SEA incidence has increased more than three-fold over the past decade at a large, high-volume, academic medical center. This retrospective, case-control study identified several attributes that could inform the early recognition of this potentially highly morbid acute infection of the central nervous system.
Keywords: CNS infection, spinal epidural abscess
Abstract
Background. Delayed recognition of spinal epidural abscess (SEA) contributes to poor outcomes from this highly morbid and potentially lethal infection. We performed a case-control study in a regional, high-volume, tertiary care, academic medical center over the years 2005–2015 to assess the potential changing epidemiology, clinical and laboratory manifestations, and course of this disorder and to identify factors that might lead to early identification of SEA.
Methods. Diagnostic billing codes consistent with SEA were used to identify inpatient admissions for abstraction. Subjects were categorized as cases or controls based on the results of spinal imaging studies. Characteristics were compared using Fisher's exact or Kruskal-Wallis tests. All P values were 2-sided with a critical threshold of <.05.
Results. We identified 162 cases and 88 controls during the study period. The incidence of SEA increased from 2.5 to 8.0 per 10 000 admissions, a 3.3-fold change from 2005 to 2015 (P < .001 for the linear trend). Compared with controls, cases were significantly more likely to have experienced at least 1 previous healthcare visit or received antimicrobials within 30 days of admission; to have comorbidities of injection drug use, alcohol abuse, or obesity; and to manifest fever or rigors. Cases were also more likely to harbor coinfection at a noncontiguous site. When available, inflammatory markers were noted to be markedly elevated in cases. Focal neurologic deficits were seen with similar frequencies in both groups.
Conclusions. Based on our analysis, it appears that selected factors noted at the time of clinical presentation may facilitate early recognition of SEA.
Spinal epidural abscess (SEA) is a relatively uncommon yet highly morbid and potentially lethal pyogenic infection of the central nervous system (CNS). Spinal epidural abscess can rapidly and unpredictably evolve to irreversible neurologic injury via pathophysiologic mechanisms that culminate in ischemic necrosis of the spinal cord [1]. Although the presence and duration of neurologic deficits appear to correlate with adverse outcomes, diagnostic delays are common [2, 3]. Early recognition is hampered by 2 main factors: symptoms may be vague and indolent, deterring patients from seeking medical attention; and the clinical manifestations classically associated with SEA may be nonspecific or inconsistently present when medical attention is initially sought [1–5].
Spinal epidural abscess appears to be seen with increasing frequency [1, 6]. Some literature supports a changing epidemiology and risk profile of this infection in the current era compared with reports from before the year 2000 [4]. In this study, we reviewed the clinical experience with SEA over the previous decade in a single, tertiary care, regional, academic medical center to assess the potential changing epidemiology, risk factors, clinical manifestations, and diagnostic and therapeutic approach to this disorder. In so doing, we hoped to identify factors that could facilitate early clinical recognition of SEA.
METHODS
Study Design
The study was a retrospective, case-control investigation of SEA at Baystate Medical Center (BMC), a 720-bed, tertiary care, regional, academic medical center currently serving a population of approximately 850 000 people in western Massachusetts. The protocol was approved by the Department of Medicine Scientific Review Committee and by the BMC Institutional Review Board.
Selection Criteria
Baystate Medical Center inpatient billing software database (McKesson Performance Analytics; McKesson Corporation, San Francisco, CA) was used to identify all adult (≥18 years) inpatient admissions from January 1, 2005 to December 31, 2015 with a principal or secondary International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code of 324.1 Intraspinal Abscess; 324.9 CNS Abscess NOS; 336.9 Spinal Cord Disease NOS; or 721.8 Spinal Disorders NEC, or ICD-10 G06.1 (after 1 October 2015). To be eligible for review, at least 1 Current Procedural Terminology (CPT) billing code was required for magnetic resonance imaging (MRI), computed tomography (CT), or myelography of the spine during the hospitalization.
Data Collection
Identifiers for eligible encounters were uploaded to a RedCap database, hosted by Tufts Clinical and Translational Science Institute (Grant Number UL1TR001064) for abstraction from the electronic medical record. Baystate Medical Center has had an integrated, inpatient electronic health record since 2004; however, some elements of the record continued to be in paper format until 2007, and therefore most records for hospitalizations earlier than 2008 required supplemental review of paper charts. Records were randomly assigned to 1 of 4 coinvestigators (A.W.A., A.H., D.L., J.Fi.) for abstraction. Before individual abstractions, the team coabstracted a 10-record (4%), randomly selected subsample of charts to ensure interobserver reliability. After all abstractions were completed, approximately 30% of records were randomly selected for overabstraction and adjudication by the principal investigator (A.W.A.). Systematic data discrepancies were subject to reabstraction by study personnel with the principal investigator's oversight and confirmation.
Case Definition
Encounters meeting study criteria (ie, principal or secondary ICD-9 diagnosis code of 324.1 Intraspinal Abscess; 324.9 CNS Abscess NOS; 336.9 Spinal Cord Disease NOS; or 721.8 Spinal Disorders NEC, or ICD-10 G06.1, plus the requisite imaging study) were defined as (1) “confirmed” SEA if imaging demonstrated an epidural lesion associated with a positive culture from the lesion or blood and (2) “probable” SEA if imaging demonstrated an epidural lesion in the absence of at least 1 positive culture from lesion or blood.
Control Definition
Controls were defined as encounters that met the same inclusion criteria as cases (ie, principal or secondary ICD-9 diagnostic codes as above plus the requisite imaging study) but with no epidural lesion identified on review of the imaging study.
Statistical Analysis
All variables were checked for completeness and plausibility using frequencies (percentage) (categorical) and means/ranges (continuous, ordinal). Univariable statistics between case/control status and each independent variable were generated using Fisher's exact test (categorical), one-way analysis of variance (Gaussian), or Kruskal-Wallis equality-of-populations rank test (non-Gaussian). All tests of significance were 2-sided with a critical threshold of <0.05. Stata/MP 14.1 (StataCorp LP) was used for all analyses.
RESULTS
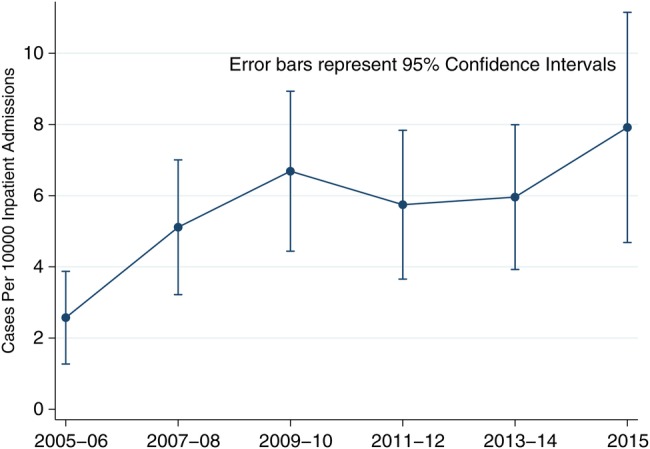
Two hundred fifty-six records were associated with a principal or secondary diagnosis of SEA; upon review, 6 lacked a qualifying imaging study. Of the 250 patients that met study inclusion criteria, 162 (65%) were classified as either confirmed (n = 132) or probable (n = 30) cases of SEA by study definitions; 88 patients (35%) were classified as controls. Both categories of SEA were considered as a single entity of “cases” for purposes of analysis. During the study period, incident SEA diagnoses increased from 2.5 to 8.0 per 10 000 admissions (Figure 1), a 3.3-fold increase from 2005 to 2015 (P < .001 for the linear trend). The proportion of cases identified in their earliest stage, ie, localized pain at the affected spinal level [1], remained flat over the study period (P = .26 for the linear trend; data not shown). Sixty-six percent of cases were diagnosed by MRI; most of these were contrast-enhanced studies. The remainder of cases was diagnosed by CT imaging. There were no differences in the relative utilization of either MR or CT imaging among study subjects by study year (P = .71 for test of linear trend; data not shown).
Figure 1.
Incidence of spinal epidural abscess per 10 000 hospital admissions from 2005 to 2015.
Table 1 details the baseline and epidemiologic characteristics and comorbidities of the study population. Although SEA cases were diagnosed in all decades of adult life, 73% occurred in those over the age of 50. Compared with controls, cases were significantly more likely to have been transferred from another healthcare facility (30.3% vs 17.1%, P = .02), to have experienced at least 1 previous healthcare visit for similar symptoms within 30 days of admission (50.6% vs 29.6%, P = .001), or to have received antimicrobials within 30 days of admission (35.2% vs 6.8%, P < .001). Cases were significantly more likely than controls to harbor comorbidities of injection drug use (20.4% vs 4.6%, P = .001), alcohol abuse (19.1% vs 8.0%, P = .03), or obesity (21.6% vs 2.3%, P < .001) but did not differ with respect to other recognized SEA risk factors (Table 1). Controls were significantly more likely to have no recognized risk factors for SEA; however, 16% of cases also had no identifiable risk factors. Very few patients had a bleeding diathesis that would confer risk for epidural hemorrhage.
Table 1.
Baseline Characteristics and Epidemiologya
| Case (n = 162) | Control (n = 88) | P Valueb | |
|---|---|---|---|
| n (%) or Median (IQR) | n (%) or Median (IQR) | ||
| Demographics | |||
| Age in years | 58.5 (49.6–69.4) | 64.2 (50.8–75.2) | .10 |
| <40 | 13 (8.0%) | 10 (11.4%) | .09 |
| 40 to <50 | 31 (19.1%) | 10 (11.4%) | |
| 50 to <60 | 44 (27.2%) | 17 (19.3%) | |
| 60 to <70 | 36 (22.2%) | 21 (23.9%) | |
| 70 to <80 | 28 (17.3%) | 17 (19.3%) | |
| 80+ | 10 (6.2%) | 13 (14.8%) | |
| Age ≥50 yr | 118 (72.8%) | 68 (77.3%) | .54 |
| %Male | 100 (61.7%) | 48 (54.6%) | .28 |
| Race | |||
| White | 118 (72.8%) | 67 (76.1%) | .68 |
| Black/AA | 16 (9.9%) | 8 (9.1%) | |
| Hispanic | 25 (15.4%) | 10 (11.4%) | |
| Other | 3 (1.9%) | 3 (3.4%) | |
| Transferred from another facility | 49 (30.3%) | 15 (17.1%) | .02 |
| ED (nontransfer) | 107 (66.0%) | 67 (76.1%) | .11 |
| Health care visit <30 d similar symptoms | 82 (50.6%) | 26 (29.6%) | .001 |
| Antimicrobial use within last 30 d | 57 (35.2%) | 6 (6.8%) | <.001 |
| Bleeding diathesis | 6 (3.7%) | 7 (8.0%) | .23 |
| Risk Factors | |||
| Alcohol abuse | 31 (19.1%) | 7 (8.0%) | .03 |
| Obesityc | 35 (21.6%) | 2 (2.3%) | <.001 |
| Chronic kidney disease | 31 (19.1%) | 9 (10.2%) | .07 |
| End-stage renal disease-HD | 13 (8.0%) | 2 (2.3%) | .09 |
| Diabetes Mellitus | 55 (34.0%) | 21 (23.9%) | .11 |
| AIDS/HIV | 1 (0.6%) | 3 (3.4%) | .13 |
| Non-HIV immune compromise | 12 (7.5%) | 5 (5.7%) | .79 |
| Injection drug use | 33 (20.4%) | 4 (4.6%) | .001 |
| Indwelling catheter | 36 (22.2%) | 13 (14.8%) | .18 |
| Spinal surgery within last 12 m | 13 (8.0%) | 3 (3.4%) | .19 |
| Spinal anesthesia within last 12 m | 1 (0.6%) | 4 (4.6%) | .05 |
Abbreviations: AA, African American; AIDS, acquired immune deficiency syndrome; ED, emergency department; HD, hemodialysis; HIV, human immunodeficiency virus; ICD-9, International Classification of Diseases, Ninth Revision; IQR, interquartile range.
a The null hypothesis for all tests is that means or proportions are equal in cases and controls.
b χ2 test for linear trend of the log odds (ordinal) or Fisher's exact (categorical).
c Obesity defined by ICD-9 diagnostic code.
Nearly half of the controls had a musculoskeletal or mechanical explanation for their clinical manifestations, such as compression fracture, herniated disc, or spinal stenosis. The final diagnoses in the remainder of controls were metastatic or primary malignancy (15%), myelopathy or myelitis (12%), cerebrovascular accident (4%), and unknown (15%). Five control patients (6%) had vertebral osteomyelitis/discitis.
Nontraumatic back pain was documented in 58% of cases vs 33% of controls; overall, back and/or neck pain was a component of the chief complaint in most cases on admission (92% of cases vs 61% of controls, P < .001; data not shown). Other symptoms and signs related to potential spinal cord impingement were seen with similar frequencies and of similar durations among cases and controls (Table 2). Although fairly common, focal neurologic deficits were seen with similar frequencies in both groups. Fever (self-reported or measured temperature ≥100.4°F) and/or rigor were observed in 62.4% of cases vs 13.6% of controls (P < .001). Only one third of cases manifested the classic clinical triad of back pain, fever, and neurologic deficit, as did 6% of controls.
Table 2.
Clinical Manifestations and Laboratory Results
| Case (n = 162) | Control (n = 88) | P Valuea | |
|---|---|---|---|
| n (%) or Median (Interquartile Range) | n (%) or Median (Interquartile Range) | ||
| Back Pain | |||
| None | 54 (33.3%) | 53 (60.2%) | <.001 |
| Nontraumatic | 94 (58.0%) | 29 (33.0%) | |
| Traumatic | 14 (8.6%) | 6 (6.8%) | |
| Duration of back pain in days | 7 (3–21) | 5 (2–14) | .18 |
| Neck pain | 38 (23.5%) | 21 (23.9%) | 1.00 |
| Duration of neck pain in days | 6 (2–2) | 5 (1–14) | .83 |
| Paresthesia | 55 (34.0%) | 39 (44.3%) | .13 |
| Duration of paresthesia in days | 4 (1–14) | 3 (1–14) | .63 |
| Radicular pain | 42 (25.9%) | 13 (14.8%) | .05 |
| Focal neurologic deficit | 68 (42.0%) | 45 (51.4%) | .18 |
| Duration of focal neurologic deficit | 4 (1–7) | 2 (1–14) | .59 |
| Bowel incontinence | 10 (6.2%) | 5 (5.7%) | 1.00 |
| Bladder incontinence | 15 (9.3%) | 11 (12.5% | .52 |
| Urinary retention | 20 (12.4%) | 16 (18.2%) | .26 |
| Fever and/or rigor | 101 (62.4%) | 12 (13.6%) | <.001 |
| Duration of fever | 2 (1–3) | 2 (1–3) | .91 |
| Acute mental status abnormalities | 41 (25.3%) | 17 (19.3%) | .35 |
| Noncontiguous Coinfections | |||
| Bacteremia | 103 (63.6%) | 7 (8.0%) | <.001 |
| Pneumoniab | 20 (12.4%) | 3 (3.4%) | .02 |
| Genitourinary | 21 (13.0%) | 6 (6.8%) | .20 |
| Osteomyelitis (remote) | 17 (10.5%) | 1 (1.1%) | .004 |
| Infective endocarditis | 12 (7.4%) | 1 (1.1%) | .04 |
| Cellulitis | 10 (6.2%) | 1 (1.1%) | .10 |
| Hepatitis C | 19 (11.7%) | 3 (3.4%) | .03 |
| Soft tissue/foreign body abscess/infection | 28 (17.3%) | 3 (3.4%) | .001 |
| Other Soft Tissue Coinfection | |||
| Noncontiguous Coinfection Categoryc | |||
| None | 75 (46.3%) | 74 (84.1%) | <.001 |
| 1 of above | 57 (35.2%) | 11 (12.5%) | |
| >1 | 30 (18.5%) | 3 (3.4%) | |
| Laboratory Values | |||
| International normalized ratio >1.1:1 | 57/124 (46.0%) | 19/64 (29.7%) | .04d |
| White blood cells >11 000 cells/L | 108/162 (66.7%) | 35/87 (40.2%) | <.001e |
| Neutrophils >76% | 102/161 (63.4%) | 38/75 (50.7%) | .09d |
| Erythrocyte sedimentation rate >50 mm/hr | 93/118 (78.8%) | 7/27 (25.9%) | <.001f |
| C-reactive protein >3 mg/L | 82/101 (81.2%) | 7/20 (35.0%) | <.001f |
| Serum creatinine >1.2 mg/dL | 58/162 (35.8%) | 14/87 (16.1%) | .001e |
| Lactate >2.2 mmol/L | 14/64 (21.9%) | 3/14 (21.4%) | 1.00d |
| Hemoglobin A1C >6.5% | 5/11 (45.5%) | 6/7 (85.7%) | .15f |
a Fisher's exact test. The null hypothesis for all tests is that means or proportions are equal in cases and controls.
b Includes asymptomatic/x-ray diagnosis.
c Excluding bacteremia.
d Small effect size (≤0.20).
e Medium effect size (>0.20 to ≤0.60).
f Large effect size (>0.6).
Noncontiguous coinfections were documented in 53.7% of cases; these included pneumonia (12.4%), distant osteomyelitis (10.5%), infective endocarditis (7.4%), and soft tissue or foreign body site (17.3%). Cases were significantly more likely than controls to harbor 1 or more of these coinfections; approximately 1 in 5 cases had more than 1 coinfection (Table 2). White blood cell counts in cases were significantly higher than those in controls (11.6/liter vs 9.5/liter, P < .001). When available for comparison, inflammatory markers (ie, erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) were markedly elevated in cases compared with controls (P < .001 for each; Table 2).
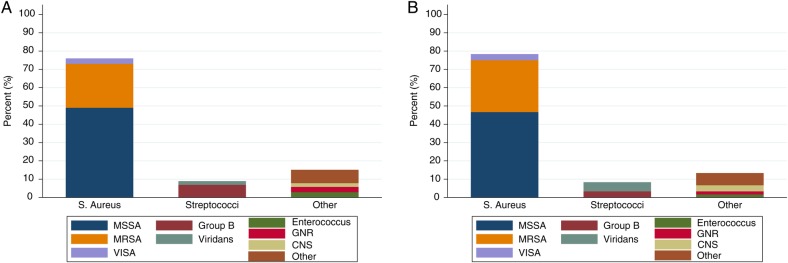
Blood cultures were positive in 63.6% of cases (Table 2); more than three quarters of these grew Staphylococcus aureus. Approximately two-thirds of the S aureus blood isolates were identified as methicillin-susceptible S aureus (MSSA); methicillin-resistant S aureus (MRSA) accounted for 31% of the remainder; and vancomycin intermediate S aureus (VISA) accounted for 5% (Figure 2). The relative proportions of SEA isolates generally mirrored those of blood isolates, albeit abscess specimens were obtained in only 95 (59%) cases. Streptococci comprised the majority of the non-S aureus blood and SEA isolates (Figure 2). Overall, 90% of blood and SEA isolates were Gram-positive cocci. For 94 cases in which both blood and SEA cultures were obtained, 57% demonstrated concordant results (including 18% with no growth in both); 18% had bacteremia with negative abscess cultures; and 22% had positive abscess cultures in the absence of bacteremia. Two cases had different organisms isolated from blood and abscess: in 1 case, Veillonella species were isolated from blood and viridans streptococci from the SEA; in the other, Mycobacterium abscessus was isolated from blood and MSSA from the abscess.
Figure 2.
Microbiologic isolates from A) blood and B) spinal epidural abscesses. Abbreviations: CNS, coagulase-negative staphylococci; GNR, Gram-negative rods; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S aureus; VISA, vancomycin intermediate S aureus.
The majority of SEA (n = 91, 56.2%) was located in the lumbar spine; the remainder of cases involved all portions of the vertebral spine in similar proportions without significant differences among anatomical locations (Table 3). Most cases affected a single anatomical level of the vertebral spine; 149 cases (92%) involved either 1 or 2 levels. “Skip” lesions involving more than 1 spinal column level occurred in 9.8% of cases. The largest proportion of cases (n = 77, 47.5%) involved the ventral epidural space (Table 3). Anatomically contiguous or proximate coinfections were common among cases: vertebral osteomyelitis was present in 97 (59.9%); discitis in 95 (58.6%); paraspinal or sacral abscesses in 87 (53.7%); and psoas abscess in 34 (21%). Approximately 70% of cases had more than 1 contiguous coinfection (Table 3).
Table 3.
Spinal Epidural Abscess Lesion Characteristics Among Cases (n = 162)
| n (%) | |
|---|---|
| Vertebral Location | |
| Cervical | 42 (25.9%) |
| Thoracic | 55 (34.0%) |
| Lumbar | 91 (56.2%) |
| Sacral | 47 (29.0%) |
| Not available | 5 (3.1%) |
| Level Count | |
| 0/Not available | 5 (3.1%) |
| 1 | 88 (54.3%) |
| 2 | 61 (37.7%) |
| 3 | 7 (4.3%) |
| 4 | 1 (0.6%) |
| Skip lesion | 16 (9.8%) |
| Orientation | |
| Dorsal | 43 (26.4%) |
| Ventral | 77 (47.5%) |
| Both | 22 (13.5%) |
| Not available | 20 (12.3%) |
| Contiguous Coinfections | |
| Vertebral osteomyelitis | 97 (59.9%) |
| Discitis | 95 (58.6%) |
| Paraspinal | 85 (52.5%) |
| Psoas | 34 (21.0%) |
| Sacral | 2 (1.2%) |
| Other soft tissue infection | 11 (6.8%) |
| Other | 23 (14.2%) |
| >1 of above | 113 (69.8%) |
Empiric antimicrobials were deployed in 96.3% of cases and in 30.7% of controls (Table 4). More than three quarters (n = 126, 77.8%) of cases received parenteral vancomycin as a component of their empiric regimen. This agent was most frequently used in combination with either piperacillin/tazobactam (n = 49, 30.3%) or ceftriaxone (n = 41, 25.3%). The initial, empiric antimicrobial therapy was judged “appropriate” by clinical abstractors, based on eventual microbiologic isolates or on most likely pathogens, in 85.2% of cases who received empiric therapy (data not shown). Levofloxacin was the most commonly used antimicrobial agent in cases in which empiric therapy was deemed to be “inappropriate”.
Table 4.
Treatments and Outcomes
| Cases (n = 162) | Control (n = 88) | P Valuea | |
|---|---|---|---|
| n (%) | n (%) | ||
| Preculture Antimicrobials | |||
| None | 6 (3.7%) | 61 (69.3%) | <.001 |
| Vancomycin | 126 (77.8%) | 16 (18.2%) | <.001 |
| Piperacillin/Tazobactam | 49 (30.3%) | 8 (9.1%) | <.001 |
| Carbapenem | 5 (3.1%) | 0 (0.0%) | .17 |
| Cefepime | 10 (6.2%) | 1 (1.1%) | .10 |
| Ceftriaxone | 41 (25.3%) | 4 (4.6% | <.001 |
| Other | 49 (30.3%) | 13 (14.8%) | .009 |
| Time to empiric antimicrobial in hours (median, IQR) | 11.1 (6.0–26.3) | 9.1 (3.7–22.4) | .40 |
| Treatmentsb | |||
| None | 1 (0.6%) | 53 (60.2%) | <.001 |
| Antibiotics only | 64 (39.5%) | 11 (12.5%) | |
| Surgery only | 1 (0.6%) | 17 (19.3%) | |
| Antibiotics and surgery | 96 (59.3%) | 7 (8.0%) | |
| Discharge Disposition | |||
| Death | 13 (8.0%) | 5 (5.7%) | .09 |
| Home | 47 (29.0%) | 39 (44.3%) | |
| Skilled nursing/long-term care facility | 99 (61.1%) | 42 (47.7%) | |
| Against medical advice | 3 (1.9%) | 2 (2.3%) | |
Abbreviations: IQR, interquartile range; IR, interventional radiology.
a Kruskal-Wallis equality of populations rank test (continuous) or Fisher's exact (categorical) test. The null hypothesis for all tests is that means or proportions are equal in cases and controls.
b Surgery consisting of 1 or more of the following: neurosurgical debridement/drainage; IR-guided needle aspiration; spinal fusion; disc replacement; laminectomy; discectomy; foraminotomy.
Fifty-nine percent of cases (n = 96) were treated with a combination of antimicrobials and an invasive modality; of the latter, 61% underwent neurosurgical drainage and debridement of the SEA (Table 4). However, 39.5% of all cases received antimicrobials as their only form of therapy. Among those with a neurosurgical procedure other than incision and drainage, laminectomy accounted for 45% of these interventions (Table 4). Of patients who survived to discharge, hospital length of stay was significantly greater in cases compared with controls (13 vs 6 days, P < .001; data not shown), and there were no significant changes in the difference over the time frame of the study. Cases were associated with a slightly but not significantly higher hospital mortality rate (8.0% vs 5.7%, P = .61). Among those who survived and were not discharged against medical advice, cases were significantly more likely to be discharged to a skilled nursing or long-term care facility versus home (67.8% vs 51.9%, P = .02; Table 4).
Cases were as likely as controls to be discharged with a neurologic deficit (39.5% vs 44.3%, P = .50; data not shown). Approximately 60% of all patients had 30-day follow-up visits, and many had persistent neurologic symptoms or signs at this time. Twelve percent of cases experienced irreversible outcomes of paraplegia or quadriplegia, which was not statistically different than controls.
DISCUSSION
This retrospective, case-control study of SEA over the past decade at a high-volume, tertiary care, academic medical center appears to represent the largest study of such patients from a single institution. Kim et al [7] recently reported a retrospective analysis of 355 SEA cases; however, these were derived over 2 decades from 2, large, tertiary academic medical centers, and the authors did not include a control group with other spinal pathologies. Based on the large volume of cases accrued over the most recent past decade and compared against a concurrent control group in our study, we are able to assess potential factors that might inform early recognition of SEA at the time of admission.
Incidence rates appear to have increased over the second half of the 20th Century, from 0.5 to approximately 2 per 10 000 hospital admissions [1, 8–10]. Although the baseline rate of 2.5 per 10 000 admissions in 2005 in this study is consistent with that in the contemporary literature, our data demonstrate a rise in SEA incidence of more than 200% from baseline to 8 per 10 000 admissions during the past decade at this institution (Figure 1), confirming [11] and surpassing most recent estimates. Because the rate of SEA detected at the earliest clinical stage did not change during this study period, the marked incidence increase is unlikely to be explained by surveillance bias at our institution [12]. Various hypotheses have been advanced to explain the increasing incidence of SEA, most notably the expansion of a comorbidly ill, aging population, and procedures and behaviors, such as injection drug use, predisposing to bacteremia [1, 3, 4, 6, 10, 11].
For these data, we considered both confirmed and probable cases as a single entity after a sensitivity analysis demonstrated that associations of epidemiologic and clinical characteristics between cases and controls remained unchanged by the inclusion or omission of probable cases. The only exception involved chronic kidney disease in which the removal of probable cases weakened a statistically borderline association with SEA. In addition, we believed that the high frequency of cases who received antimicrobials proximate to admission may have unfavorably impacted the yield of microbial isolation and hence falsely inflated the probable (vs confirmed) case category.
The predominance of males (61.7%) and those over the age of 50 years (72.8%) among SEA cases in this study is consistent with findings reported in the extant literature [4, 5, 7, 13, 14]. Our data demonstrate that 23.5% of cases occurred in patients >70 years of age, and although this is consistent with previous studies [7, 15], 34% of control patients with noninfectious spinal diseases were also in this age group, calling into question the discriminatory utility of age in this population (Table 1). Nevertheless, several epidemiologic features did distinguish cases from controls. Cases were significantly more likely to have predisposing conditions of alcohol abuse, injection drug use, or obesity (Table 1). Although the former 2 findings are well described risk factors [3–5, 9, 14], obesity has not previously been shown to predispose to SEA. Because obesity has been demonstrated to confer risk in several other infectious diseases [16, 17], it may impact SEA through a similar, yet unknown mechanism. Diabetes mellitus, often cited as an important risk factor for SEA in uncontrolled series [1–5, 7, 14, 18], was highly prevalent in both cases and controls in this study with no statistical difference observed.
More than half of our cases sought healthcare for symptoms at least once in the 30 days before admission. The majority of these received antimicrobial therapy, presumably for a suspected infection at some site. These episodes represent missed opportunities at recognizing SEA. Such missed opportunities have been documented to occur in 30%–57% of cases in previous studies [2, 3]. The nonspecific nature, inconsistent character, and variable pace of progression of the clinical manifestations of SEA contribute to the high rate of delayed diagnosis and affirm the need for clinical predictors to enhance early recognition of this entity [1, 11].
Aside from nontraumatic back pain and radicular pain, the presence and duration of other neurologic symptoms and signs did not distinguish SEA cases from controls in this study (Table 2). In particular, the presence of focal neurologic deficits, commonly regarded as a prevalent manifestation of SEA [4, 9, 10, 13, 18], was not significantly different between the groups, suggesting the lack of diagnostic reliability of neurologic symptoms and signs in this disorder [2, 11, 18]. We found fever and/or rigor to be significantly more likely in cases, consistent with the 33%–67% frequency reported elsewhere [3–5, 9–11, 13, 14, 18, 19]. In addition, only 33% of our cases exhibited the classic clinical triad of fever, back pain, and neurologic deficit; others have also reported this to be of relatively low prevalence [2, 11].
Cases in our study were significantly more likely than controls to harbor infection in at least 1 noncontiguous site (Table 2). Infections such as pneumonia, distant osteomyelitis, infective endocarditis, and soft tissue abscess or foreign body-site infection would potentially be evident at initial clinical presentation and could inform the early recognition of SEA in the presence of other predictive factors. These findings, in concert with the significance of known bacteremia as a predisposition to SEA, support the hypothesis that any potential source of bacteremia may portend risk [1, 4, 11, 20].
Leukocytosis and elevated ESR and CRP levels have been shown to be present in most patients with SEA reported in case series [4, 7, 11, 18]. We have confirmed this in our case control study using high threshold levels for ESR and CRP elevations (Table 2). The incorporation of elevated levels of inflammatory markers as a nodal point in a clinical decision guideline for the early recognition of SEA has been shown to reduce diagnostic delays [21]. Unfortunately, levels of inflammatory markers in this retrospective study were not consistently available, especially in the control group, thus limiting our ability to rigorously assess their relative predictive value.
Both the anatomic (Table 3) and the microbiologic characteristics (Figure 2) of our SEA cases demonstrate general concordance with those in other series, suggesting no significant evolution in these attributes over the past several decades [3–5, 7, 9–11, 14, 18, 22–25]. The vast majority of our cases were caused by Gram-positive cocci, largely S aureus; MRSA was isolated in a significant minority. Thus, the empiric use of vancomycin in patients with potential SEA is appropriate. The low prevalence of SEA caused by Gram-negative organisms in our study may reflect the relatively small number of cases receiving hemodialysis or who had experienced preceding spinal intervention and may therefore have increased risk for infection due to these pathogens [1, 26].
To our knowledge, this study represents the largest SEA case series from a single institution. Because we collected a large number of cases over a contemporary time frame with a concomitant control group, we are able to assess potential predisposing factors and clinical associations in a more rigorous manner than in the extant literature, making our findings potentially more generalizable. To make this case control study as clinically applicable as possible, we chose to restrict our study to individuals in whom SEA was a diagnostic consideration either upon presentation or during their hospital course. Those cases in whom SEA was ruled out became the control group. By restricting the study base to such patients, we hoped to reduce the risk of residual confounding and selection bias [27]. Although it is possible that our sampling strategy may have overlooked highly occult SEA cases, it seems more likely that these would have declared themselves at some point in our study time frame, and that these patients would have been cared for at our institution because it is the only tertiary referral center in the region.
In addition to the potential limitations inherent in our control group selection, our study has several other limitations. Given its retrospective nature, a large proportion of cases and controls lacked inflammatory markers that showed promise as discriminating factors. Furthermore, correlations between treatment and outcomes cannot be assumed to be causal and thus serve descriptive purposes only.
CONCLUSIONS
Because of the rising incidence of SEA, the insidious nature and nonspecific initial clinical manifestations of the infection, and the established, direct correlation between diagnostic delays and deleterious neurologic consequences [1, 2, 5, 28], early recognition is essential to optimizing patient outcomes. Our data suggest that patients presenting with new or acute worsening of chronic back or neck pain who have established risk factors for bacteremia or fever or have sought healthcare or used antimicrobials within 30 days, are obese, or harbor a noncontiguous source of infection be considered for immediate spinal imaging to rule out SEA. Significant elevations of inflammatory markers may also serve to stratify those patients who would benefit from immediate spinal imaging. Future work will involve applying our data to the development and prospective evaluation of a clinical predictive model for risk stratification towards the identification of SEA at the earliest opportunity.
Acknowledgments
We thank Reva Kleppel for administrative support and Dr. Dan Skiest for thoughtful discussions and careful review of the manuscript.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Darouiche RO. Spinal epidural abscess. N Engl J Med 2006; 355:2012–20. [DOI] [PubMed] [Google Scholar]
- 2.Davis DP, Wold RM, Patel RJ et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med 2004; 26:285–91. [DOI] [PubMed] [Google Scholar]
- 3.Nussbaum ES, Rigamonti D, Standiford H et al. Spinal epidural abscess: a report of 40 cases and review. Surg Neurol 1992; 38:225–31. [DOI] [PubMed] [Google Scholar]
- 4.Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev 2000; 23:175–204. [DOI] [PubMed] [Google Scholar]
- 5.Shweikeh F, Saeed K, Bukavina L et al. An institutional series and contemporary review of bacterial spinal epidural abscess: current status and future directions. Neurosurg Focus 2014; 37:E9. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamohan P, Berger JR. Spinal epidural abscess. Curr Infect Dis Rep 2014; 16:436. [DOI] [PubMed] [Google Scholar]
- 7.Kim SD, Melikian R, Ju KL et al. Independent predictors of failure of nonoperative management of spinal epidural abscesses. Spine J 2014; 14:1673–9. [DOI] [PubMed] [Google Scholar]
- 8.Baker AS, Ojemann RG, Swartz MN, Richardson EP. Spinal epidural abscess. N Engl J Med 1975; 293:463–8. [DOI] [PubMed] [Google Scholar]
- 9.Darouiche RO, Hamill RJ, Greenberg SB et al. Bacterial spinal epidural abscess: review of 43 cases and literature survey. Medicine (Baltimore) 1992; 71:369–85. [PubMed] [Google Scholar]
- 10.Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurg 1990; 27:177–84. [PubMed] [Google Scholar]
- 11.Rigamonti D, Liem L, Sampath P et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol 1999; 52:189–97. [DOI] [PubMed] [Google Scholar]
- 12.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics . Gaithersburg, MD; Aspen Publishers, Inc.; 2000. [Google Scholar]
- 13.Curry WT, Hoh BL, Amin-Hanjani S, Eskandar EN. Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol 2005; 63:364–71. [DOI] [PubMed] [Google Scholar]
- 14.Patel AR, Alton TB, Bransford RJ et al. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J 2014; 14:326–30. [DOI] [PubMed] [Google Scholar]
- 15.Adogwa O, Karikari IO, Carr KR et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15-year institutional perspective and review of the literature. J Neurosurg Spine 2014; 20:344–9. [DOI] [PubMed] [Google Scholar]
- 16.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis 2006; 6:438–46. [DOI] [PubMed] [Google Scholar]
- 17.Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis 2011; 53:413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang HJ, Lin HJ, Liu YC, Li CM. Spinal epidural abscess—Experience with 46 patients and evaluation of prognostic factors. J Infect 2002; 45:76–81. [DOI] [PubMed] [Google Scholar]
- 19.Joshi SM, Hatfield RH, Martin J, Taylor W. Spinal epidural abscess: a diagnostic challenge. Br J Neurosurg 2003; 17:160–3. [DOI] [PubMed] [Google Scholar]
- 20.Danner RL, Hartman BJ. Update of spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis 1987; 9:265–74. [DOI] [PubMed] [Google Scholar]
- 21.Davis DP, Salazar A, Chan TC, Vilke GM. Prospective evaluation of a clinical decision guideline to diagnose spinal epidural abscess in patients who present to the emergency department with spine pain. J Neurosurg Spine 2011; 14:765–70. [DOI] [PubMed] [Google Scholar]
- 22.Arko L IV, Quach E, Nguyen V et al. Medical and surgical management of spinal epidural abscess: a systematic review. Neurosurg Focus 2014; 37:E4. [DOI] [PubMed] [Google Scholar]
- 23.Khanna RK, Malik GM, Rock JP, Rosenblum ML. Spinal epidural abscess: evaluation of factors influencing outcome. Neurosurgery 1996; 39:958–64. [DOI] [PubMed] [Google Scholar]
- 24.Chen SH, Chang WN, Lu CH et al. The clinical characteristics, therapeutic outcome, and prognostic factors of non-tuberculous bacterial spinal epidural abscess in adults: a hospital-based study. Acta Neurol Taiwan 2011; 20:107–13. [PubMed] [Google Scholar]
- 25.Soehle M, Wallenfang T. Spinal epidural abscess: clinical manifestations, prognostic factors, and outcomes. Neurosurg 2002; 51:79–87. [DOI] [PubMed] [Google Scholar]
- 26.Lewis SS, Sexton DJ. Metastatic complications of bloodstream infections in hemodialysis patient. Semin Dialysis 2013; 26:47–53. [DOI] [PubMed] [Google Scholar]
- 27.Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case-control studies. I. Principles. Am J Epidemiol 1992; 9:1019–28. [DOI] [PubMed] [Google Scholar]
- 28.Tuchman A, Pham M, Hsieh PC. The indications and timing for operative management of spinal epidural abscess: literature review and treatment algorithm. Neurosurg Focus 2014; 37:E8. [DOI] [PubMed] [Google Scholar]