportion. The samples were analyzed by infrared spectroscopy, as shown in Fig. 2.
Figure 2.
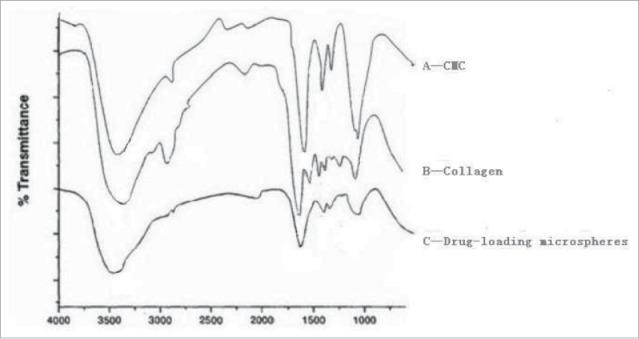
IR spectrums of carboxymethyl chitosan, collagen, and drug-loaded microspheres.
In the IR spectrum of carboxymethyl chitosan (A), 3420 cm−1 absorption peak is the stretching vibration of amino group and hydroxyl group absorption peaks, 1603 cm−1 absorption peak is carboxyl asymmetric stretching vibration absorption peak, 1417 cm−1 absorption peak is carboxyl symmetric stretching vibration absorption peak, In IR spectrum a of collagen(B), 1655 cm−1 absorption peak is characteristic absorption peak of the amide I, 1545 cm−1 absorption peak is characteristic absorption peak of the amide II, 1354 cm−1 absorption peak is characteristic absorption peak of the amide III. 3412 cm−1 absorption peak is amino and hydroxyl stretching vibration absorption peak.6 Comparing the infrared light spectrum of the carboxymethyl chitosan, collagen, and drug-loaded microspheres, we can see that the absorption peaks of the -NH2 and -OH stretching vibration in carboxymethyl chitosan and collagen molecules moved to 3399 cm−1. This indicates that carboxymethyl chitosan and collagen have relatively strong intermolecular hydrogen bond interactions. Compared with the IR spectrums of carboxymethyl chitosan and drug-loaded microspheres, we can see that in the IR spectrum of collagen (C) 1641 cm−1 had a new characteristic absorption peak, which was a -C = N stretching vibration absorption peak. This indicates that a Schiff base generated through carboxymethyl chitosan and glutaraldehyde crosslinking reaction. In addition, the 1603 cm−1 absorption peak was a carboxyl asymmetric stretching vibration absorption peak in the IR spectrum of carboxymethyl chitosan (A), are moved to 1619 cm−1 in drug-loaded microspheres IR spectrum, carboxyl symmetric stretching vibration absorption peak from 1417 cm−1 moved to 1412 cm−1, this further demonstrates between carboxymethyl chitosan and collagen exist the relatively strong intermolecular forces.
Determining microsphere swelling properties
A microsphere swelling property test was conducted to determine the pH value of the solvent's effect on microspheres. Figure 3 shows the test data, which provides a reference for future pharmaceutical preparations.
Figure 3.

Drug-loaded microsphere swelling curve.
The structure of carboxymethyl chitosan-collagen contains a large number of aminos and carboxyls; these groups may be ionized. The dissociation degree of aminos and carboxyls will change with the solvent pH; thus, the inside and outside ionic strength of the microspheres will change as the pH changes. According to Daonan balance,5 microsphere osmotic pressure will change as the pH changes, which causes the microspheres to swell or shrink. Figure 3 shows the swelling curve. The swelling process of carboxymethyl chitosan-collagen blend microspheres is the performance of a typical pH-sensitive response behavior: the microsphere swelling degree is the minimum between pH = 3–4; the microspheres swelling degree is the maximum of about pH = 9. The reason for this may be that the pH between 3–4 is near the isoelectric point of the microspheres, which causes the dissociation of the carboxyls and aminos to achieve a balance. Thus, the number of positive and negative charges are equal, the free ion concentration drops to the lowest point, and the microspheres osmotic pressure also drops to the lowest point. This causes the minimum degree of microsphere swelling; deviation from isoelectric point, the swelling degree of microspheres increases also.
In vitro release experiments
The United States FDA recommends that an in vivo drug dissolution medium should be composed of the following: 1 pH artificial gastric juice, 6.8 pH artificial intestinal fluid, 4.5 pH buffer solution, and distilled water. This solution will enable the investigation of microsphere properties in vitro release.
Diffusion and dissolution are 2 kinds of microsphere major release mechanisms. In the pre-release stage, the release mechanism is generally diffusion; during this period, the release curve is steep and fast. In the late release stage, which generally features dissolution, the release curve is less steep. As can be seen from Fig. 4, in the artificial gastric juice and intestinal media release curve, the sudden release phenomenon was more pronounced when the 1 h dissolution rate reached 66%. The main reason may be because ligustrazine is a water-soluble drug. In the release medium, microspheres swell, then ligustrazine is released from the tunnel, so it releases faster. In the late stage, the microspheres mainly release the drugs through dissolution, so the release curve is gentler.
Figure 4.

Drug-loaded microspheres in different media release curves.
Conclusions
In this paper, ligustrazine hydrochloride carboxymethyl chitosan-collagen microspheres were prepared by the emulsification–crosslinked method. The preparation process was as follows: the Ligustrazine to microencapsulated material ratio should be 1:2, the carboxymethyl chitosan to collagen ratio was 1:2, the oil phase volume to water phase volume ratio was 5:1, the span-80 dosage was 0.5%, the stirring speed was 600 r/min, and crosslinking glutaraldehyde 3 ml. The crosslinking reaction occurred for 3 h, then the filtration was dried to obtain the pale yellow microspheres, which were round, smooth, and uniform in size. The microsphere yield was 86.09%, the envelop rate reached 54.08%, the carrier rate reached 14.16%, and the preparation was very stable.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the topic of biologically active substances and functional food, Beijing Key Laboratory (ZK70201401), 2014 Beijing Natural Science Foundation - Beijing Institute of Science and Technology jointly funded by the projects L140006 and the National Natural Science Foundation of China (Grant No. 11475020).
References
- [1].Dan WH, Xia ChF. Leather chemistry and technology (first volume). 2nd ed. Chengdu University of Science, Light Industry College: Light Industry Press; 1987; 131p. [Google Scholar]
- [2].Reich G. Collagen report: A review about the present state. Lender 1995; 46:192-9. [Google Scholar]
- [3].Wang LJ, Chen R. Collagen/chitosan composite sponge dressings epidermal growth factor thrombin Development. J Guangdong Pharm Coll 2004; 20:471-2. [Google Scholar]
- [4].Xu H, Ding PT, Zheng JM. Non-diffusion-controlled release of drugs in polymer systems. J Med 2000; 35:710-4. [Google Scholar]
- [5].Jeans R, Ponder RK. Preparation of gelatin micro spheres of bloodying. Int J Pharm 2004; 35:177-9. [Google Scholar]
- [6].Mi WL, Chen CT, Tseng YC, Kuna CY, Shun SS. Iron (III)-Carboxymethyl chitin mesosphere for the PH-sensitive release of 6- mere atop urine. Jamul of Controlled Release 1997; 44:19-32; http://dx.doi.org/ 10.1016/S0168-3659(96)01502-7 [DOI] [Google Scholar]


