ABSTRACT
Schizandrin B is extracted from Schisandra chinensis (Turcz.) Baill. This study evaluated the photoprotective effect of Schizandrin B on oxidative stress injury of the skin caused by UVB-irradiation and the molecular mechanism of the photoprotective effect of Schizandrin B, and we firstly found that Schizandrin B could block Cox-2, IL-6 and IL-18 signal pathway to protect damage of skin cells given by UVB-irradiation. In the research, we found that Schizandrin B can attenuate the UVB-induced toxicity on keratinocytes and dermal fibroblasts in human body, and can outstandingly eliminated intracellular ROS produced by UVB-irradiation. These results demonstrate that Schizandrin B can regulate the function of decreasing intracellular SOD's activity and increasing the expression level of MDA in HaCaT cells result from the guidance of UVB, and it markedly reduced the production of inflammatory factors such as Cox-2, IL-6 or IL-18, decreased the expression level of MMP-1, and interdicted degradation process of collagens in UVB-radiated cells. Therefore, skin keratinocytes can be effectively protected from UVB-radiated damage by Schizandrin B, and UVB-irradiation caused inflammatory responses can be inhibited by attenuating process of ROS generating.
KEYWORDS: collagen, COX-2, photoaging, ROS, Schizandrin B, UVB-irradiation
Introduction
UV radiation, a particular UVB-irradiation of which wavelength was 280∼320 nm, can hazarded human health through bring out cyclobutane pyrimidine dimers (CPDs), inflammatory reaction, cellular damage, immunosuppression and ultimate skin cancer.1,2 Skin cells secreted reactive oxygen species (ROS) product after being acted on by UVB-irradiation.3,4 The high expression level of ROS can effectively oxidate DNA, cellular proteins and lipids, result in activating a number of signaling pathways and release many kinds of inflammatory mediators.5 Basal cell layers of epidermis were mostly influenced by UVB-irradiation, thus keratinocytes that was one of typical prime cell populations of basal layer, was primarily affected by UVB-irradiation. Fibroblasts were also primarily affected by UVB-irradiation and can created typical responses of UVB-irradiation photo-damage through releasing and increasing various proinflammatory activators.6-11 Inflammation caused by UV radiation activates various matrix-degrading matrix metalloproteases (MMPs), which leads to collagen degradation and cellular apoptosis. Especially, MMP-1 was the main endogenous factor that degraded dermal collagen in the process of human skin senility.12-14 Type I collagen was the primary composition of extracellular matrix (ECM).When its expression level was reduced, then had a close relevance with skin senility.
In previous studies, we can see that UV-irradiation had an essence that can take strongly oxidative and photo-oxidative damages on skin.15 Therefore, antioxidants that clear up ROS have been proposed as photoprotective agents.16,17
More and more skin care products contained botanical agents, which have the potential antioxidant and the properties of anti-inflammatory and anti-carcinogenic, which can avoid damages of UV radiation damge to skin.9 Moreover, the extractive of traditional Chinese medicine were proved to effectively avianize oxi-dative cellular damage of UV radiation. Andnzo[a,c] cyclooctadiene lignans were extracted from Schisandra berry and can effectively stop skin cancer from spreading.
Schizandrin B, a Dibenzo (a,c) cyclooctene lignans isolated from Fructus schizandrae, is an anti-hepatotoxic, antiasthmatic, antidiabetic, sedative and tonic agent.18,19 It was claimed that unfavorable influence of stress factors can be resisted against increasingly by Schizandrin B besides its adaptogenic activity.11 In China, the Schizandra berry had been taking into treating patients who were suffering infections arose by chronic virus hepatitis B since the 1970s.The lipid peroxidation of cell membranes could be inhibited and the ability of resisting reactive oxygen species of cells by enhancive superoxide dismutase (SOD) with catalase activities could be reinforced both by lots of Schizandra lignans.18
The activity of succedaneums were comparably familiar with that of vitamin E, particularly with liver microsomes.19 Schisandrin B was used to be a hepatoprotectant to depress or eliminate toxicity of xenobiotic agents to diabetic animals' cells.19 The free radical scavenging activity (FRSA), which acted on hydroxyl radical and superoxides in schisandrol A and B, was functionally familiar with that of vitamin E and C, and its eliminating efficiency of hydroxyl radical was twice as high as that of superoxide anion in 2 vitamins.19 However, inhibitory actions of Schizandrin B in photoaging have yet to be studied. In our study, we have evaluated the agent for its antioxidant and cell membrane stability properties in order to prevent and treat photo-damage and photoageing. Our research result shows that Schizandrin B has a potential protective effect on skin cell damage by UVB-irradiation.
In this paper, the activities of Schizandrin B which inhibited against collagen degradation and skin cells' UVB-irradiation inflammatory response were elaborated. It was investigated if the synthesis and secretion of collagenolytic MMP, inflammatory interleukin 6 and 18(IL-6and18) in the UVB-exposed human keratinocytes (HaCaT) and dermal fibroblasts (FB) could be regulated by Schizandrin B.
Experimental
Cell culture
Human dermal fibroblasts and melanoma cell were obtained from the Blood Institute of the China Union Medical University. The human keratinocyte HaCaT cell line was purchased from KeyGEN Biotechnology (Changchun, China). All cells were cultured in Dulbecco's modified Eagle's media (DMEM, Sigma-Aldrich Co., Ltd, St. Louis, MO) containing 10% FBS (Life Technologies, Grand Island, NY), 2 mmol/L of glutamine, 100 U/mL of penicillin, and 100 µg/mL of streptomycin with an experimental environment of 37°C and 5% CO2.
UVB-irradiation and treatment
UVB-irradiation
In the UVB-irradiation experiments, the medium was detached from culture plates and cells were washed twice by phosphate buffered saline (PBS) in order to avoid radiation procedure being influenced by medium components. The cells were covered by a thin covering of PBS without or with test compounds, and then acted on by UVB-irradiation chamber which had a set of 5 UVB lamps on the 15 cm above cells. The doses of UVB-irradiation were calculated with a radiometer. Control samples were given a non-irradiated dealt with
Healthy dermal fibroblasts and keratinocyte HaCaT were propagated in the 70% DMEM according to 1×104 cells per well in 96-wells plates. Cells were seeded in serum-free DMEM for 24 h, then washed by PBS and exposed under 312 nm of UVB-irradiation at the successive does of 0∼36 MJ/cm2. The does of lamps irradiation were metered with a UV-meter. The optimum irradiation dose was estimated at 30 mJ/cm2, therefore this dose of UV-B was used in further experiments.
Cell viability assay
The UVB-irradiated cell viabilities were assayed by thiazolyl blue tetrazolium blue (MTT, Sigma Co., Ltd, St. Louis, MO) on the conditions of Schizandrin B or no Schizandrin B. Culture medium was cleared up and the 1 mg/mL of MTT was added to react for 4 h at 37°C. The liquid supernatant was detruded and then dimethylsulfoxide (DMSO Sigma Co., Ltd, St. Louis, MO) was added to decomposed the rest formazan product. The cell intensity was detected colorimetrically at 550 nm.
Detection of intracellular ROS
Cells were taken a UVB-irradiated pretreatment and then dyed for avoiding the direct fluorescent-dyed photooxidation. HaCaT cells were irradiated under 30 mJ/cm2 of UVB with or without Schizandrin B, then acted on by 5 µm of DCFH-DA. The intensity of DCF fluorescence was metered by a Shimadza UV-260 spectrofluorophotometer. Samples were observed under a confocal laser-scanning microscope.
Measurement of SOD, MDA release
HaCaT cells were pretreated individually with and without of Schizandrin B for 2∼4 h. Then they were irradiated with 30 mJ/cm2 of UVB-irradiation for 24 h. After all, expression levels of SOD and MDA of supernatant and cells were measured by assay kit (Beyotime Co., Ltd, Jiangsu, China).
Western blot
Conditioned medium containing FB cell lysates were gathered. Total protein concentration was measured by Bradford assay. Protein equal amounts were transferred onto PVDF membrane which was blocked by 5% skim milk mixed with TBS/T for 90 min and incubated by first antibodies, and then analyzed by 10% SDS-PAGE. Monoclonal anti-α procollagen aminoterminal extension peptide antibody was used at a 1:250 dilution (Santa Cruz Biotechnology, Santa Cruz, CA) under 4°C in an overnight response. Membranes were then washed with TBST and incubated for 1 h with a secondary antibody before being visualized by DAB. Images were taken by western blots for quantitative analysis. Band intensities images were scanned to quantify by Gel-Pro Analyzer 3.1 Automated Digitizing System. Each experiment was repeated 3 times.
RT-PCR
Total RNA of HaCaT was extracted. The reverse transcription of RNA-to-cDNA was implemented with a synthesis kit. The sequences of primers and cycling conditions were shown in Table 9.
Table 9.
The sequences of primers and cycling conditions.
| Gene | Primers | DNA sequence | cycling condition |
|---|---|---|---|
| IL-18 | sense | 5′-AGGAATAAAGATGGCTGCTGAAC-3′ | 95°C 50s, 60°C 1min, 72°C 45s, for 32cycles |
| antisense | 5′-GCTCACCACAACCTCTACCTCC-3′ | ||
| IL-6 | sense | 5′-CTACATTTGCCGAAGAGCCCT-3′ | 95°C 30s,58°C 30s, 72°C 1min, for 35 cycles |
| antisense | 5′-CTCGACGGCATCTCAGCC-3′ | ||
| COX-2 | sense | 5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′ | 94°C 56s, 50°C 90s,72°C 2min, for 30 cycles |
| antisense | 5′-AGATCATCTCTGCCTGAGTATCTT-3′ | ||
| MMP-1 | sense | 5′-ACGGATACCCCAAGGACATCT-3′ | 95°C 30s,55°C 30s,72°C 1 min, for 35 cycles |
| antisense | 5′-CTCAGAAAGAGCAGCATCGATATG-3′ | ||
| β-actin | sense | 5′-GGCCATCTCTTGCTCGAAGT-3′ | 94°C 30s, 56°C 30s, 72°C 1min, for 30 cycles |
| antisense | 5′-GCCCAGAGCAAGAGAGGCAT-3′ |
Reacted products were electrophoresed with 1.5% agarose gel and dyed with ethidium bromide. Strengths of signals were quantified with a densitometric program and its software kit of quantity one analysis, which were produced by Bio-Rad Laboratories. Increased or decreased ratios of each gene were respectively detected after the intensity of β-actin was normalized.
Statistical analysis
All of experimental data were gathered and statistically analyzed with student's t-test Data. All of Statistical analysis were handled with SPSS for windows (SPSS Inc., Chicago, IL). Results were expressed in the way of means ± SEM(). While P value < 0.05, stands for statistically significant difference.
Results and discussion
Cell viability of Schizandrin B after UVB-irradiation acting on HaCaT
The cytotoxic effect of Schizandrin B HaCaT cells acted on by UVB-irradiation was detected by MTT assay. The result showed that cell viability can not be reduced by different Schizandrin B concentrations that were from 0.1 μmol/L to 10 μmol/L for 24 h (Fig. 1, Table 1).Cell viability of HaCaT was decreased to nearly 80% survival at the dose of 30 mj·cm−2 after UVB treating. Along with the dose of UVB increase, cell viability of HaCaT was further reducing. The optimum irradiation dose was found to be 30 mJ/cm2, therefore this dosage of UV-B were used in further experiments (Table 2).
Figure 1.
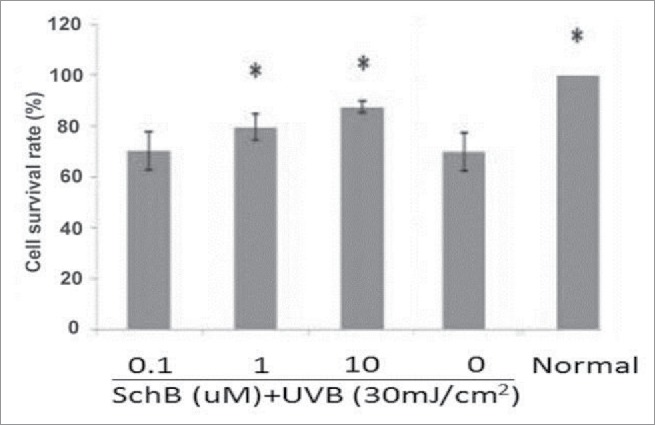
Schizandrin B protected from oxidative damage in UVB-induced HaCaT. Normal: Cells were treated with 0.1% DMSO. * p < 0.05, compared with control.
Table 1.
Cytotoxic activity of Schizandrin B on HaCaT. ( ± s,n ≥ 3).
| Schizandrin B (μmol/L) | Cell survival rate |
|---|---|
| Normal | 100.00 ± 0.00 |
| 0.1 | 103.21 ± 2.05 |
| 1 | 102.89 ± 4.24 |
| 10 | 100.13 ± 2.81 |
Note: p>0.1; compared with Normal.
Table 2.
The effect of UVB on HaCaT cell viability ( ± s, n = 3).
| UVB-irradiation (mJ/cm2) | Cell survival rate |
|---|---|
| 0 | 100.00 ± 0.00 |
| 6 | 103.55 ± 1.38 |
| 12 | 103.40 ± 4.27 |
| 18 | 100.38 ± 4.39 |
| 24 | 96.98 ± 2.28 |
| 30 | 70.05 ± 4.75* |
| 36 | 56.71 ± 1.73* |
Note:
p < 0.05; compared with Normal.
The effect of that Schizandrin B protected cell viability of HaCaT from reduction induced by UVB was detected by the MTT analysis. In the Table 2 show, the survival rate of irradiated non-Schizandrin-B cells was 69.71% while the cell viabilities of cells pretreated with at 0.1, 1, and 10 μmol/L of Schizandrin B were respectively 70.26%, 79.63%, and 87.57% before exposing to UVB-irradiation. When HaCaT cells were pretreated by Schizandrin B in concentrations between 0.1 μmol/L and 10 μmol/L, and their cell viabilities were dose-dependently increased after UVB-irradiation. This result showed Schizandrin B could provide significant protection to human skin cells against damage caused by UVB-irradiation.
Effect of Schizandrin B on intracellular ROS of after UVB-irradiation
The generation of intracellular ROS can be determinated by DCFH-DA, because it could pass through the cell membrane freely. The DCFH-DA could be hydrolyzed by the esterase activity of cells DCF, which could be oxidized by peroxides and then constituted fluorescent 2′, 7′-dichlorofluorescin, which can be used during experiment that needed spectrofluorometer and confocal laser scanning microscopy.20,21 The effect of that Schizandrin B cleared up intracellular ROS induced by UVB-irradiation was detected to show in Table 3 and Fig. 2. The level of ROS of UVB-irradiation cells was 177.47% as much as that of non-UVB-irradiation control cells. However, the cells were acted on by Schizandrin B undering UVB-irradiation, and the levels of intracellular ROS were reduced to 128.57% at 0.1 μmol/L, 106.64% at 1 μmol/L, and 89.01% at 10 μmol/L, respectively. Figure 2 shows highlighted green fluorescence in UVB-exposed HaCaT cells, and that Schizandrin B could reduce green fluorescence in UVB-exposed HaCaT cells. The inhibition was enhanced in a dose-dependent fashion.
Table 3.
Generation of ROS of HaCaT treated with SchB for different dose( ± s,n = 3).
| Group | Fluorescence intensity(%) |
|---|---|
| UVB (−) | 100 ± 0 |
| UVB (+) | 177.47 ± 23* |
| 0.1 μmol/L SchB + UVB (+) | 128.57 ± 33* |
| 1 μmol/L SchB + UVB (+) | 106.04 ± 21* |
| 10 μmol/L SchB + UVB (+) | 89.01 ± 22* |
Note:
p < 0.05; compared with Normal.
Figure 2.
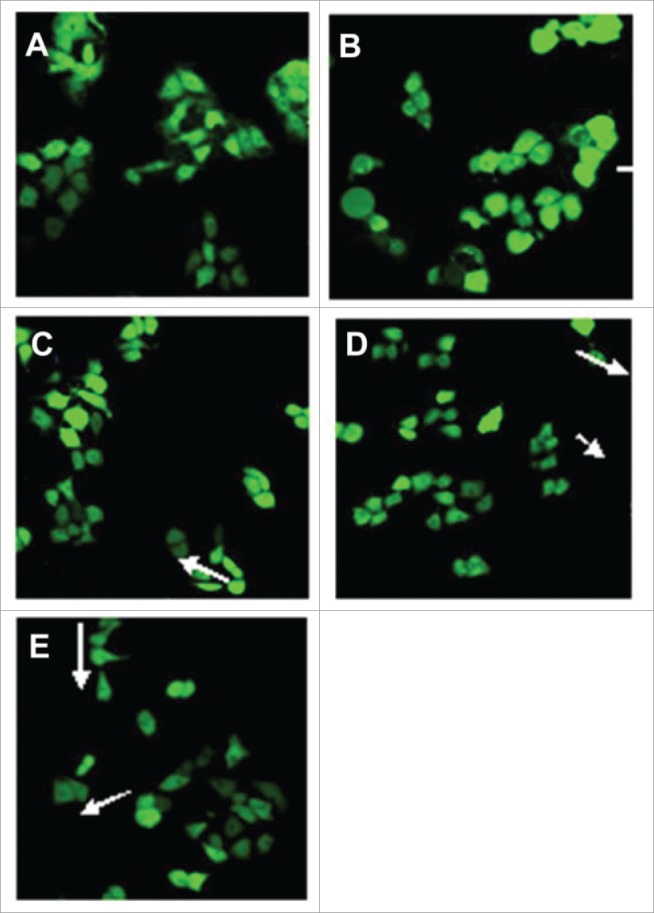
Morphological appearance of HaCaT cells treated with different doses of Schizandrin B by Confocal microscopy detection (×400): A HaCaT cells treated with 0.1% DMSO; B HaCaT cells treated with 0.1%DMSO + UVB; C HaCaT cells treated with 0.1 mol/L SchB + UVB; D HaCaT cells treated with 1 μmol/L SchB + UVB; E HaCaT cells treated with 10 μmol/L SchB + UVB.
Schizandrin B can decrease activity of intracellular SOD and increase level of MDA of UVB-induced HaCaT
SOD could purge oxyradicals and reduce oxidative stress,22,23 and MDA was thought as experimental marker that could preciously show the level of lipid peroxidation by oxyradicals inducement. Thus, the activity decreasing of SOD and the level increasing of MDA were used to indicate to show the oxidative damage degree. To evaluate antioxidant activity of Schizandrin B and SOD, an MDA assay was performed in this experiment. HaCaT cells were pretreated with Schizandrin B after a 2 h and exposure to 30 mJ/cm2 of UVB-irradiation.Cells were incubated for 24 h to measure SOD and MDA secretion. The result revealed expression levels of intracellular SOD and MDA in HaCaT could be regulated by Schizandrin B treatment.The result showed that Schizandrin B treatment can effectively increase the level of intracellular SOD activity and decrease the level of MDA at 10 μmol/L (Figs. 3, 4). So, we can conclude that Schizandrin B had a strong antioxidant effect.
Figure 3.
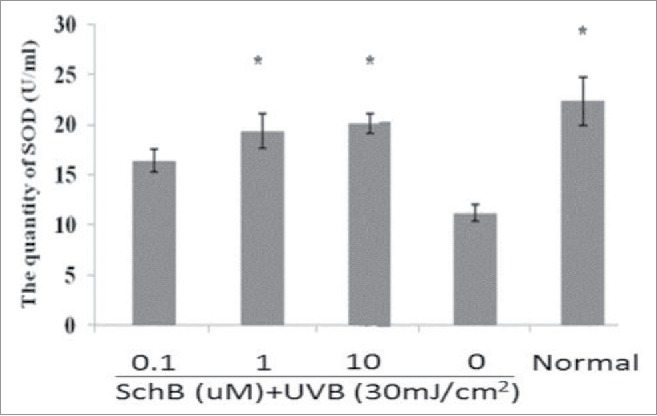
Measurement of SOD on UVB-induced cellular fluid. Normal: Cells were treated with 0.1% DMSO. *p < 0.05, compared with 0μmol/L SchB group.
Figure 4.
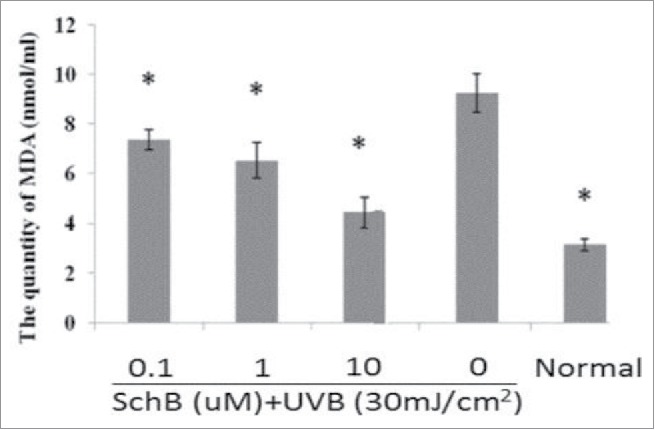
Measurement of MDA on UVB-induced cellular fluid. Normal: Cells were treated with 0.1% DMSO. *p< 0.05, compared with 0μmol/L SchB group.
Effects of Schizandrin B on expression levels of COX-2, IL-6, IL-18 mRNA in UVB-irradiation-induced HaCaT
In order to analyze if Schizandrin B could apply an anti-inflammation to UVB-damaged HaCaT, expression levels of COX-2, IL-6 and IL-18 were detected. Extra-expressed level of COX-2 protein can evaluate the degree of inflammatory after being catalyzedly bio-synthetized PGE2.24,25 The results showed that COX-2 expression was markedly enhanced in HaCaT cells after 8 h or 12 h of UVB-irradiation. This enhancement was gradually cut down with increasing dosages of Schizandrin B. At 10 μmol/L, the COX-2 expression was nearly abolished at 8 h and 12h after UVB-irradiation. (Fig. 5, Table 4)
Figure 5.
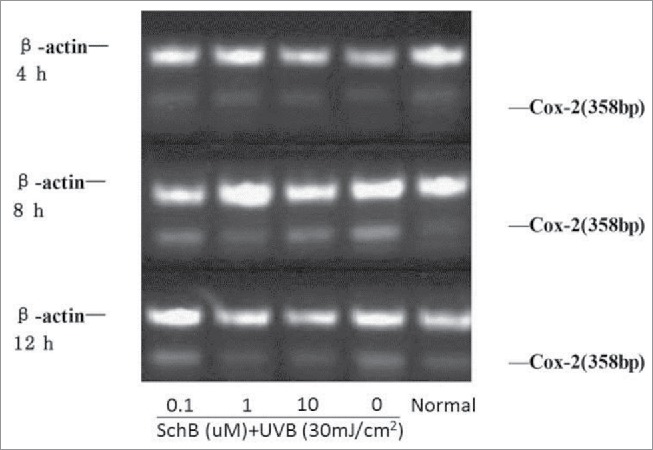
Effect of Schizandrin B on Cox-2 expression in HaCaT Cells after UVB-irradiation. Normal: Cells were treated with 0.1% DMSO.
Table 4.
Effect of Schizandrin B on Cox-2 expression in HaCaT Cells after UVB-irradiation ( ± s,n = 3).
| ration of IOD(cox-2/β-actin) |
|||
|---|---|---|---|
| Group | 4 h | 8 h | 12 h |
| Normal | 0.24 ± 0.022 | 0.26 ± 0.019* | 0.24 ± 0.015* |
| 0μmol/L SchB | 0.26 ± 0.017 | 0.45 ± 0.038 | 0.34 ± 0.021 |
| 0.1 μmol/L SchB + UVB | 0.26 ± 0.028 | 0.40 ± 0.027 | 0.36 ± 0.024 |
| 1 μmol/L SchB + UVB | 0.25 ± 0.019 | 0.28 ± 0.033* | 0.25 ± 0.023* |
| 10 μmol/L SchB + UVB | 0.28 ± 0.021 | 0.27 ± 0.023* | 0.28 ± 0.021* |
Note:
p < 0.05; compared with Normal.
IL-6 can preciously evaluate the degree of inflammatory reaction coming from body stress response to injurious stimulis, such as UVB lights causing a severe sunburn reaction. Some reports showed that the level of IL-6 of human keratinocyte cells were significantly increase in exposure under UVB-irradiation. IL-18 was a kind of functional proteins containing IFN-γ factor, and was secreted by lipopolysaccharide activated macrophages or Kupffer cells, its molecular weight was 18 kDa.26,27 Much more IFN-γ can be produced by th1 cells and NK cells of immune system with the action effect of IL-18.The cytotoxicity of NK cells was strengthened and the proliferation of th1 cells was activate by IL-18.28 In recent papers, it assumed that IL-18 can easily bring out malignant skin tumors and was a main product of UVB-irradiation. Expression levels of IL-6and18 of HaCaT can be significiantly enhanced at 8h and 12h of UVB-irradiation. Schizandrin B can significantly reduce the IL-6, IL-18 expression in HaCat cells after UVB-irradiation. (Figs. 6, 7; Table 5, 6)
Figure 6.
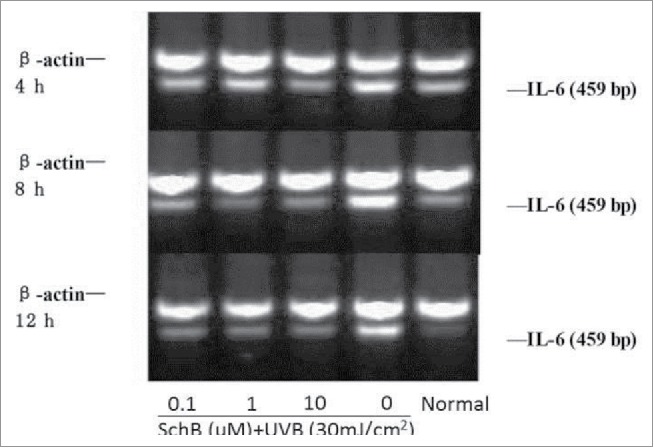
Effect of Schizandrin B on IL-18 expression in HaCaT Cells after UVB-irradiation. Normal: Cells were treated with 0.1% DMSO.
Figure 7.
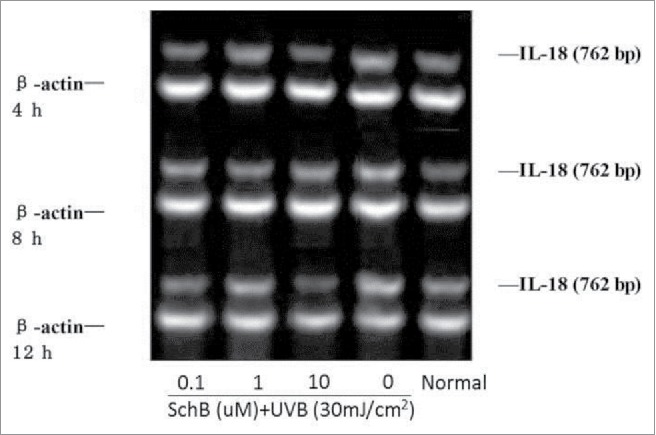
Effect of Schizandrin B on IL-18 expression in HaCaT Cells after UVB-irradiation. Normal: Cells were treated with 0.1% DMSO.
Table 5.
Effect of Schizandrin B on IL-6 expression in HaCaT Cells after UVB-irradiation (± s, n ≥ 3).
| ration of IOD (IL-6/β-actin) | |||
|---|---|---|---|
| Group |
4 h |
8 h |
12 h |
| Normal | 0.65 ± 0.11* | 0.53 ± 0.15* | 0.23 ± 0.06* |
| 0μmol/L SchB | 0.97 ± 0.12 | 0.99 ± 0.16 | 0.88 ± 0.19 |
| 0.1 μmol/L SchB + UVB | 0.69 ± 0.13* | 0.53 ± 0.11* | 0.38 ± 0.09* |
| 1 μmol/L SchB + UVB | 0.83 ± 0.14 | 0.35 ± 0.15* | 0.37 ± 0.08* |
| 10 μmol/L SchB + UVB | 0.58 ± 0.09* | 0.47 ± 0.13* | 0.32 ± 0.11* |
Note:
p < 0.05; compared with Normal.
Table 6.
Effect of Schizandrin B on IL-18 expression in HaCaT Cells after UVB-irradiation (± s, n ≥ 3).
| Ratio of IOD (IL-18/β-actin) | |||
|---|---|---|---|
| Group |
4 h |
8 h |
12 h |
| Normal | 0.507 ± 0.124* | 0.439 ± 0.075* | 0.562 ± 0.063* |
| 0μmol/L SchB | 0.724 ± 0.131 | 0.668 ± 0.064 | 0.902 ± 0.084 |
| 0.1 μmol/L SchB + UVB | 0.465 ± 0.119* | 0.550 ± 0.076* | 0.495 ± 0.075* |
| 1 μmol/L SchB + UVB | 0.575 ± 0.087* | 0.491 ± 0.059* | 0.666 ± 0.072* |
| 10 μmol/L SchB + UVB | 0.435 ± 0.019* | 0.503 ± 0.088* | 0.426 ± 0.063* |
Note:
p < 0.05; compared with Normal.
Effect of Schizandrin B on expression levels of MMP-1 mRNA in FB cells induced by UVB
MMP-1, -3and -9 of hearth human epidermis can be induced by UVB-irradiation.
MMP-1 can directly contribute to photoaging through degrading collagen.29 So, UVB-induced photoaging can effectively depressed by inhibiting MMP-1 expression of FB cells. The result revealed that the effect of that Schizandrin B depressed expression level of MMP-1 of FB cells was dose-dependent in this paper (Fig. 8, Table 7).
Figure 8.
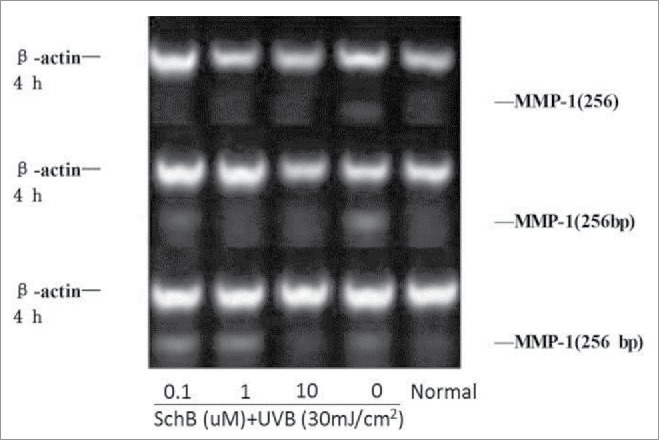
Effect of Schizandrin B on MMP-1 expression in FB Cells after UVB-irradiation. Normal: Cells were treated with 0.1% DMSO.
Table 7.
Effect of Schizandrin B on MMP-1 expression in FB Cells after UVB-irradiation ( ± s, n ≥ 3).
| ration of IOD (MMP-1/β-actin) | |
|---|---|
| Group |
24 h |
| Normal | 0.46 ± 0.06* |
| 0μmol/L SchB | 0.60 ± 0.08 |
| 0.1 μmol/L SchB + UVB | 0.43 ± 0.07* |
| 1 μmol/L SchB + UVB | 0.37 ± 0.05* |
| 10 μmol/L SchB + UVB | 0.33 ± 0.04* |
Note:
p < 0.05; compared with Normal.
Effect of Schizandrin B on expression level of type 1 pro-collagen mRNA
Collagen can majorly maintain the elasticity of skin connective tissues consisting of multiple structures.29 Fibrillar collagen and elastin were damaged and decomposed by collagenolytic MMP enzymes, and then dermal strength and resiliency were lost.30 MMP inhibition can eliminate UV-triggered photo-damage. To examine whether the expression level of pro-collagen were enhanced by Schizandrin B and dermal fibroblast was well treated by 2 h of 0.1 ∼10 μmol/L of Schizandrin B and then 30 mj/cm2 of UVB-irradiation. The amount of synthesis of pro-collagen was detected with western blot. Result revealed that 60% of synthesis of pro-collagen were cut down. However, the reduced amount of synthesis of pro-collagen caused by UVB-irradiation can be restored by Schizandrin B, and the result of restoration depend on the doses of UVB-irradiation. Taken together, the expression level of type 1 pro-collagen can be effectively incresased by Schizandrin B, so the result depent on the dose of Schizandrin B. Thus, Schizandrin B can be an important additive to produce anti-aging cosmetics (Fig. 9, Table 8).
Figure 9.
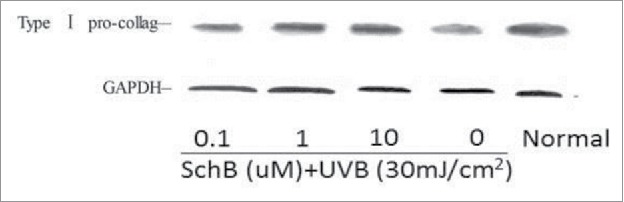
Effect of Schizandrin B on Type I pro-collage expression in FB Cells after UVB-irradiation. Normal: Cells were treated with 0.1% DMSO.
Table 8.
Effect of Schizandrin B on Type I pro-collage expression in FB cells. ( ± s, n = 3).
| Ratio of IOD (type I pro collage/GAPDH) | |
|---|---|
| Normal | 1.263 ± 0.122* |
| 0μmol/L SchB | 0.787 ± 0.131 |
| 0.1 μmol/L SchB + UVB | 1.322 ± 0.167* |
| 1 μmol/L SchB + UVB | 1.229 ± 0.134* |
| 10 μmol/L SchB + UVB | 0.887 ± 0.133 |
Note:
p < 0.05; compared with Normal.
In the experimental results of this paper, it showed that anti-oxidant UVB-irradiation damages of Skin fibroblast and epidermal keratinocytes can be obviously reduced by Schizandrin B. The functionary mechanism of that Schizandrin B depressed the UVB-irradiation-caused oxidative cellular damage may be as follows: a scavenging effect on the intracellular ROS generated by UVB-irradiation; reduced expression levels of COX-2, IL-6 and IL-18; reduced expression level of MMP-1; reduced degradation of collagen. Therefore, Schizandrin B could be a potential UVB-protective agent for use in sunscreen cream.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
All of the researches referring to this paper were supported by the National Natural Science Foundation of China (No.81072964).
Notes on contributors
Researches on molecular genetic were carried out, and related sequence alignments were assisted to complete, and research manuscripts were recorded and drafted by CB and GCG. Immunoassays were carried out by HJ. The task of sequence alignment was participated in by CH. Whole structure of researches were assistedly designed and main statistical analysis were carried out by HJ. Many significant assistances and coordinations were given by LT on the concept and design of research and drafting of manuscript. The final manuscript was agreed on by all authors.
References
- [1].Kappes UP, Luo D, Potter M, Schulmeister K, Rünger TM. Runger.Short- and long-wave UV light (UVB and UVA) induce similar mutations in human skin cells. J Invest Dermatol 2006; 126(3):667-75; PMID:16374481; http://dx.doi.org/ 10.1038/sj.jid.5700093 [DOI] [PubMed] [Google Scholar]
- [2].Ji C, Yang B, Yang Z, Tu Y, Yang YL, He L, Bi ZG. Ultra-violet B (UVB)-induced skin cell death occurs through a cyclophilin D intrinsic signaling pathway. Biochem Biophys Res Commun 2012; 425(4):825-9; PMID:22892127; http://dx.doi.org/ 10.1016/j.bbrc.2012.07.160 [DOI] [PubMed] [Google Scholar]
- [3].Van Laethem A, Claerhout S, Garmyn M, Agostinis P. The sunburn cell:regulation of death and survival of the keratinocyte. Int J Biochem Cell Biol 2005; 37(8):1547-53; PMID:15896663; http://dx.doi.org/ 10.1016/j.biocel.2005.02.015 [DOI] [PubMed] [Google Scholar]
- [4].Ito T, Seo N, Yagita H, Tsujimura K, Takigawa M, Tokura Y. Alterations of immune functions in barrier disrupted skin by UVB irradiation. J Dermatol Sci 2003; 33(3):151-9; PMID:14643520; http://dx.doi.org/ 10.1016/S0923-1811(03)00177-4 [DOI] [PubMed] [Google Scholar]
- [5].Fischer TW, Zbytek B, Sayre RM, Apostolov EO, Basnakian AG, Sweatman TW, Wortsman J, Elsner P, Slominski A. Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J Pineal Res 2006; 40(1):18-26; PMID:16313494; http://dx.doi.org/ 10.1111/j.1600-079X.2005.00273.x [DOI] [PubMed] [Google Scholar]
- [6].Belletti S, Uggeri J, Gatti R, Govoni P, Guizzardi S. Polydeoxyribonucleotide promotes cyclobutane pyrimidine dimer repair in UVB-exposed dermal fibroblasts. Photodermatol Photoimmunol Photomed 2007; 23(6):242-9; http://dx.doi.org/ 10.1111/j.1600-0781.2007.00320.x [DOI] [PubMed] [Google Scholar]
- [7].Lee YR, Noh EM, Han JH, Kim JM, Hwang JK, Hwang BM, Chung EY, Kim BS, Lee SH, Lee SJ, et al.. Brazilin inhibits UVB-induced MMP-1/3 expressions and secretions by suppressing the NF-κB pathway in human dermal fibroblasts. Eur J Pharmacol 2012; 674(2-3):80-6; PMID:22044921; http://dx.doi.org/ 10.1016/j.ejphar.2011.10.016 [DOI] [PubMed] [Google Scholar]
- [8].Yang B, Ji C, Chen X, Cui L, Bi Z, Wan Y, Xu J. Protective effect of astragaloside IV against matrix metalloproteinase-1 expression in ultraviolet-irradiated human dermal fibroblasts. Arch Pharm Res 2011; 34(9):1553-60; PMID:21975818; http://dx.doi.org/ 10.1007/s12272-011-0918-1 [DOI] [PubMed] [Google Scholar]
- [9].Ji C, Yang YL, Yang Z, Tu Y, Cheng L, Chen B, Xia JP, Sun WL, Su ZL, He L, et al.. Perifosine sensitizes UVB-induced apoptosis in skin cells: new implication of skin cancer prevention? Cell Signal 2012; 24(9):1781-9; PMID:22584119; http://dx.doi.org/ 10.1016/j.cellsig.2012.05.003 [DOI] [PubMed] [Google Scholar]
- [10].Shan SJ, Xiao T, Chen J, Geng SL, Li CP, Xu X, Hong Y, Ji C, Guo Y, Wei H, et al.. Kanglaite attenuates UVB-induced down-regulation of aquaporin-3 in cultured human skin keratinocytes. Int J Mol Med 2012; 29(4):625-9; PMID:22211241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Adil MD, Kaiser P, Satti NK, Zargar AM, Vishwakarma RA, Tasduq SA. Effect of Emblica officinalis (fruit) against UVB-induced photo-aging in human skin fibroblasts. J Ethnopharmacol 2010; 132(1):109-14; PMID:20688142; http://dx.doi.org/ 10.1016/j.jep.2010.07.047 [DOI] [PubMed] [Google Scholar]
- [12].Liu S, Mizu H, Yamauchi H. Molecular response to phototoxic stress of UVB-irradiated ketoprofen through arresting cell cycle in G2/M phase and inducing apoptosis. Biochem Biophys Res Commun 2007; 364(3):650-5; PMID:17964538; http://dx.doi.org/ 10.1016/j.bbrc.2007.10.046 [DOI] [PubMed] [Google Scholar]
- [13].Liu S, Mizu H, Yamauchi H. Photoinflammatory responses to UV-irradiated ketoprofen mediated by the induction of ROS generation, enhancement of cyclooxygenase-2 expression, and regulation of multiple signaling pathways. Free Radic Biol Med 2010; 48(6):772-80; PMID:20036733; http://dx.doi.org/ 10.1016/j.freeradbiomed.2009.12.014 [DOI] [PubMed] [Google Scholar]
- [14].Chen CL, Liou SF, Chen SJ, Shih MF. Protective effects of Chlorella-derived peptide on UVB-induced production of MMP-1 and degradation of procollagen genes in human skin fibroblasts. Regul Toxicol Pharmacol 2011; 60(1):112-9; PMID:21397653; http://dx.doi.org/ 10.1016/j.yrtph.2011.03.001 [DOI] [PubMed] [Google Scholar]
- [15].Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol 2006; 126(12):2565-75; PMID:17108903; http://dx.doi.org/ 10.1038/sj.jid.5700340 [DOI] [PubMed] [Google Scholar]
- [16].Bae JY, Choi JS, Kang SW, Lee YJ, Park J, Kang YH. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp Dermatol 2010; 19(8):e182-90; PMID:20113347; http://dx.doi.org/ 10.1111/j.1600-0625.2009.01044.x [DOI] [PubMed] [Google Scholar]
- [17].Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci 2010; 58(2):85-90; PMID:20399614; http://dx.doi.org/ 10.1016/j.jdermsci.2010.03.003 [DOI] [PubMed] [Google Scholar]
- [18].Opletal L, Sovova H, Bartlova M. Dibenzo[a,c]cyclooctadiene lignans of the genus Schisandra: importance, isolation and determination. J Chromatogr B Analyt Technol Biomed Life Sci 2004; 812(1-2):357-71; PMID:15556508; http://dx.doi.org/ 10.1016/S1570-0232(04)00646-4 [DOI] [PubMed] [Google Scholar]
- [19].Lee MS, Chao J, Yen JC, Lin LW, Tsai FS, Hsieh MT, Peng WH, Cheng HY. Schizandrin protects primary rat cortical cell cultures from glutamate-induced apoptosis by inhibiting activation of the MAPK family and the mitochondria dependent pathway. Molecules 2012; 18(1):354-72; PMID:23271470; http://dx.doi.org/ 10.3390/molecules18010354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wu CL, Chou HC, Cheng CS, Li JM, Lin ST, Chen YW, Chan HL. Proteomic analysis of UVB-induced protein expression- and redox-dependent changes in skin fibroblasts using lysine- and cysteine-labeling two-dimensional difference gel electrophoresis. J Proteomics 2012; 75(7):1991-2014; PMID:22270008; http://dx.doi.org/ 10.1016/j.jprot.2011.12.038 [DOI] [PubMed] [Google Scholar]
- [21].Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal 2005; 7(7-8):964-72; PMID:15998251; http://dx.doi.org/ 10.1089/ars.2005.7.964 [DOI] [PubMed] [Google Scholar]
- [22].Hong CE, Lyu SY. Anti-inflammatory and anti-oxidative effects of korean red ginseng extract in human keratinocytes. Immune Netw 2011; 11(1):42-9; PMID:21494373; http://dx.doi.org/ 10.4110/in.2011.11.1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. Is eczema really on the increase worldwide? J Allergy Clin Immunol 2008; 121(4):947-54; PMID:18155278; http://dx.doi.org/ 10.1016/j.jaci.2007.11.004 [DOI] [PubMed] [Google Scholar]
- [24].Iovine B, Iannella ML, Gasparri F, Giannini V, Monfrecola G, Bevilacqua MA. A comparative analysis of the photo-protective effects of soy isoflavones in their aglycone and glucoside forms. Int J Mol Sci 2012; 13(12):16444-56; PMID:23211668; http://dx.doi.org/ 10.3390/ijms131216444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Byun S, Park J, Lee E, Lim S, Yu JG, Lee SJ, Chen H, Dong Z, Lee KW, Lee HJ. Src kinase is a direct target of apigenin against UVB-induced skin inflammation. Carcinogenesis 2013; 34(2):397-405; PMID:23161610; http://dx.doi.org/ 10.1093/carcin/bgs358 [DOI] [PubMed] [Google Scholar]
- [26].Monfrecola G, Gaudiello F, Cirillo T, Fabbrocini G, Balato A, Lembo S. Nicotinamide downregulates gene expression of interleukin-6, interleukin-10, monocyte chemoattractant protein-1, and tumour necrosis factor-α gene expression in HaCaT keratinocytes after ultraviolet B irradiation. Clin Exp Dermatol 2013; 38(2):185-8; PMID:23397947; http://dx.doi.org/ 10.1111/ced.12018 [DOI] [PubMed] [Google Scholar]
- [27].Gruchlik A, Jurzak M, Chodurek E, Dzierzewicz Z. Effect of Gly-Gly-His, Gly-His-Lys and their copper complexes on TNF-α-dependent IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm 2012; 69(6):1303-6; PMID:23285694 [PubMed] [Google Scholar]
- [28].Hwang EC, Lim MS, Im CM, Kwon DD, Lee HJ, Nguyen-Pham TN, Lee YK, Lee JJ. Generation of potent cytotoxic T lymphocytes against castration-resistant prostate cancer cells by dendritic cells loaded with dying allogeneic prostate cancer cells. Scand J Immunol 2013; 77(2):117-24; PMID:23126536; http://dx.doi.org/ 10.1111/sji.12007 [DOI] [PubMed] [Google Scholar]
- [29].Hwang BM, Noh EM, Kim JS, Kim JM, Hwang JK, Kim HK, Kang JS, Kim DS, Chae HJ, You YO, et al.. Decursin inhibits UVB-induced MMP expression in human dermal fibroblasts via regulation of nuclear factor-κB. Int J Mol Med 2013; 31(2):477-83; PMID:23232935 [DOI] [PubMed] [Google Scholar]
- [30].Liao PL, Li CH, Chang CY, Lu SR, Lin CH, Tse LS, Cheng YW. Anti-ageing effects of α-naphthoflavone on normal and UVB-irradiated human skin fibroblasts. Exp Dermatol 2012; 21(7):546-8; PMID:22716253; http://dx.doi.org/ 10.1111/j.1600-0625.2012.01517.x [DOI] [PubMed] [Google Scholar]


