Supplemental Digital Content is Available in the Text.
Key Words: HIV, testing, awareness, antiretroviral therapy, viral load, Africa
Abstract
Introduction:
Identifying gaps in HIV testing and treatment is essential to design specific strategies targeting those not accessing HIV services. We assessed the prevalence and factors associated with being HIV untested, unaware, untreated, and virally unsuppressed in KwaZulu-Natal, South Africa.
Methods:
Cross-sectional population-based survey. People aged 15–59 years were eligible. Interviews, HIV testing, and blood collection for antiretroviral drug presence test, CD4, and viral load were done at the participants' home.
Results:
Of the 5649 individuals included, 81.4% (95% CI: 79.8 to 82.9) had previously been tested. HIV prevalence was 25.2%. HIV-positivity awareness rate was 75.2% (95% CI: 72.9 to 77.4). Of all unaware, 73.3% of people were aged <35 years and 68.7% were women. Antiretroviral therapy coverage was 75.0% (95% CI: 72.0 to 77.8) among those eligible for treatment (CD4 < 350, PMTCT-B) and 53.1% (95% CI: 50.4 to 55.7) among all HIV-positive individuals. Viral load was <1000 copies per milliliter in 57.1% of all HIV-positive individuals. Although 66.3% and 71.7% of people with viral load ≥1000 copies per milliliter were people aged <35 years and women respectively, men had 4.4, 1.8, 1.6, and 1.7 times the odds of being untested, unaware, untreated, and virally unsuppressed. In addition, people with more than 1 sexual partner had 1.3, 2.2, and 1.9 times the odds of being untested, unaware, and untreated.
Conclusions:
The majority of HIV-positive people unaware of their status, untreated, and virally unsuppressed were individuals aged <35 years and women. However, men were disproportionately untested, unaware HIV positivity, untreated, and virally unsuppressed. In this context, HIV testing and treatment should be prioritized to target young people and women, whereas novel strategies are necessary to reach men.
INTRODUCTION
The HIV epidemic does not affect populations homogeneously. Assessing the prevalence of HIV testing, antiretroviral therapy (ART), and viral suppression in the population and identifying specific groups with higher risk of being untested, unaware of their HIV-positive status, untreated and with a detectable viral load is essential to set priorities and design specific strategies targeting those not accessing HIV services. KwaZulu-Natal (KZN) is the province with the highest HIV prevalence and incidence in South Africa and high investment in terms of HIV testing and treatment. In 2012, HIV prevalence in this province was 27.9% in the age group 15–49 years1 and 37% of HIV-positive people had initiated ART.2
Although women have higher HIV prevalence and ART coverage,1 men are less likely to start ART and have lower survival rates.3–5 However, little is known about other socio-demographic and behavioral characteristics that may be linked to being untreated. In addition, if specific groups are less likely to test and to have an undetectable viral load, this could also have consequences for interventions such as test and treat. Population-based surveys are helpful to identify gaps in HIV testing and treatment, and the demographic characteristics of population groups most at risk of not being diagnosed or treated. In addition, measurement of HIV viral load in these surveys allows the identification of demographic characteristics of groups with high potential for transmission of HIV.6,7
We assessed the prevalence of HIV testing, HIV-positivity awareness, ART and viral suppression, and factors associated with being HIV untested, unaware, untreated, and virally unsuppressed in Mbongolwane and Eshowe, KZN, South Africa.
METHODS
Design and Population
This was a cross-sectional population-based survey. A 2-stage stratified cluster probability sampling was used for the selection of households. In total, 125 clusters of 25 households each were selected from 14 administrative units called Wards. The number of clusters per Ward was selected with probability proportional to population aged 15–59 years according to the 2011 Census.8 Google Earth maps from 2011 with exhaustive identification of the households were used to sample the households to be visited by choosing randomly the first household and then sequentially the closest to the first/previous one. Field staff used Global Positioning System (GPS) receivers to find the geographic coordinates of each household. People aged 15–59 years living in Mbongolwane and Eshowe Health Service Areas, uMlalazi Municipality, in KZN province were eligible for enrolment in the study. Those who signed a written informed consent were included.
Study Setting
Mbongolwane is a rural area and Eshowe the main town of the municipality. According to the 2011 Census,8 61,179 people aged 15–59 were living in 25,106 households in the area covered by the survey. The KZN Department of Health (DOH) over the last 10 years has conducted an HIV program in the province that includes HIV testing, HIV care, and ART. Since 2011, large-scale HIV-testing activities and decentralization of ART initiation, including nurse-initiated and managed ART (NIMART), have been implemented in the province. Médecins Sans Frontières (MSF) has supported the DOH HIV and TB programs in the area of Mbongolwane and Eshowe, with the aim of decreasing HIV and TB incidence, morbidity, and mortality. This support has included prevention activities (condom distribution, voluntary medical male circumcision, and health promotion activities), large-scale community-based HIV counseling and testing, implementation of point-of-care CD4 testing, and training and mentoring of health staff in facilities in support to decentralized ART initiation and TB/HIV integration. The survey was conducted after 2 years of gradual implementation of Médecins Sans Frontières support interventions in the area; full-scale implementation was only reached in 2014. No previous survey had been done in these communities.
Procedures
The survey was conducted between July and October 2013. Before the start of the survey, activities were organized to inform the community (meetings with DOH authorities, medical staff, and traditional leaders, distribution of leaflets, and home visits in the area that were going to be surveyed in the following days). Households were visited up to 3 times using different times of the day or days of the week in case nobody was at home or an eligible individual was absent. In addition, households with eligible individuals not interviewed were revisited once more at early/late hours of the day or during the weekend at the end of the survey. The head of the household was asked to list the members of the family/household, the age of each of them, and whether the person lived most of the time in that household or not. Individuals aged 15–59 years and living in the household were eligible for the study and were interviewed individually. Those who signed the informed consent were included. Face-to-face interviewer-administered questionnaires were used to collect information on socio-demographics, sexual history, and ART. HIV rapid tests were done on site by certified lay counselors using Determine Rapid HIV-1/2 Antibody as a screening test, followed if positive by Uni-Gold Rapid HIV test kit for confirmation. The results of the test were given to the participant. HIV testing on site was optional and anonymous tests done in the laboratory were also proposed to the participants. In addition, venous blood specimens were collected by nurses at the participants' home for antiretroviral drug presence test, CD4, and viral load. Participants were informed that the results of these tests would be available at the closest health centre and were given a letter to collect them. HIV positivity was determined using the on-site rapid test result confirmed by ELISA at the laboratory, or only the laboratory result for the participants who chose anonymous testing. Dry blood spot (DBS) were prepared in the laboratory using whole blood collected in EDTA tube to spot DBS Cards (Whatman 903). Each spot on the DBS card was spotted with 50 μL of whole blood. Qualitative testing for antiretroviral drug levels including nevirapine, efavirenz, and lopinavir was performed for all HIV-positive participants using DBS samples and a LC MS/MS qualitative assay for the drug presence determinations. This technique detects presence or absence of the drugs down to 0.2 μg/mL. Viral load was performed using a NucliSens EasyQ HIV-1 v2.0 assay from BioMerieux.
Ethics
Written informed consent was sought before the enrolment of participants. The protocol was approved by the University of Cape Town Human Research Ethics Committee (HREC), the Health Research Committee of the Health Research and Knowledge Management Unit of KZN DOH, and the “Comité de Protection de Personnes,” Paris, France.
Definitions
ART coverage: proportion of HIV-positive individuals on ART (as per blood test) among HIV-positive individuals eligible for ART (already on ART or in need of ART). In need of ART according to the National Guidelines at the time of the survey: individuals with CD4 below 350 cells per microliter and as per PMTCT option B (ART during pregnancy and breastfeeding period). Viral suppression: individuals HIV positive with viral load below 1000 copies per milliliter.
Outcomes
Untested (probability that a person had never been tested for HIV), unaware (probability that a person who was HIV positive was not aware that she/he was HIV-infected), untreated among all HIV positives (probability that a person who was HIV positive was not on ART as per blood test), untreated among HIV positives eligible for ART (probability that a person who was HIV positive and in need of ART according to the National Guidelines at the time of the survey was not on ART as per blood test), unsuppressed among all HIV positives (probability that a person who was HIV positive was not virally suppressed), and unsuppressed among individuals on ART (probability that a person who was on ART was not virally suppressed).
Predictors
Age (15–24, 25–34, 35–59 years), sex (women, men), marital status (married/living together, not married, separated/divorced/widowed), number of sexual partners in the year preceding the survey (0, 1, more than 1), level of education (no schooling, primary/secondary, tertiary), area of residence (rural, urban/farms), mobility (nonmigrant, migrant/visitor), and ART (positive, negative as per blood test).
Data Analyses
All statistical analyses were adjusted for clustering at the level of Ward and household. Descriptive analyses are presented here with 95% confidence intervals (CIs). Categorical variables were compared using proportional test. We used multivariable logistic regression models to identify factors associated with being HIV untested, unaware of being HIV positive, untreated with ART, and virally unsuppressed. Each model was adjusted for age, sex, marital status, number of sexual partners in the year preceding the survey, level of education, area of residence, and mobility. ART was included in the model exploring factors associated with being virally unsuppressed. Main ART coverage analysis was done as per the national guidelines at the time of the survey. In addition, ART coverage using other ART initiation strategies was also explored. Data were entered and checked using EpiData version 3.1 and analyzed using Stata 13 (Stata Corp., College Station, TX).
RESULTS
Survey Response Rates
In total, 2377 households were included out of 3566 visited (Fig. 1). Main reasons for not including a household were: vacant/destroyed/not a household (13.5%), no one at home (9.6%), and refusal (7.7%). Of the 6688 eligible individuals (59.9% women, 40.1% men), 5649 (84.5%) were included. The inclusion rate was 87.8% among women and 79.5% among men. All individuals included answered to the questionnaire and provided full blood samples for HIV biomarker tests.
FIGURE 1.
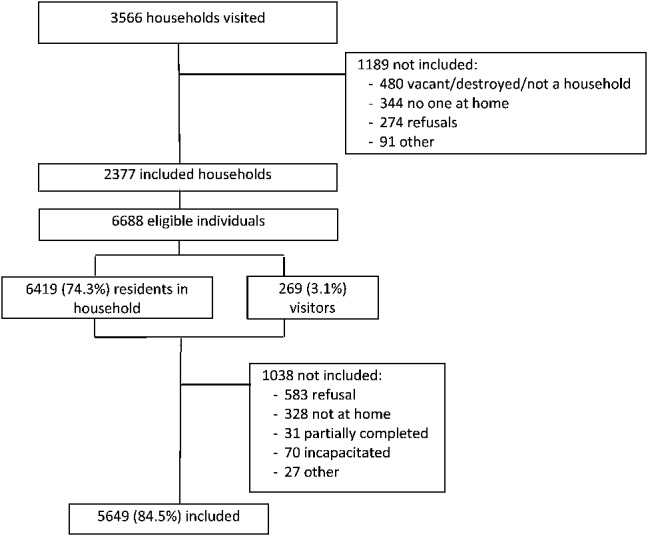
Flow chart of eligibility and inclusions.
Description of the Surveyed Population
The median age was 26 years (IQR: 19–40) and 3518 (62.3%) were women. Seventy-five percent of the participants had never been married, 89% had completed at least primary school, 36.3% were students, and 28.9% were unemployed. The majority of the participants lived in rural areas, 15% lived in urban or semiurban areas, and 2% in farms. Concerning the participants' mobility, 761 (13.5%) had moved their residence in the previous 10 years (migrants) and 246 (4.4%) were visitors.
HIV Testing and Positive HIV Status Awareness
The proportion of people who had an HIV test was 81.4% (95% CI: 79.8 to 82.9), higher in women compared with men: 88.4% (95%CI: 86.8 to 89.9) versus 69.8% (95% CI: 67.2 to 72.4). Individuals aged less than 35 years and men and accounted for 75.5% and 63.3% of the total individuals untested. HIV-testing prevalence among HIV-negative participants was 77.1% (95% CI: 75.2 to 78.8) compared with 94.2% (95% CI: 92.9 to 95.3) among HIV-positive individuals. Although the overall HIV prevalence was 25.2% [(95% CI: 23.6 to 26.9), 30.9% (95% CI: 29.0 to 32.9) in women and 15.9% (95% CI: 14.0 to 18.0) in men], the proportion of HIV-positive people among nontested participants was 7.8% (95% CI: 6.2 to 9.8) compared with 29.2% (95% CI: 27.5 to 30.9) among tested. HIV prevalence among nontested participants was lower than among tested in individuals aged 15–24 years [3.4% (95% CI: 2.2 to 5.3) versus 11.9% (95% CI: 10.5 to 13.5)] and in women and in men separately (data not shown). Participants having had an HIV test reported being tested a median of 3 times (IQR: 2–4) and 48.2% had their last test done in the previous 6 months. A majority of people were tested in the public sector, 78.6%, or by Médecins Sans Frontières, 18.9%.
Overall, 75.2% (95% CI: 72.9 to 77.4) of people with HIV were aware of their HIV-positive status. Individuals aged less than 35 years and women accounted for 73.3% and 68.7% of the total unaware. Young people and older men had the lowest prevalence of awareness: 56.4% in people aged 15–24 years, 56.2% in men aged 25–34 years, and 66.0% in men aged 55–59 years (see Fig.ure 1, Supplemental Digital Content, http://links.lww.com/QAI/A838).
Antiretroviral Treatment and Viral Suppression
ART coverage was 75.0% (95% CI: 72.0 to 77.8) according to the National Guidelines at the time of the survey, 64.3% (95% CI: 61.5 to 67.0) according to the 2013 WHO guidelines, and 53.1% (95% CI: 50.4 to 55.7) according to a test and treat strategy. Of the 247 participants eligible and not on ART, 161 (65.2%) were women and 164 (66.4%) were younger than 35 years. Those eligible according to the 2013 WHO guidelines or the test and treat strategy and not on treatment had similar age/sex profiles (Fig. 2). The median time since ART initiation for the participants on ART was 31.0 (IQR: 12.4–61.0) months. Of all patients on ART, 37.1% had initiated treatment between 2012 and 2013. ART coverage was lower in men than in women (63.9% versus 78.5%, P < 0.001) and among participants 15–24 years and 25–34 years compared with those older than 35 years (52.2%, 69.5%, and 84.3%; P < 0.001).
FIGURE 2.
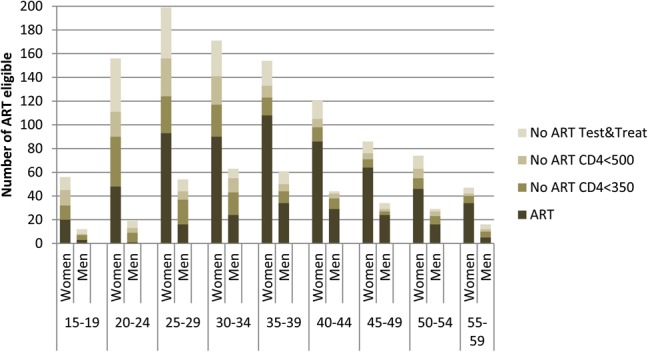
Number of HIV positives on ART and not on ART by gender and age group according to: national guidelines (CD4 < 350 and PMTCT B) at the time of the survey, 2013 WHO recommendations (CD4 < 500 and PMTCT B+), and test and treat strategy.
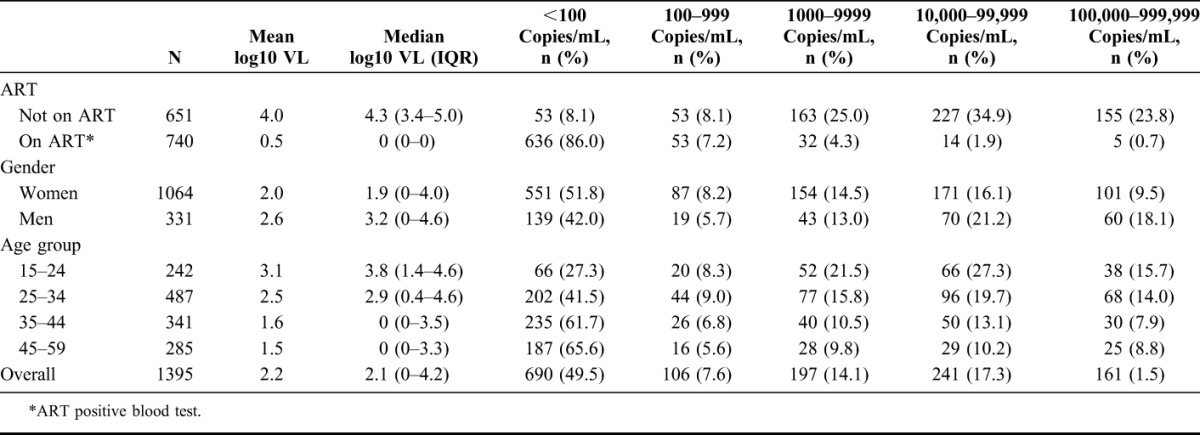
Of all HIV-positive individuals, 57.1% (796/1395) had a viral load of less than 1000 copies per milliliter (Table 1). Of all individuals with a viral load above 1000 copies per milliliter, 71.1% were women and 66.3% were aged less than 35 years. The proportion of HIV-positive participants with a viral load less than 1000 copies per milliliter increased with age, from 35.5% in the group aged 15–24 year to 50.5% in the group aged 25–34 years, and 69.7% in the group aged 35–59 years. Conversely, men and people under 35 years were the groups with the highest proportion of people with more than 100,000 copies per milliliter: 18.1% of men versus 9.5% of women (P < 0.001) and 14.5% of people aged less than 35 years versus 8.3% of those aged more than 35 years (P < 0.001). Nevertheless, in absolute numbers, the majority of participants with more than 100,000 copies per milliliter were women (62.7%, 101/161). Viral suppression was 93.1% among participants with antiretroviral drugs detected in blood. In this group, 2.6% of the individuals had more than 10,000 copies per milliliter; whereas among people not on treatment (ART blood test negative), this proportion reached 58.7%. Among participants who self-reported being on ART, 90.4% were virally suppressed. The viral load distribution in this group was: 518 (84.6%) <100 copies per milliliter, 35 (5.7%) 100–999 copies per milliliter, 27 (4.4%) 1000–9999 copies per milliliter, and 32 (5.2%) ≥10,000 copies per milliliter.
TABLE 1.
Viral Load Distribution in the HIV-Positive Population According to Age, Gender, and ART Intake
Factors Associated With Being Untested, Unaware, Untreated, and Virally Unsuppressed
Men, individuals younger than 35 years, and individuals with more than 1 sexual partner in the year preceding the survey had higher risk of being untested in the overall population and among HIV positives, a higher risk of being unaware of their HIV status, and untreated with antiretroviral drugs (Tables 2 and 3). Men had 4.4 times the odds of being untested and HIV-positive men had 1.8 and 1.6 times the odds of being unaware and untreated respectively compared with women. Individuals aged 15–24 years had 1.6 times the odds of being untested and HIV-positive individuals aged 15–24 years had 5.3 and 5.9 times the odds of being unaware and untreated, respectively, compared with those aged 35–59 years. Individuals with more than 1 sexual partner had 1.3 times the odds of being untested and HIV-positive individuals with more than 1 sexual partner had 2.2 and 1.9 times the odds of being unaware and untreated, respectively, compared to those with 1 sexual partner. People living in an urban area and people who were mobile did not have an increased risk of being untested, unaware, or untreated.
TABLE 2.
Factors Associated With Being Untested for HIV in the Overall Population and Unaware of HIV-Positive Status
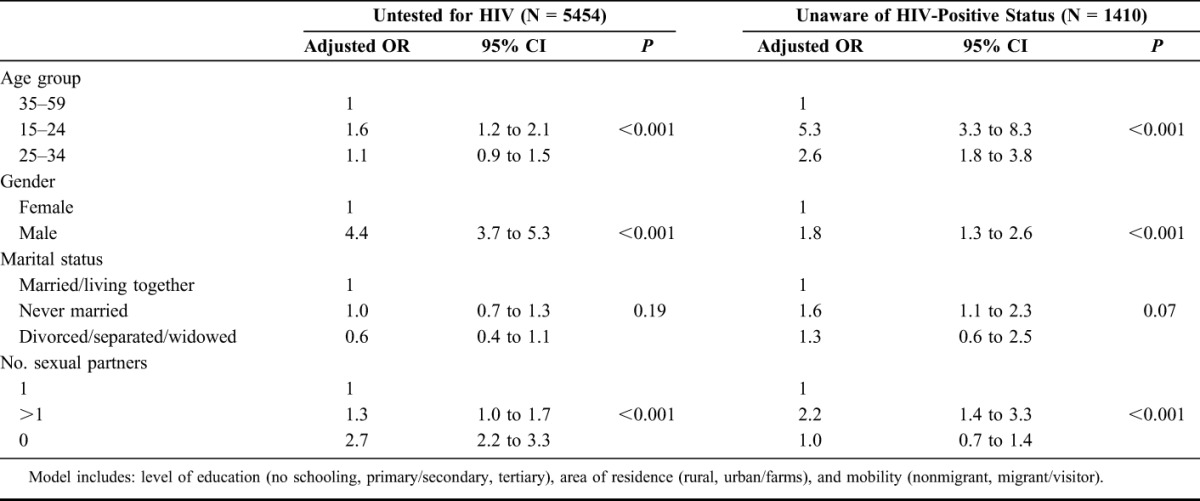
TABLE 3.
Factors Associated With Being Untreated and Virally Unsuppressed Among HIV-Positive People
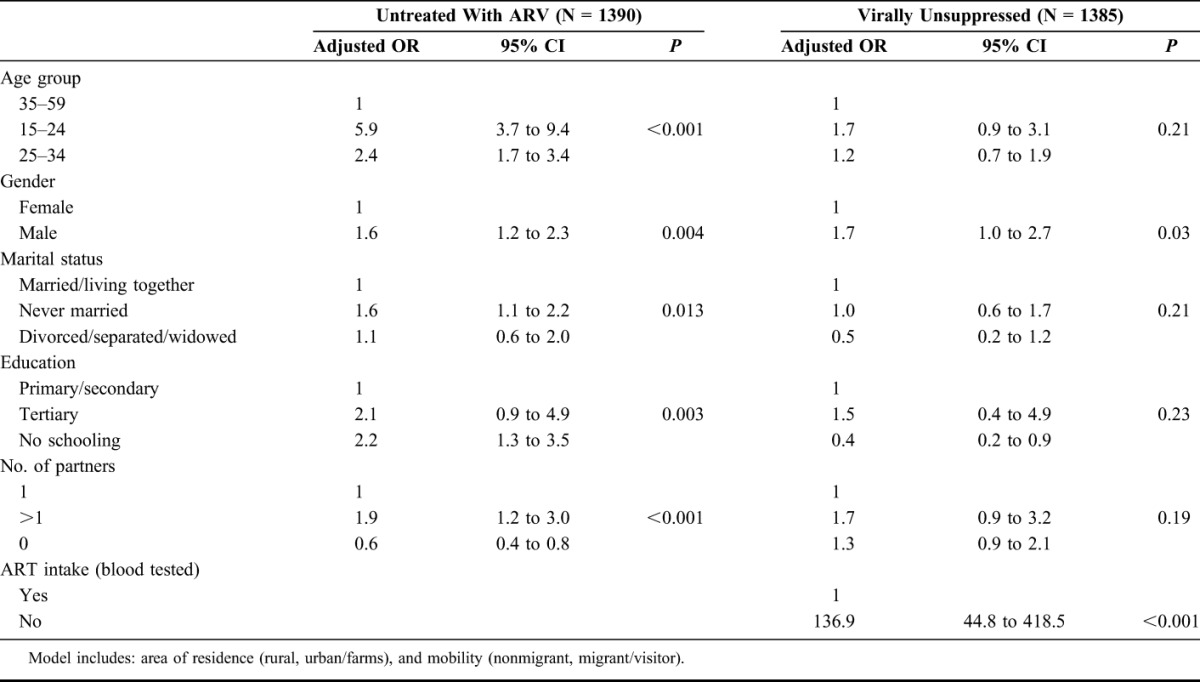
The same characteristics were found associated with being untreated among people in need of ART according to the National Guidelines at the time of the survey: men (aOR = 2.0, 95% CI: 1.4 to 2.8), individuals younger than 35 years (15–24 y aOR = 2.7, 95% CI: 1.8 to 4.1; 25–34 y aOR = 1.6, 95% CI: 1.1 to 2.3), and people with more than 1 sexual partner (aOR = 1.5, 95% CI: 1.0 to 2.3) had an increased risk of being untreated. The gender effect (men aOR = 1.7, 95% CI: 1.0 to 2.7) persisted in the risk of being virally unsuppressed after adjustment for socio-demographic characteristics and ART intake in a model including all HIV-positive individuals (Table 3). The model showed a nonstatistically significant increased odds of being virally unsuppressed for individuals younger than 35 years and people with more than 1 sexual partner. No factor was associated with being virally unsuppressed in a model including only people on ART.
DISCUSSION
This population survey identified that despite good overall testing and treatment coverage in this area, certain demographic subgroups remain at higher risk for being untested, untreated and virally unsuppressed, hence being at high risk for adverse health outcomes and having a high potential for transmitting HIV. Individuals aged less than 35 years and men accounted for most of the people untested (75.5% and 63.3% respectively). Individuals aged less than 35 years and women accounted for most of the HIV-positive people unaware (73.2% and 68.7% respectively), in need of treatment (66.4% and 65.2% respectively), and with a viral load above 1000 copies per milliliter (66.3% and 71.1% respectively). Potential reasons for this include lower access to testing and treatment in people aged less than 35 years and the higher HIV prevalence in women and the higher proportion of women in the population in this area (62.3%). In addition, people with more than 1 sexual partner had increased odds of being untested, unaware, and untreated.
These results highlight that the first priority is to increase access to testing and treatment for young people and women, and the need to adapt HIV testing strategies to better target men, as supported by other findings.9–11 Their particular needs (eg, fear, stigma-related issues, working hours, and location of services) should be taken into consideration because acceptability of HIV testing and reasons for refusal may differ by gender and age.12 Community strategies to increase HIV testing and linkage to care have been successful in other contexts.13 Other innovative strategies such as HIV self-testing or HIV testing and provision of ART at the workplace and in schools could benefit young people and men.14–16 These approaches have been found to be cost effective in modelling,17,18 and they should be further investigated and implemented for the benefit of individuals and public health.6,7,19 The provision of HIV services in the population focusing on specific groups at risk, but less likely to have been tested and treated, is extremely important to cover existing gaps and continue impacting the current epidemic. However, given that most of the HIV-positive people unaware of their status were women, a strategy of targeting men (at the expense of women) would be targeting the minority of persons needing care, and a balance must be struck with this in mind.
An interesting finding is that people nontested have a much lower prevalence of HIV than the general population. The proportion of people tested was higher among HIV-positive individuals compared with HIV-negative individuals. This suggests that there is a selection of people at higher risk for being HIV positive into testing. Although this is encouraging and expected, it suggests that blanket, population-based testing strategies may be poorly efficient with high investment and low detection of undiagnosed HIV people. This finding has implications for test and treat strategies.
ART coverage according to the National Guidelines at the time of the survey (CD4 eligibility at <350 or pregnant) was relatively high at 75% but represents only 53% coverage if a “test and treat” strategy was in place. In other African settings, ART coverage varied largely from 2%–8% in sites from Zimbabwe and Tanzania to 58%–68% in sites from Malawi, Uganda, or Kenya.20,21 Over half of the HIV-positive population had a viral load below 1000 copies per milliliter, a relatively high proportion in a population with high HIV prevalence. However, the cross-sectional design excludes deceased people who may be more likely to have had higher viral loads.5 This may partially explain differences from cohort studies, with lower viral suppression prevalence. In addition, although in this study viral suppression was high, 93%, in individuals with antiretroviral drugs detected in their blood, modeling work suggests that as the number of people on ART increase, people with treatment failure and/or defaulting ART will play an increasingly important role in transmission.22 We can expect that by increasing HIV testing, linkage to care and ART initiation, median viral load would decrease in the HIV-positive population13 and the proportion of virally suppressed individuals among those eligible for ART would increase.23 In addition to this, strategies to improve retention on ART and adherence to ART are needed.
The proportion of people not on ART and virally suppressed was relatively high, 8% or 16% depending on the threshold used for suppression (100 or 1000 copies per milliliter). HIV positivity of these individuals was confirmed using 2 different ELISA tests. Fourteen of the 106 patients with viral load below 1000 copies per milliliter and negative ART blood-test reported that they had been treated with ART in the past but stopped treatment. Excluding these patients, 14% of the patients not initiated on ART would be virally suppressed using a threshold of 1000 copies per milliliter. Of the 92 patients with viral load below 1000 and no history of ART use, 8, 17, and 67 patients had CD4 <350, 350–499 and ≥500, respectively. Repeating viral load testing on stored samples of patients with CD4 <500 found the same results. Similar findings have been reported in another setting in South Africa where 17.2% and 26.5% of people not on ART had viral loads less than 50 copies per milliliter and 1500 copies per milliliter, respectively.24 It is possible that a proportion of these patients virally suppressed belong to the group of HIV controllers, who may have different immunological and virological profiles.25
People with more than 1 sex partner were more likely to be untested, unaware of their HIV-positive status, and untreated and they are probably also more likely to be virally unsuppressed (although this association was not statistically significant). This adverse selection implies that there may be important limits to test and treat: the people we most want to start therapy and stay on it are the least likely to do so. This result has important implications for the prospects of Treatment as Prevention (TasP), and these parameters could be used to revise existing mathematical models as most of them do not account for the potential correlation between HIV risk behavior and likelihood of being on ART and virally suppressed.
This study has a number of limitations. The cross-sectional design prevented a view over time of the studied parameters and the survey, by definition, excluded deceased people. However, this study provides us with a picture of the people most at risk of being untested, untreated, and virally unsuppressed at a given time. In addition, the study was not designed to include people who may be frequent visitors to the area but who are not residents, unless they were present in the house the day of the survey. These groups may have specific risks or play a role in transmission though this should not impact the prevalence of HIV care and treatment observed in the population living in the area. Other types of studies are needed to describe HIV dynamics in migrants or nonresidents.26
This community survey helped to reveal gaps in HIV services provided in the area and identify who needs to be targeted for HIV testing and treatment. The majority of HIV-positive people unaware of their status, and untreated and virally unsuppressed were individuals aged less than 35 years and women. However, men were disproportionately untested, unaware of their HIV-positive status, untreated, and virally unsuppressed. In this context, HIV testing and treatment activities should be prioritized to target young people and women, and a right balance should be found between increasing access to test and treat for the bulk of people untested and untreated and specific strategies for those who are more difficult to reach.
ACKNOWLEDGMENTS
The authors thank the participants and the community of Mbongolwane and Eshowe for their collaboration. We are grateful to the Epicentre study field team for their work and the Médecins Sans Frontières field team for their support. Thanks to Madurai S and the rest of Global Clinical and Viral laboratories team for their collaboration and work performing part of the laboratory tests.
Footnotes
The Division of Clinical Pharmacology at the University of Cape Town is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Bethesda, MD, USA) under Award Number UM1 AI068634, UM1 AI068636, and UM1 AI106701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Presented as a part in the Conference on Retroviruses and Opportunistic Infections (CROI), February 23–26, 2015, Seattle, WA.
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Shisana O, Rehle T, Simbayi LC, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town, South Africa; HSRC Press, 2014. [Google Scholar]
- 2.Tanser F, Bärnighausen T, Grapsa E, et al. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4255272&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassett IV, Regan S, Chetty S, et al. Who starts antiretroviral therapy in Durban, South Africa?… Not everyone who should. AIDS. 2010;24(suppl 1):S37–S44. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3521614&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornell M, Schomaker M, Garone DB, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9:e1001304 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3433409&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bor J, Rosen S, Chimbindi N, et al. Mass HIV treatment and sex Disparities in Life Expectancy: demographic Surveillance in rural South Africa. Tsai AC, editor. PLoS Med. 2015;12:e1001905 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4658174&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermund SH, Fidler SJ, Ayles H, et al. Can combination prevention strategies reduce HIV transmission in generalized epidemic settings in Africa? the HPTN 071 (PopART) study plan in South Africa and Zambia. J Acquir Immune Defic Syndr. 2013;63(suppl 2):S221–S227. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3739051&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes R, Ayles H, Beyers N, et al. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment - a study protocol for a cluster randomised trial. Trials. 2014;15:57 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3929317&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Statistics South Africa. South African National Census 2011. Pretoria, South Africa; Statistics South Africa, 2012. [Google Scholar]
- 9.Dokubo EK, Shiraishi RW, Young PW, et al. Awareness of HIV status, prevention knowledge and condom use among people living with HIV in Mozambique. PLoS One. 2014;9:e106760 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4164358&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ettarh RR, Kimani J, Kyobutungi C, et al. Correlates of HIV-status awareness among adults in Nairobi slum areas. Afr J AIDS Res. 2012;11:337–342. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25860192. [DOI] [PubMed] [Google Scholar]
- 11.Cremin I, Cauchemez S, Garnett GP, et al. Patterns of uptake of HIV testing in sub-Saharan Africa in the pre-treatment era. Trop Med Int Health. 2012;17:e26–e37. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3443375&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naik R, Tabana H, Doherty T, et al. Client characteristics and acceptability of a home-based HIV counselling and testing intervention in rural South Africa. BMC Public Health. 2012;12:824 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3487909&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnabas RV, van Rooyen H, Tumwesigye E, et al. Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. Lancet HIV. 2014;1:e68–e76. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25601912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensen B, Lewis JJ, Schaap A, et al. Factors associated with HIV-testing and acceptance of an offer of home-based testing by men in rural Zambia. AIDS Behav. 2015;19:492–504. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25096893. [DOI] [PubMed] [Google Scholar]
- 15.van Rooyen H, Tulloch O, Mukoma W, et al. What are the constraints and opportunities for HIVST scale-up in Africa? Evidence from Kenya, Malawi and South Africa. J Int AIDS Soc. 2015;18:19445 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4369555&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choko AT, MacPherson P, Webb EL, et al. Uptake, Accuracy, Safety, and linkage into care over two Years of promoting Annual self-testing for HIV in Blantyre, Malawi: a Community-Based Prospective Study. Bangsberg DR, editor. PLOS Med. 2015;12:e1001873 Available at: http://www.ncbi.nlm.nih.gov/pubmed/26348035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JA, Sharma M, Levin C, et al. Cost-effectiveness of community-based strategies to strengthen the continuum of HIV care in rural South Africa: a health economic modelling analysis. Lancet HIV. 2015;2:e159–e168. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4384819&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer-Rath G, Pienaar J, Brink B, et al. The impact of Company-level ART provision to a mining Workforce in South Africa: a cost-benefit analysis. PLoS Med. 2015;12:e1001869 Available at: http://www.ncbi.nlm.nih.gov/pubmed/26327271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rooyen H, McGrath N, Chirowodza A, et al. Mobile VCT: reaching men and young people in urban and rural South African pilot studies (NIMH Project Accept, HPTN 043). AIDS Behav. 2013;17:2946–2953. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3597746&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wringe A, Floyd S, Kazooba P, et al. Antiretroviral therapy uptake and coverage in four HIV community cohort studies in sub-Saharan Africa. Trop Med Int Health. 2012;17:e38–e48. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22943378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odhiambo JO, Kellogg TA, Kim AA, et al. Antiretroviral treatment scale-up among persons living with HIV in Kenya: results from a nationally representative survey. J Acquir Immune Defic Syndr. 2014;66(suppl 1):S116–S122. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24732815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson LF, Chiu C, Bekker LG, et al. What Will it Take to Achieve Virtual Elimination of HIV Transmission in South Africa? 8th International AIDS Society Conference on HIV Pathogenesis. Vancouver, Canada: Treatment and Prevention; 2015. [Google Scholar]
- 23.van Rooyen H, Barnabas RV, Baeten JM, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64:e1–e8. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3744613&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kranzer K, Lawn SD, Johnson LF, et al. Community viral load and CD4 count distribution among people living with HIV in a South African Township: implications for treatment as prevention. J Acquir Immune Defic Syndr. 2013;63:498–505. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4233323&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Côrtes FH, Passaes CPB, Bello G, et al. HIV controllers with different viral load cutoff levels have distinct virologic and immunologic profiles. J Acquir Immune Defic Syndr. 2015;68:377–385. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4334695&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath N, Eaton JW, Newell M-L, et al. Migration, sexual behaviour, and HIV risk: a general population cohort in rural South Africa. Lancet HIV. 2015;2:e252–e259. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26280016. [DOI] [PMC free article] [PubMed] [Google Scholar]


