ABSTRACT
The objective is to explore the effect of testosterone on the proliferation and collagen synthesis of neonatal rat cardiac fibroblasts (CF) induced by Angiotensin II (Ang II) and the underlying mechanisms. Derived from neonatal rats, the CFs were divided into 4 groups: the control group, Ang II group, testosterone group, and testosterone + Ang II group in vitro. Cell cycle distribution, collagen counts, and phosphorylated extracellular signal-regulated kinase (ERK1/2) (p - ERK1/2) expression were assessed by flow cytometry, VG staining, and immunocytochemistry, respectively. The Ang II group had a much higher proportion of cells in the S-phase, higher collagen contents, and a higher p - ERK1/2 expression level than either the control or testosterone group. However, these factors were significantly reduced in the testosterone + Ang II group as compared to the Ang II group. In terms of cells in the S-phase and the collagen contents, there was not a significant difference between the testosterone group and the control. However, the protein expression of p-ERK1/2 was significantly increased in the testosterone group as compared to the control. Testosterone inhibits the proliferation and collagen synthesis of CF induced by Ang II. The underlying mechanism may involve the ERK1/2 signaling pathway.
KEYWORDS: angiotensin II, cardiac fibroblasts, ERK1/2, testosterone
Introduction
Cardiac fibroblasts (CF) play an important role in maintaining the structure and function of the heart.1,2 Some pathogenic factors can greatly increase the number of CFs and the synthesis of collagen, which eventually leads to myocardial fibrosis.3 Furthermore, cardiac fibrosis plays a pivotal role in the progression of hypertension, coronary heart disease, heart failure and other cardiovascular diseases, and it initiates myocardial remodeling.3 As an important biological effector in the Renin-angiotensin system (RAS), Ang II contributes to collagen synthesis and CF proliferation, which plays an important role in myocardial fibrosis hypertension.4,5
Testosterone regulates numerous physiological processes, and evidence suggests that it plays a critical role in myocardial fibrosis. However, it is undetermined whether testosterone positively or negatively affects myocardial fibrosis,6,7,8, and the underlying mechanism remains unclear. In this study, we observed the effects of testosterone on the cell cycle, collagen synthesis, and phosphorylation ERK1/2 protein expression with or without Ang II-induced rats cardiac fibrosis. The goal of the present study is to provide experimental evidence for testosterone application in myocardial fibrosis.
Materials and methods
The main reagent and instrument
The following reagents were used in this study: Dulbecco's modified Eagle's medium (DMEM) medium (Hyclone Company, US), newborn fetal serum (NFS) (Hyclone Company, US), angiotensin II, propyl iodide (PI), RNA enzymes, testosterone (Sigma Company, US), p-ERK1/2 (4370s, CST), Goat Anti-Mouse IgG antibody (Cell Signaling Company, US), trypsin (Difco Company, US), collagenase (Invitrogen Company, US), VG staining kits (Amresco Company, US), immunohistochemical kit, rat against vimentin monoclonal antibodies, the amino benzidine (Amresco Company, US), and some other reagents for pure market analysis. The following instruments were used in this study: flow cytometry instrument (Epics-XP-MCL Company, US), CO2 incubator (Scientific Company, US), inverted phase contrast microscope (Olympus company, CK2 type, Japan), and high-speed centrifuge at low temperature (Heraeus Company, Biofuge28 RS type, Germany).
Extraction, subculture, and identification of CF
Neonatal Sprague-Dawley rats (1–2 d old) were purchased from the Experimental Animal Center of Zhengzhou University (clean level, No:0008508). All experimental protocols were approved by Zhengzhou University's Review Committee for the Use of Animal Subjects.
Primary and passaged cultured CF
After being dissociated from the heart tissue by trypsin and collagenase, the cells were pre-plated into culture flacons at 37°C for 2 h in order to remove the suspended cells. The majority of the adherent cells were fibroblasts. CFs were routinely cultured in DMEM supplemented with 10% NCS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cell culture was achieved in a humidified 5% CO2 thermostatic incubator (Scientific) at 37°C with media replenishment every 3 d. When the cells became confluent, they were harvested with 0.25% trypsin/ethylenediaminetetraacetic acid and replated (passaged) with a split of 1:3. The medium was changed every 2 d. Cells in passage 3 or 4 were used in all of the experiments. The CF purity was assessed by morphological observation under an inverted phase contrast microscope and immunocytochemistry using antibody for vimentin.
As shown in Figure 1, the cardiac fibroblasts were identified by immunocytochemistry, at SPx400. The CFs were long spindle-shaped cells, and more than 98% of the cells contained brown particles, indicating that more than 98% of the cells were CFs. Therefore, the cellular density fits the requirement of the subsequent test.
Figure 1.
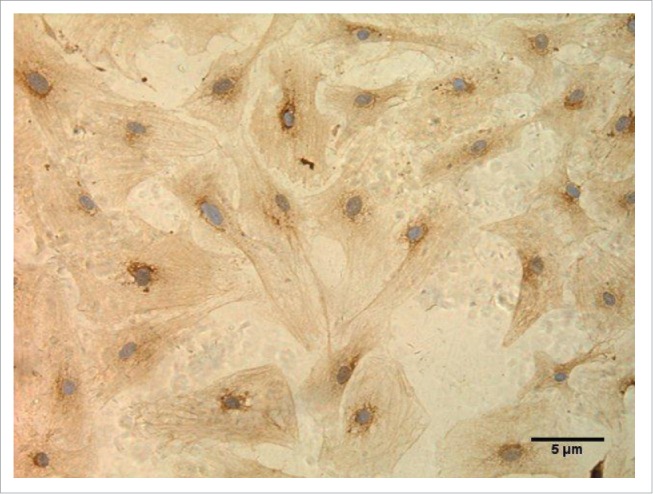
Cardiac fibroblasts were identified by immunocytochemistry. (SP × 400).
Cell group
After 24 hours of starvation with serum-free DMEM, the cells were divided into 4 groups: the control group (the cells were cultured in serum-free DMEM), the Ang II group (the cells were cultured in serum-free DMEM containing 10−6 mol/L Ang II), the testosterone group (the cells were cultured in serum-free DMEM containing 30 nmol/L testosterone), and the testosterone and Ang II co-incubation group (the cells were cultured in serum-free DMEM containing 30 nmol/L testosterone and 10−6 mol/L Ang II).
Cell cycle assay
The CFs were incubated for 24 hours. After which, they were harvested, washed in cold PBS (pH 7.4), and fixed with 70% (v/v) ethanol at −20°C for 30 min. After the ethanol was removed, the cells were incubated with PBS containing RNase at 37°C for 30 min, and then, they were stained for 30 min with 0.005% PtdIns. Fluorescence was measured with flow cytometry. The results represented a minimum of 3000 cells assayed for each determination. The experiment was performed 6 times.
Collagen synthesis detection
The cells were cultured on sheet glasses in 6-well flat-bottomed microtiter plates. After becoming confluent, the cells were incubated for 24 h. The medium was removed, and the glasses were washed. Afterwards, the cells were stained with hematoxylin and washed. Then, the cells were processed with VG staining, differentiated by absolute ethyl alcohol, dehydrated by gradient alcohol, transparentized by xylol, and finally sealed by object slides.
The slides were observed, and pictures were taken with an Olympus CK2 microscope. The extracellular optical density of collagen was measured (Integral Optical Density) by Biosins Digital Imaging Systems.
p-ERK1/2 protein expression
The cells were cultured on sheet glasses in 6-well flat-bottomed microtiter plates in the same method as described above. After becoming confluent, the cells were incubated for 30 min. After the medium was removed, the cells were washed with cold PBS and then immobilized by paraformaldehyde. Next, they were incubated in 3% H2O2, sealed by normal goat serum, and then exposed to p-ERK 1/2 solution for 3 h. They were then incubated with the biotinylated second antibody at room temperature, incubated with HRP-Streptavidin at room temperature, stained by DAB, and washed exhaustively. They were then re-stained by hematoxylin, dehydrated by gradient alcohol, transparentized by xylol, and finally sealed by object slides.
The slides were observed, and pictures were taken with an Olympus CK2 microscope. The extracellular optical density of p-ERK1/2 was measured (Integral Optical Density) by Biosins Digital Imaging Systems.
Statistical analysis
Data were analyzed using the statistical package SPSS17.0. All results were expressed as the mean and standard deviation (mean ± SD). The difference was analyzed for significance by factorial analysis. A value of P < 0.05 was considered statistically significant.
Results
S-phase distributions in different groups
As shown in Table 1, there were significantly increased S-phase (DNA synthesis phase) distributions in the Ang II group as compared to the control (P < 0.01). However, there was no significant difference between the testosterone group and the control group (P > 0.05). Finally, the testosterone and Ang II co-incubation group had significantly reduced S-phase distributions as compared to the Ang II group (P < 0.01).
Table 1.
S phase distributions in different groups (n = 6).
| AngII group |
||
|---|---|---|
| Testosteron | − | + |
| − | 15.14 ± 1.14 | 32.57 ± 4.66 |
| + | 18.43 ± 0.68 | 22.73 ± 0.31 |
F AngII group=30.403, P (vs the control) = 0.001, F Testosterone group
= 5.812, P (vs the control) > 0.05, F co-incubation group = 18.073, P (vs AngII group) = 0.003.
Collagen content
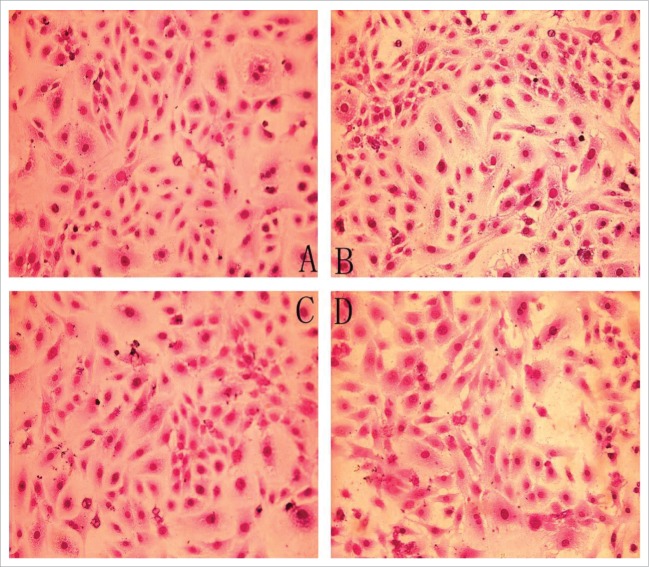
Figure 2 details the collagen content in the 4 groups. As shown in Table 2, after 24 h incubation with Ang II, the collagen of CF was significantly increased as compared to the control (P < 0.01). However, no significant difference was detected between the testosterone group and the control group (P > 0.05). Finally, the collagen was decreased in the testosterone and Ang II co-incubation group as compared with the Ang II group (P < 0.01)
Figure 2.
Collagen content in 4 groups (VG staining ×200) (A) Control group, (B) AngII group, (C) Testostorone group, (D) Co-incubation group.
Table 2.
Collagen content in different groups (n = 6).
| AngII |
||
|---|---|---|
| Testosteron | − | + |
| − | 120.68 ± 4.43 | 150.74 ± 3.8 |
| + | 118.44 ± 3.9 | 119.5 ± 6.93 |
F AngII group = 10.365, P (vs the control) = 0.002, F Testosterone group = 4.533, P (vs the control) > 0.05, F co-incubation group = 42.049, P (vs AngII group) < 0.001.
p-ERK1/2 protein expression
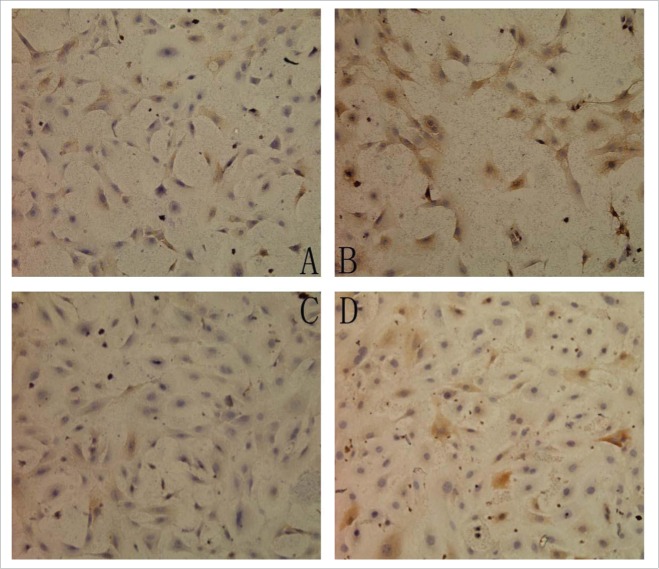
Figure 3 shows the p-ERK1/2 expression in the 4 groups. As shown in Table 3, the protein expression of p-ERK1/2 was significantly increased in the Ang II group (P < 0.01) and testosterone group (P< 0.05) as compared with the control group. However, the testosterone and Ang II co-incubation group had reduced p-ERK1/2 protein expression as compared with the Ang II group (P < 0.01).
Figure 3.
The expression of p-ERK1/2 in 4 groups (SP ×200) (A) Control group, (B) AngII group, (C) Testostorone group, (D) Co-incubation group.
Table 3.
The expression of p-ERK1/2 in different groups (n = 6).
| AngII group |
||
|---|---|---|
| Testosteron | − | + |
| − | 115.12 ± 7.25 | 130.53 ± 10.15s |
| + | 121.85 ± 4.35 | 116.8 ± 4.93 |
F AngII group =174.762, P (vs the control) < 0.001, F Testosterone group = 184.828, P (vs the control) < 0.001, F co-incubation group = 152.240, P (vs AngII group) < 0.001.
Discussion
Myocardial fibrosis is primarily characterized as CF proliferation, collagen I and II deposition, and an extracellular matrix protein concentration.9 Additionally, it represents an important pathogenesis of various heart diseases, including heart failure, arrhythmia, sudden cardiac death, and other serious complications.10 Several investigations have previously demonstrated that Ang II, through cell receptors, promotes the growth of CFs and the expression of extracellular matrix protein. These factors converge to play an important role in myocardial remodeling, which is considered to be through the coupling reaction of intracellular signal transduction pathways.4
Testosterone is a steroid hormone and is one of the most important androgens in men. It plays a significant role in immune function and in maintaining male sexuality. Furthermore, it has been reported since 1990 that testosterone plays a role in the cardiovascular system, and additional investigations on this topic have been conducted. However, the results from different study groups vary greatly. Existing studies have confirmed that a low serum testosterone level positively correlates with the incidence of cardiovascular events.10,11 Moreover, exogenous testosterone is associated with adverse cardiovascular events.12 In addition, studieshave also found that testosterone has a protective effect on the cardiovascular system, such as possessing anti-inflammatory properties, regulating blood lipids, reducing atherosclerosis, dilating blood vessels, converting Ang II-induced vascular remodeling, and so on.13-15
The mitogen-activated protein kinase (MAPK) system is the focal point and common pathway for the transmission from extracellular signals to an intracellular signal, and it is involved in the biological effect of Ang II. Furthermore, ERK1/2 and its active form p-ERK1/2 represent one of the key downstream signal MAPKs.16 Generally, immunoprecipitation can be used to accurately detect ERK activation levels but not the intracellular distribution of ERK during the activation process. In fact, there is a nuclear transfer process during ERK activation. To assess the level of activated ERK, immunohistochemistry has been used in many studies to detect the p-ERK level, which is the activated form of ERK; however, the intracellular distribution can be clearly detected.
In our study, it was observed that 10−6 mol/L Ang II can obviously increase the proportion of CFs in the S-phase (DNA synthesis) and collagen synthesis. At the same time, it can obviously enhance p-ERK1/2 protein expression, thereby indicating that Ang II can promote CF proliferation through ERK1/2 pathways, which is consistent with previous investigations.3,4,17 Additionally, the testosterone and Ang II co-incubation group experienced a more significant reduction of CFs in the S-phase proportion, collagen synthesis, and p-ERK1/2 expression than did the Ang II group alone. This result indicates that testosterone might suppress Ang II-induced CF proliferation and collagen synthesis, and its potential mechanism might be related to ERK1/2 inhibition.
There was no statistical difference for either the proportion of CFs in the S-phase or the collagen synthesis between the testosterone group and the control group; however, the protein expression of p-ERK1/2 was increased in the testosterone group as compared to the control. These results demonstrate that testosterone as a growth hormone exerts a growth-promoting effect on myocardial cells, the mechanism of which is involved in the p-ERK signaling pathway. However, testosterone in physiological doses is sufficient to cause biological effects that arise after ERK activation, such as an increase in CFs in the S-phase and excessive collagen synthesis.
It should be noted, however, that a very interesting phenomenon occurs when there is an excessive stimulation of Ang II. In that case, testosterone will have an antagonistic biological effect on Ang II, which is due to the interference of ERKs. This is why the testosterone receptor antagonist group was not included in this study. Here, the aim was to observe the effects of testosterone on Ang II-mediated biological effects, rather than testosterone on CFs.
Of course, some studies have reported that testosterone has a negative effect on the cardiovascular system. These studies ignored the differences between in vivo and in vitro culture. Testosterone in vivo cells enact a positive effect on the cardiovascular system through the conversion of testosterone to estradiol, whereas testosterone in vitro cells negatively impact the cardiovascular system due to the inability to convert testosterone to estrogen. In addition, varying doses of testosterone impact the cardiovascular system in different ways.18,19
Conclusion
In conclusion, testosterone might suppress Ang II-induced CF proliferation and collagen synthesis in vitro, thus potentially playing an important role in cardiac fibrosis inhibition. Additionally, the underlying mechanism might be related to the inhibition of ERK1/2 signaling pathways.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res 2005;65(1):40; PMID:15621032; http://dx.doi.org/ 10.1016/j.cardiores.2004.08.020 [DOI] [PubMed] [Google Scholar]
- [2].Banerjee I, Yekkala K, Borg TK, Baudino TA. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann N Y Acad Sci 2006;1080:76; PMID:17132776; http://dx.doi.org/ 10.1196/annals.1380.007 [DOI] [PubMed] [Google Scholar]
- [3].Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Therap 2009;123(2):255; PMID:19460403; http://dx.doi.org/ 10.1016/j.pharmthera.2009.05.002 [DOI] [PubMed] [Google Scholar]
- [4].Ohlsson C, Barrett-Connor E, Bhasin S, Orwoll E, Labrie F, Karlsson MK, Tivesten Å. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men: the MrOS (osteoporotic fractures in men) study in Sweden. J Am Coll Cardiol 2001;58(16):1674-81; PMID:21982312; http://dx.doi.org/ 10.1016/j.jacc.2011.07.019 [DOI] [PubMed] [Google Scholar]
- [5].Wang LP, Wang Y, Zhao LM, Li GR, Deng XL. Angiotensin II upregulates KCa3.1 channels and stimulates cell proliferation in rat cardiac fibroblasts. Biochem Pharmacol 2013;85(10):1486; PMID:23500546; http://dx.doi.org/ 10.1016/j.bcp.2013.02.032 [DOI] [PubMed] [Google Scholar]
- [6].Olson ER, Shamhart PE, Naugle JE, Meszaros JG. Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase C delta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension 2008;51(3):704; PMID:18195168; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.107.098459 [DOI] [PubMed] [Google Scholar]
- [7].Herring MJ, Oskui PM, Hale SL, Kloner RA. Tetosterone and the cardiovascular system: a comprehensive review of the basic science literature. Am Heart Assoc 2013;2(4):e271; PMID:23842280; http://dx.doi.org/ 10.1161/JAHA.113.000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Potenza M, Shimshi M. Male hypogonadism: The unrecognized cardiovascular risk factor. J Clin Lipidol 2008;2(2):71-8; PMID:21291723; http://dx.doi.org/ 10.1016/j.jacl.2008.01.011 [DOI] [PubMed] [Google Scholar]
- [9].Cheng S, Vasan RS. Advances in the epidemiology of heart failure and left ventricular remodeling. Circulation 2011;124(20):e516; PMID:22083151; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.111.070235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ruige JB, Mahmoud AM, De BD, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart 2011;97(11):870; PMID:21177660; http://dx.doi.org/ 10.1136/hrt.2010.210757 [DOI] [PubMed] [Google Scholar]
- [11].Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011;96(10):3007; PMID:21816776; http://dx.doi.org/ 10.1210/jc.2011-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Choong K. Adverse events associated with testosterone administration. N Engl J Med 2010;363(2):109; PMID:20592293; http://dx.doi.org/ 10.1056/NEJMoa1000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chung CC, Kao YH, Chen YJ, Chen YJ. Androgen modulates cardiac fibrosis contributing to gender differences on heart failure. Aging Male 2013;16(1):22; PMID:23356882; http://dx.doi.org/ 10.3109/13685538.2012.754008 [DOI] [PubMed] [Google Scholar]
- [14].Nettleship JE, Jones TH, Channer KS, Jones RD. Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse: an effect that is independent of the classic androgen receptor. Circulation 2007;116(21):2427; PMID:17984376; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.107.708768 [DOI] [PubMed] [Google Scholar]
- [15].Ikeda Y, Aihara KI, Yoshida S, Sato T, Yagi S, Iwase T, Akaike M. Androgen-androgen receptor system protects against angiotensin II-induced aascular remodeling. Endocrinology 2009;150(6):2857; PMID:19196803; http://dx.doi.org/ 10.1210/en.2008-1254 [DOI] [PubMed] [Google Scholar]
- [16].Kawaguchi H, Kitabatake A. Altered signal transduction system in hypertrophied myocardium: angiotensin II stimulates collagen synthesis in hypertrophied hearts. J Card Fail 1996;2(4):S13; PMID:8951556; http://dx.doi.org/ 10.1016/S1071-9164(96)80054-6 [DOI] [PubMed] [Google Scholar]
- [17].Zhang W, Chen XF, Huang YJ, Chen QQ, Bao YJ, Zhu W. 2,3,4′,5-Tetrahydrox-ystilbene-2-O-β-d-glucoside inhibits angiotensin II-induced cardiac fibroblast proliferation via suppression of the reactive oxygen species-extracellular signal-regulated kinase 1/2 pathway. Clin Exp Pharmacol Physiol 2012;39(5):429; PMID:22340635; http://dx.doi.org/ 10.1111/j.1440-1681.2012.05692.x [DOI] [PubMed] [Google Scholar]
- [18].Zhang L, Wu SZ, Ruan YJ, Hong L, Xing XW, Lai WY. Testosterone therapy delays cardiomyocyte aging via an androgen receptor-independent pathway. Braz J Med Biol Res 2011;44(11):1118-24; PMID:21971687; http://dx.doi.org/ 10.1590/S0100-879X2011007500128 [DOI] [PubMed] [Google Scholar]
- [19].Singh AB, Hsia S, Alaupovic P, Sinha-Hikim I, Woodhouse L, Buchanan TA, Bhasin S. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metabol 2002;87(1):136-43; PMID:11788637; http://dx.doi.org/ 10.1210/jcem.87.1.8172 [DOI] [PubMed] [Google Scholar]