Abstract
Objective:
To better understand the epidemiology of tuberculosis (TB)/HIV coinfection in the European Union (EU) and European Economic Area (EEA) for planning of prevention and control measures.
Design:
Analysis of surveillance data.
Methods:
We performed an analysis of the 2014 TB and AIDS data to assess the burden of TB/HIV coinfection and we applied multivariable logistic regression to evaluate predictors for coinfection.
Results:
Twenty-one of 31 EU/EEA countries reported HIV testing results for 64.6% of the 32 892 notified TB cases. Of those, 1051 (4.9%) were reported as HIV-positive. Males [adjusted odds ratio (aOR) 1.25; 95% confidence interval (CI) 1.07–1.46] and those in age group 25–44 years were more frequently coinfected. TB cases originating from the WHO African region had the highest proportion of coinfection (aOR 3.28 versus origin in EU/EEA; 95% CI 2.35–4.57). TB treatment was completed successfully by 57.9% of HIV-positive TB cases and 83.5% of HIV-negative cases. In 2014, 3863 cases of AIDS were reported by 29 EU/EEA countries; 691 (17.9%) of these cases presented with TB as an AIDS-defining illness. Persons who had acquired HIV through injecting drug use had higher odds of TB as an AIDS-defining illness (aOR 1.78 versus heterosexual route of transmission; 95% CI 1.37–2.32).
Conclusion:
TB/HIV coinfection is a substantial problem in the EU/EEA. The occurrence of TB in HIV-positive cases and the low TB treatment success rate suggest that international guidelines for prevention and treatment of TB in HIV-infected adults need to be better implemented.
Keywords: AIDS, coinfection, Europe, European Union, HIV, tuberculosis
Introduction
In 2014, an estimated 2.0 million persons were newly infected with HIV at a global level [1]. In the same year, an estimated 9.0 million people developed tuberculosis (TB) worldwide [2]. Of these, 1.1 million (12%) had both HIV and TB, and 360 000 died from HIV-associated TB [2]. Most coinfected cases were reported in the WHO African region (433 000 HIV-positive TB cases) and the southeast Asian region (59 000 HIV-positive TB cases). Compared with these regions, the burden of TB and HIV is much lower in the European Union and European Economic Area (EU/EEA). In 2014, about 30 000 new HIV infections and 58 000 TB cases were reported [3,4]. There is a close link between the global TB and HIV situation and the situation in the EU/EEA as 27% of all TB cases and 37% of new HIV cases in the EU/EEA are of foreign origin [3–5].
Information on the burden of TB/HIV coinfection is critical for planning and evaluating TB/HIV prevention and control activities. Prevention measures include testing of TB patients for HIV coinfection and excluding TB infection and/or disease in HIV-positive patients; both measures are strongly recommended by the WHO [6]. In 2013, globally 64 countries reported a total of 5.5 million people enrolled in HIV care who were screened for TB [2]. The degree of implementation of this recommendation in the EU/EEA is unknown. TB patients testing positive for HIV should be provided with combined antiretroviral therapy (cART), which greatly improves their survival and quality of life [7]. Testing and preventatively treating HIV and AIDS patients for TB reduces TB incidence in HIV-positives [8] and may also reduce TB incidence in the general population [9].
The mandate of the European Centre for Disease Prevention and Control (ECDC) includes supporting the 31 member states of the EU/EEA with epidemiological surveillance of infectious diseases [10,11]. To fulfil this obligation, ECDC has collected case-based information on TB, HIV infections and new AIDS diagnoses on an annual basis since 2007. We analysed TB and AIDS surveillance data to assess the burden of TB/HIV coinfection in the EU/EEA and to evaluate predictors for coinfection, including associations between TB drug resistance and HIV infection and the outcome of TB treatment and HIV infection. By looking at HIV/TB coinfection from two different angles, we hope to gain new insights for better disease surveillance, prevention and control.
Methods
Data sources
Designated national surveillance institutions report TB and new AIDS diagnosis surveillance data to a common database [The European Surveillance System (TESSy)] hosted by ECDC. The detailed data collection methods and definitions are described elsewhere [3,12]. The surveillance system contains data up to 2014. TB surveillance data were extracted on 1 October 2015 and new AIDS diagnosis data on 5 November 2015.
Data included and definitions
TB cases were defined according to the case definition published by the European Commission [13]. For each TB case, information on age, sex and geographic origin was collected or derived. We further assigned the origin of TB cases to one of the seven WHO regions (http://www.who.int/about/regions/en/). In addition, information was collected on the TB treatment history (new/previously treated), site of disease (pulmonary/extrapulmonary) and drug resistance pattern. Cases with both pulmonary and extrapulmonary TB were classified as pulmonary TB. Multidrug resistance (MDR) was defined as resistance to at least isoniazid and rifampicin and extensively drug-resistant (XDR) TB as resistance to at least isoniazid and rifampicin, to any fluoroquinolone and to any of the three second-line injectables (amikacin, capreomycin and kanamycin). For TB cases with TB sensitive to isoniazid or rifampicin, treatment outcome (success, failed, died, lost to follow-up or still on treatment) was collected 12 months after the start of treatment. For MDR-TB and XDR-TB cases, treatment outcome was collected 24 and 36 months after start of treatment, respectively.
AIDS cases were defined according to the European case definition, which includes the presence of an AIDS-defining condition at clinical diagnosis [13]. For each AIDS case reported, the following information was collected or derived: date of HIV diagnosis, age, sex, geographic origin (grouped into the same categories as described for TB), route of transmission, time between HIV and AIDS diagnosis, outcome (alive or dead) and the presence of AIDS-defining illness(es) at AIDS diagnosis including pulmonary or extrapulmonary TB.
Data analysis
We performed a descriptive analysis of the TB data from EU/EEA countries that reported case-based TB data to TESSy for both HIV-positive and HIV-negative TB cases. Descriptive analysis was also performed for case-based AIDS data reported to TESSy, for cases with TB reported at AIDS diagnosis as well as cases without TB reported at AIDS diagnosis. For both TB and AIDS, we used the most recent complete data available, that is diagnoses made during 2014. In 2014, TB final treatment outcomes were reported for non-MDR-TB cases notified in 2013, for MDR-TB (excluding XDR TB) cases notified in 2012 and for XDR-TB cases notified in 2011.
Further analysis was restricted to the 20 countries reporting both on HIV status of TB cases and on TB as an AIDS-defining illness. In our analysis of the TB data, we compared HIV-positive TB cases and HIV-negative TB cases by sex, age, origin, treatment history, site of disease, classification of TB, drug resistance pattern and treatment outcome. For the AIDS data, we compared cases with and without TB reported as an AIDS-defining illness by sex, age, origin, HIV transmission route, outcome and time between HIV and AIDS diagnosis, if known. Risk factors for coinfection were identified by disease-specific univariate analysis. If for any univariate association, P value 0.1 or less by chi-square test, the variable was included in disease-specific logistic regression models applying backward elimination based on maximum likelihood estimates. In the TB model, interaction terms were considered for geographic origin and age as well as for age and site of disease. In the AIDS model, interaction terms were considered for sex and route of HIV transmission. Statistical associations were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Data analysis was performed using STATA 14 software (StataCorp, College Station, Texas, USA).
Both the TB surveillance and new AIDS diagnosis data contain anonymized information. Therefore, informed consent was not considered to be necessary, and the research proposal was not submitted to an ethical review board.
Results
Tuberculosis perspective
In 2014, 21 of 31 EU/EEA countries reported case-based data on HIV status of TB cases (Fig. 1). In these 21 countries, 21 243 (64.6%) of 32 892 TB cases were reported to have undergone HIV testing, and 1051 (4.9%) of those with known HIV status were reported as HIV-positive. Four countries had a reporting completeness for HIV status of less than 50%: Czech Republic (28.4%), Denmark (1.9%), Hungary (3.5%) and Ireland (26.6%). Among the 17 countries with at least 50% reporting completeness, the proportion of coinfected cases was highest in Latvia (19.5%) followed by Malta (17.1%), Portugal (14.7%) and Estonia (10.1%).
Fig. 1.
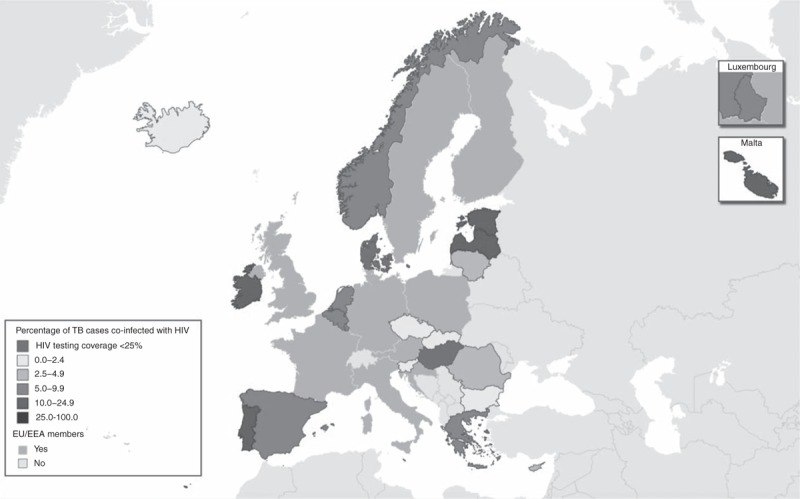
Percentage of HIV-positive cases among tuberculosis cases with known HIV status, by country, European Union and European Economic Area, 2014.
In the 20 countries that were included in the logistic regression analysis, HIV infection was more frequently reported in male TB cases than in female TB cases (5.2 versus 4.3%; OR 1.23; 95% CI 1.07–1.41) (Table 1). The most affected age group was the one from 25 to 44 years with 8.3% reported as HIV-positive. Most HIV-positive TB cases originated from the European region (78.4%), whereas 5.4% originated from the African region, 1.8% from the American region and 2.4% from other regions. For 12.0% of cases, the region of origin was unknown. TB cases originating from the African region had the highest probability of being coinfected with HIV (OR 4.91; 95% CI 3.65–6.61). Of 316 TB cases from the African region, 56 (17.7%) were coinfected with HIV.
Table 1.
Risk factors for HIV infection in tuberculosis cases reported in 20 European Union and European Economic Area countriesa.
| Variable | HIV-positive TB cases, N (%) | HIV-negative TB cases, N (%) | Univariate analysis, OR (95% CI) | Multivariable analysis, OR (95% CI) |
| Total | 1028 (4.9) | 19 791 (95.1) | ||
| Sex (N = 20 810) | ||||
| Male | 736 (5.2) | 13 305 (94.8) | 1.23 (1.07–1.41) | 1.25 (1.07–1.46) |
| Female | 291 (4.3) | 6478 (95.7) | 1 | 1 |
| Age groups (N = 20 815) | ||||
| 0–14 | 2 (0.3) | 705 (99.7) | 0.03 (0.01–0.12) | 0.03 (0.01–0.12) |
| 15–24 | 82 (3.6) | 2201 (96.4) | 0.41 (0.32–0.52) | 0.45 (0.35–0.57) |
| 25–44 | 641 (8.3) | 7088 (91.7) | 1 | 1 |
| 45–64 | 281 (4.0) | 6694 (96.0) | 0.46 (0.40–0.54) | 0.43 (0.36–0.50) |
| ≥65 | 21 (0.7) | 3100 (99.3) | 0.07 (0.05–0.12) | 0.07 (0.04–0.11) |
| Region of origin (N = 20 163) | ||||
| Europe | ||||
| EU/EEA | 787 (4.2) | 17 940 (95.8) | 1 | 1 |
| Non-EU/EEA | 19 (8.5) | 205 (91.5) | 2.11 (1.31–3.40) | 1.91 (1.15–3.17) |
| Africa | 56 (17.7) | 260 (82.3) | 4.91 (3.65–6.61) | 3.28 (2.35–4.57) |
| Americas | 18 (8.1) | 204 (91.9) | 2.01 (1.24–3.27) | 1.44 (0.86–2.40) |
| Other regions | 25 (3.7) | 649 (96.3) | 0.88 (0.58–1.32) | 0.64 (0.42–0.98) |
| TB treatment history (N = 20 588) | ||||
| New | 796 (4.6) | 16 537 (95.4) | 1 | 1 |
| Previously treated | 190 (5.8) | 3065 (94.2) | 1.29 (1.09–1.51) | 1.39 (1.16–1.66) |
| Site of disease (N = 20 731) | ||||
| Pulmonary | 781 (4.5) | 16 572 (95.5) | 1 | 1 |
| Extrapulmonary | 245 (7.3) | 3133 (92.7) | 1.66 (1.43–1.92) | 1.81 (1.50–2.19) |
| Classification of TB (N = 20 819) | ||||
| Confirmed | 676 (4.6) | 13 881 (95.4) | 1 | 1 |
| Probable | 103 (7.2) | 1326 (92.8) | 1.59 (1.29–1.98) | 1.53 (1.19–1.97) |
| Possible | 249 (5.2) | 4584 (94.8) | 1.12 (0.96–1.29) | 1.17 (0.98–1.39) |
| Drug resistance pattern (N = 10 469) | ||||
| Sensitive | 290 (3.3) | 8530 (96.7) | 1 | |
| Resistant to any anti-TB drug (not MDR) | 49 (6.0) | 764 (94.0) | 1.88 (1.38–2.58) | |
| MDR | 50 (7.1) | 655 (92.9) | 2.25 (1.65–3.06) | |
| XDR | 12 (9.2) | 119 (90.8) | 2.97 (1.62–5.43) | |
CI, confidence interval; EEA, European Economic Area; EU, European Union; MDR, multidrug-resistant; OR, odds ratio; TB, tuberculosis; XDR, extensively drug-resistant.
aCountries reporting on HIV status of TB cases and on TB as AIDS-defining illness in 2014: Belgium, Bulgaria, Czech Republic, Cyprus, Denmark, Estonia, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Norway, Portugal, Romania, Slovakia, Slovenia and Spain.
The majority of the 1028 HIV-positive TB cases were newly diagnosed (N = 796, 77.4%) and had pulmonary TB (N = 781, 76.0%). Previously treated TB cases were more frequently HIV-positive than new TB cases (5.8 versus 4.6%; OR 1.29; 95% CI 1.09–1.51), and extrapulmonary TB cases were more frequently HIV-positive than pulmonary TB cases (7.3 versus 4.5%; OR 1.66; 95% CI 1.43–1.92).
Of the 20 819 TB cases with an HIV test result, the TB diagnosis was confirmed by a positive laboratory test in 14 557 (69.9%). Of those, 10 469 (71.9%) had a drug susceptibility testing result: 401 (59.3%) of the HIV-positive TB cases and 10 068 (72.5%) of the HIV-negative TB cases. Of the 401 HIV-positive TB cases, the majority (N = 290, 72.3%) had drug-sensitive TB, 49 (12.2%) had TB that was resistant to one or more anti-TB drugs but not MDR-TB, 50 (12.5%) were MDR-TB cases (excluding XDR-TB) and 12 (3.0%) were diagnosed with XDR-TB. An HIV-positive test result was more frequent in cases with MDR-TB (OR 2.25; 95% CI 1.65–3.06) and XDR-TB (OR 2.97; 95% CI 1.62–5.43) than among TB cases with drug-sensitive TB.
All variables tested in the univariate model were significant at the P 0.10 or less level and thus eligible for inclusion in the logistic regression model. However, the variable Mycobacterium tuberculosis drug resistance pattern was excluded from the multivariable logistic regression model due to low drug susceptibility testing coverage. The results of the multivariable model were comparable with the results of the univariate analysis (Table 1). None of the tested interaction terms was significant.
A total of 20 200 TB cases reported in 2013 with a known HIV status had a treatment outcome reported. Of those cases, 968 (4.8%) were HIV-positive (Table 2). By 2014, TB treatment was completed successfully by 560 (57.9%) HIV-positive TB cases, a significantly lower treatment success rate than the 83.5% treatment success rate of HIV-negative TB cases (P < 0.001). While on TB treatment, a greater proportion of HIV-positive TB cases died than in HIV-negative TB cases (133 or 13.7% versus 1080 or 5.6%; P < 0.001). Also, the proportion of persons still on treatment after 12 months or not evaluated were both higher in HIV-positive TB cases, respectively, 6.9 versus 1.6% (P < 0.001) and 11.8 versus 3.2% (P < 0.001). Of the 49 HIV-positive MDR-TB cases reported in 2012 by 11 EU/EEA countries reporting case-based data on treatment outcome after 24 months, 17 (34.7%) completed treatment successfully, whereas 294 (43.8%) of 671 HIV-negative MDR-TB cases completed treatment successfully (P = 0.063). The treatment outcome analysis for XDR-TB cases reported in 2011 by 10 EU/EEA countries reporting case-based data on treatment outcome after 36 months showed that two (28.6%) of seven HIV-positive XDR-TB cases and 27 (31.0%) of 87 HIV-negative XDR-TB cases finished treatment successfully (P = 0.373).
Table 2.
Tuberculosis treatment outcome in the 2013 treatment cohort of reported tuberculosis cases with tuberculosis disease sensitive to isoniazid or rifampicin in 16 European Union and European Economic Area countries reporting on HIV status.
| Tuberculosis treatment outcome | HIV-positive TB cases, N (%) | HIV-negative TB cases, N (%) | TB cases with unknown HIV status, N (%) | Total number of TB cases, N (%) |
| Total | 968 (100.0) | 19 232 (100.0) | 11 154 (100.0) | 31 354 (100.0) |
| Success | 560 (57.9)a | 16 068 (83.5)a | 8403 (75.9) | 25 031 (79.9) |
| Died | 133 (13.7)a | 1080 (5.6)a | 1077 (9.0) | 2290 (7.3) |
| Failed | 19 (2.0) | 298 (1.5) | 191 (2.0) | 508 (1.6) |
| Lost to follow-up | 75 (7.7)a | 876 (4.6) | 639 (5.4) | 1590 (5.1) |
| Still on treatment | 67 (6.9)a | 299 (1.6)a | 178 (1.0) | 544 (1.7) |
| Not evaluated | 114 (11.8)a | 611 (3.2)a | 666 (6.7) | 1391 (4.4) |
TB, tuberculosis. Member states: Belgium, Bulgaria, Czech Republic, Estonia, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Norway, Portugal, Romania, Slovakia, Slovenia and Spain.
aSignificantly different between HIV-positive and HIV-negative patients, P value <0.001.
AIDS perspective
In 2014, 3863 cases of AIDS were reported by 29 countries in the EU/EEA. After Pneumocystis pneumonia, TB was the second most common AIDS-defining illness with 691 (17.9%) AIDS cases presenting with TB. Among these coinfected cases, 497 (71.9%) were reported as pulmonary TB and 194 (28.1%) as extrapulmonary TB. The highest proportions of TB as an AIDS-defining illness were reported by Malta (N = 3, 75% of AIDS diagnoses), Romania (N = 166, 43.9%), Latvia (N = 71, 41.5%) and Lithuania (N = 14, 37.8%), whereas in Cyprus, Greece, Slovenia and Slovakia no AIDS cases were reported as presenting with TB as AIDS-defining illness in 2014 (Fig. 2).
Fig. 2.
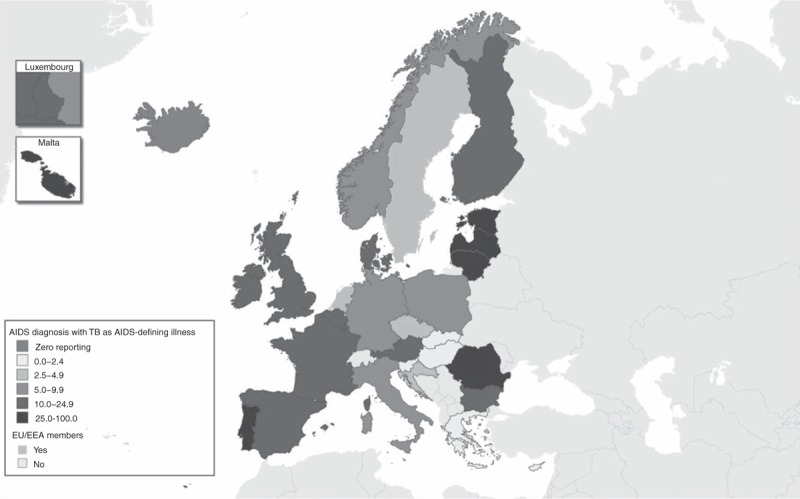
Percentage of AIDS diagnoses with tuberculosis reported as AIDS-defining illness, by country, European Union and European Economic Area, 2014.
Further analysis was restricted to the same 20 countries for which complete HIV and TB data were reported to the surveillance system. These countries reported 1777 (46.0%) of all AIDS cases and 436 (63.1%) of all AIDS cases presenting with TB as an AIDS-defining illness in the EU/EEA in 2014 (Table 3). Of the 1777 AIDS cases, 436 (24.5%) had TB as an AIDS-defining illness at AIDS diagnosis: 336 (77.1%) pulmonary TB and 100 (22.9%) extrapulmonary TB.
Table 3.
Risk factors for tuberculosis in AIDS cases reported in 2014 in 20 European Union and European Economic Areaa countries.
| Variable | AIDS cases with TB, N (%) | AIDS cases with no reported TB, N (%) | Univariate analysis, OR (95% CI) | Multivariable analysis, OR (95% CI) |
| 436 (24.5) | 1341 (75.5) | |||
| Sex (N = 1776) | ||||
| Male | 334 (24.9) | 1008 (75.1) | 1.07 (0.84–1.39) | |
| Female | 102 (23.5) | 332 (76.5) | 1 | |
| Age groups (N = 1776) | ||||
| 0–14 | 8 (27.6) | 21 (72.4) | 0.95 (0.42–2.16) | 1.08 (0.19–6.16) |
| 15–24 | 59 (32.8) | 121 (67.2) | 1.27 (0.91–1.80) | 1.05 (0.72–1.53) |
| 25–44 | 272 (27.6) | 712 (72.4) | 1 | 1 |
| 45–64 | 91 (17.8) | 421 (82.2) | 0.57 (0.43–0.74) | 0.56 (0.42–0.76) |
| ≥65 | 6 (8.4) | 65 (91.64) | 0.24 (0.10–0.56) | 0.26 (0.10–0.67) |
| Region of origin (N = 1665) | ||||
| Europe | ||||
| EU/EEA | 352 (26.4) | 983 (73.6) | 1 | |
| Non-EU/EEA | 12 (16.7) | 60 (83.3) | 0.56 (0.30–1.05) | |
| Africa | 46 (31.1) | 102 (68.9) | 1.26 (0.87–1.82) | |
| Americas | 10 (15.9) | 53 (84.1) | 0.53 (0.27–1.04) | |
| Other regions | 7 (14.9) | 40 (85.1) | 0.49 (0.22–1.1) | |
| Route of transmission (N = 1569) | ||||
| Heterosexual | 184 (25.3) | 543 (74.7) | 1 | 1 |
| Injecting drug use | 166 (41.1) | 238 (58.9) | 2.06 (1.59–2.67) | 1.78 (1.37–2.32) |
| Sex between men | 33 (8.1) | 373 (91.9) | 0.26 (0.18–0.39) | 0.25 (0.17–0.36) |
| Other | 9 (28.1) | 23 (71.6) | 1.15 (0.52–2.54) | 0.86 (0.17–4.43) |
| HIV diagnosis (N = 1274) | ||||
| ≤90 days before AIDS diagnosis | 196 (24.4) | 607 (75.6) | 1 | |
| >90 days before AIDS diagnosis | 154 (32.7) | 317 (67.3) | 1.50 (1.17–1.93) | |
| Outcome (N = 1105) | ||||
| Alive | 227 (29.6) | 539 (70.4) | 1 | |
| Dead | 80 (23.6) | 259 (76.4) | 0.73 (0.55–0.98) | |
CI, confidence interval; EEA, European Economic Area; TB, tuberculosis; EU, European Union; OR, odds ratio.
aCountries reporting on both HIV status of TB cases and on TB as AIDS-defining illness in 2014: Belgium, Bulgaria, Czech Republic, Cyprus, Denmark, Estonia, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Norway, Portugal, Romania, Slovakia, Slovenia and Spain.
In 2014, 334 (24.9%) of the 1342 newly diagnosed male AIDS cases were reported to have TB and 102 (23.5%) of the 434 female new AIDS cases (Table 3). The highest proportion of TB as an AIDS-defining illness was among 15–24-year-olds (32.8%) and the proportion was lower in those aged more than 45 years.
Most new AIDS cases reported with TB as an AIDS-defining illness came from the European region [364 cases (85.2%) of the 427 cases for whom data on origin was available]; the majority of these originated from EU/EEA countries. Fewer cases originated from the African region (10.8%), the Americas (2.3%) and from other regions (1.6%). For 6.3% of all AIDS cases and 2.1% of AIDS cases with TB reported at AIDS diagnosis, the region of origin was unknown. Cases originating from Africa had the highest proportion of TB at AIDS diagnosis (31.1%; OR 1.26, 95% CI 0.87–1.82).
The most common route of HIV transmission among AIDS cases with TB at AIDS diagnosis in 2014 was heterosexual contact (46.9% of the 392 cases with known route of transmission), followed by injecting drug use (42.4%), sex between men (8.4%) and other routes of transmission (2.3%). Compared with persons infected by heterosexual HIV transmission, persons with injecting drug use–related transmission were more likely to have TB as an AIDS-defining illness (OR 2.06; 95% CI 1.59–2.67), whereas MSM were less likely to have TB (OR 0.26; 95% CI 0.18–0.39), Table 3.
The majority (63.0%) of cases were diagnosed with HIV within 90 days of the AIDS diagnosis. Cases who were diagnosed with HIV more than 90 days before receiving their AIDS diagnosis were more likely to have TB reported as an AIDS-defining illness (OR 1.50; 95% CI 1.17–1.93). Among the 1105 cases diagnosed with AIDS in 2014 for which information on outcome was available at the time of report, 69.3% were reported as alive and 30.7% as having died (Table 3). Of the 732 known deaths among AIDS cases reported by the 20 countries in 2014, 235 (32.1%) were in cases with TB diagnosis (data not shown).
In multivariable analysis, the factors remaining significant included age (aOR 0.56; 95% CI 0.42–0.76 for 45–64-year-olds and aOR 0.26; 95% CI 0.10–0.67 for ≥65-year-olds as compared with 25–44-year-olds) and acquiring HIV through injecting drug use (aOR 1.78; 95% CI 1.37–2.32) and sex between men (aOR 0.25; 95% CI 0.17–0.36).
Discussion
We present the first analysis of TB-HIV coinfection in EU/EEA countries using unlinked TB and AIDS surveillance data. TB-HIV coinfection is not insignificant in the EU/EEA with 1051 TB cases reported with HIV infection (4.9% of all TB cases with a known HIV status) and 436 new AIDS cases with TB as AIDS-defining illness (24.5% of all reported new AIDS cases) in 20 countries in 2014. The USA reported a similar proportion of TB cases with HIV infection in 2014, that is 6% [14], whereas the United Kingdom, which does not report HIV status of TB cases to ECDC and was therefore not included in our analysis, estimated the TB incidence among people living with HIV to be 2.1 per 100 person-years [15]. Proportions of TB cases with an HIV test result who were HIV-positive varied between different WHO regions, from 2.3% in the west eastern Pacific region to 39% in the African region [16].
The TB surveillance data analysis revealed that TB-HIV coinfection was most common in the age group 25–44 years and in cases of foreign origin, especially in cases originating from the African region. This has also been described in studies analysing HIV cohort data in Europe [17–21]. Among AIDS cases, persons who had acquired HIV through injecting drug use were significantly more likely to have TB as an AIDS-defining condition than those that had acquired HIV via other modes of transmission. Injecting drug use has also been reported as a risk factor for TB in HIV cohort studies [17,19]. Also, injecting drug use is a known factor associated with MDR-TB [22]. Other risk factors believed to be associated with the development of TB in HIV-positive persons such as antiretroviral treatment receipt and adherence, low CD4+ cell count and high viral load [17–19,23] were not included in our analysis as this information was not available for a large number of the cases. Trials have found a significant protective effect of initiation of ART on the risk of TB among HIV-positive persons [24,25]. In our study, the majority of new AIDS diagnoses coincide with newly diagnosed HIV. These persons were not yet on treatment when diagnosed with AIDS. This is reflective of late diagnosis among a large proportion of people in whom symptomatic AIDS, in some cases TB symptoms, are the reason that HIV is tested for and diagnosed.
The proportion of HIV coinfected was higher in extrapulmonary than in pulmonary TB cases. The same finding has been reported in other settings [26,27]. Extrapulmonary TB may be difficult to diagnose [28]; therefore, health workers taking care of HIV-positive individuals need to be vigilant to the possibility of extrapulmonary TB, so that it is diagnosed and treated promptly [29]. Drug-resistant TB cases were more frequently HIV-positive. HIV-positive cases with drug-resistant TB frequently have unsuccessful treatment outcomes as is shown in our analysis and by other cohort studies [30]. It is therefore of paramount importance that all TB–HIV coinfected cases are tested for resistance to anti-TB drugs and, if found resistant, are treated appropriately and promptly.
The development of TB in newly diagnosed and notified AIDS cases might be prevented if HIV-positive individuals are screened for latent TB infection as is recommended [31,32] and if those with positive screening results are put on TB preventive therapy [33]. These recommendations do not appear to be fully implemented in Europe. In 2014, 142 197 newly diagnosed HIV infections were reported in the WHO European region [4], and 21 000 were provided with TB preventive therapy [16]. In a prospective cohort study in Spain, one-third of the HIV-positive cases without a history of TB were screened for latent TB infection, and of the cases with a positive screening result, approximately half received treatment for latent TB infection [34]. A cohort study in Switzerland showed that 69% of the patients without a history of TB were screened for latent TB infection, and 37% of those who tested positive received a full course of preventive treatment [35]. Screening for latent TB infection remains important, even though HIV-positive individuals who receive cART have a lower risk of TB [24]. Furthermore, additional large discrepancies in the organization of HIV and TB care have been documented across the European region, with suboptimal levels of colocation of HIV and TB care services, screening of HIV-positive patients for TB and provision of opiate substitution treatment to promote HIV and TB treatment adherence in drug-dependent individuals in some regions of Europe [36].
Individuals with TB–HIV coinfection had worse TB treatment outcomes than TB cases without HIV infection. Recommendations state that coinfected cases should receive the same TB treatment regimen as noncoinfected cases [37] and should start cART as soon as possible, but definitely within 8 weeks of beginning treatment for TB [38,39]. Information about whether cART was started in TB-HIV coinfected cases is reported to TESSy in an aggregated format by seven EU/EEA countries [3]. In 2014, cART was initiated in 81% of the 445 TB-HIV coinfected cases notified by these countries.
Our study used surveillance data to obtain an overview of the epidemic of TB/HIV coinfection in the EU/EEA. Surveillance data are collected during routine programmatic management of TB and HIV/AIDS and have limitations that may compromise the representativeness of the findings. First of all, only two-thirds of EU/EEA countries report on TB/HIV coinfection to the joint ECDC and WHO regional office for Europe TB surveillance system [3]. Due to the anonymous reporting of HIV cases and/or patient confidentiality legislation, seven EU/EEA countries could not record HIV status for individual TB patients [40]. Nonreporting countries include France, Germany, Poland and the United Kingdom, which together account for 40% of all TB cases in the EU/EEA [3].
Furthermore, although most countries had national policies recommending testing of TB patients for HIV infection, the proportion of patients actually tested varied widely, from 5 to 90% [40]. Within the ECDC/WHO HIV and AIDS surveillance system, all 31 EU/EEA countries report AIDS data. TB is reported as an AIDS-defining illness at AIDS diagnosis, by all EU/EEA countries except Sweden and the Netherlands; however, even in countries reporting, it is unknown whether all AIDS patients were tested for TB [6,41]. TB contracted or diagnosed after the initial AIDS diagnosis, or TB diagnosed among persons who have not yet developed AIDS is not reported within the EU/EEA-level HIV and AIDS surveillance system.
Before wide access to ART, underreporting for AIDS cases within EU/EEA surveillance was estimated to range from 0 to 25% [42]. Although more recent estimates are not available, underreporting of AIDS is likely more common as ART is more widely available and the focus of surveillance has shifted from HIV to AIDS in many EU/EEA countries. Thus, the number of individuals with a new AIDS diagnosis and TB as AIDS-defining illness is anticipated to be higher than reported here [43,44].
The TB and new AIDS diagnosis surveillance systems at EU/EEA level do not contain a single unique identifier that would allow direct deterministic linking of the TB and new AIDS diagnosis surveillance data. Linkage using age and additional variables is complicated due to the fact that the TB diagnosis and new AIDS diagnosis may not be reported in the same year. Future studies may assess whether probabilistic linkage is feasible and allows for assessing the overlap in the two surveillance systems [45].
In conclusion, the analysis of TB and new AIDS diagnosis surveillance data provides a more complete picture of the epidemiology of TB–HIV coinfection in the EU/EEA by combining the findings of two incomplete databases. In addition, the two databases provided complementary information on risk factors for coinfection. The surveillance of TB and HIV at the EU/EEA level needs to be improved to provide a more comprehensive picture of the problem that can better guide prevention and control efforts. All TB cases should be tested for HIV infection and HIV-positive cases should be evaluated for TB. The available data suggest that screening of TB cases for HIV infection, earlier diagnosis and screening of HIV positive-individuals for latent TB infection and the prompt initiation of treatment for latent TB infection and of cART for those with HIV infection is important and needs to be better implemented in the EU/EEA.
Acknowledgements
The authors would like to thank the following nominated contact points for surveillance:
ECDC Operational Contact Points for TB surveillance from the EU/EEA member states included in this analysis: Peter Henrik Andersen, Margaret Trude Arnesen, Thorsteinn Blondal, Domnica Ioana Chiotan, Edita Davidavičienė, Irene Demuth, Raquel Duarte, Ourania Kalkouni, Maria Koliou, Gábor Kovács, Joan O’Donnell, Analita Pace Asciak, Elena Rodríguez Valín, Erika Slump, Ivan Solovič, Petra Svetina, Tonka Varleva, Piret Viiklepp, Irina Lucenko, Jiří Wallenfels and Maryse Wanlin.
ECDC Operational Contact Points for HIV surveillance from all EU/EEA member states reporting AIDS data in 2014: Maria Axelsson, Hans Blystad, Haraldur Briem, Saulius Čaplinskas, Irma Čaplinskienė, Susan Cowan, Valerie Delpech, Mercedes Diez Ruiz-Navarro, Maria Dudas, Barbara Gunsenheimer-Bartmeyer, Derval Igoe, Irena Klavs, Maria Koliou, Šarlote Konova, Kirsi Liitsola, Florence Lot, Marek Maly, Mariana Mardarescu, Helena Cortes Martins, Jackie Maistre Melillo, Tanya Melillo, Georgios Nikolopoulos, Kate O’Donnell, Eline Op de Coul, Dimitra Paraskeva, Tatjana Nemeth Blazic, Magdalena Rosinska, Kristi Ruutel, Andre Sasse, Daniela Schmid, Jean-Claude Schmit, Gudrun Sigmundsdottir, Barbara Suligoi, Peter Truska and Tonka Varleva (all names are in alphabetical order).
Sources of support: The authors are employed by the European Centre for Disease Prevention and Control. No additional sources of support were used.
Author contribution: M.J.v.d.W. and A.P. jointly planned the analysis. A.P. and C.K. performed the analysis. M.J.v.d.W. and A.P. wrote the first draft of manuscript and selected the references. C.K., V.H., P.Z. and A.J.A.-G. made comments at various stages of the analysis and the manuscript text. All authors have seen the final manuscript and agree with the conclusions drawn.
Conflicts of interest
There are no conflicts of interest.
References
- 1.UNAIDS. How AIDS changed everything – MDG6: 15 years, 15 lessons of hope from the AIDS response. Geneva: UNAIDS; 2015. [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.European Centre for Disease Prevention and Control, World Health Organization Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe, 2016. Stockholm: European Centre for Disease prevention and Control; 2016. [Google Scholar]
- 4.European Centre for Disease Prevention and Control, World Health Organization Regional Office for Europe. HIV/AIDS surveillance in Europe 2014. Stockholm: European Centre for Disease Prevention and Control; 2015. [Google Scholar]
- 5.Kodmon C, Zucs P, van der Werf MJ. Migration-related tuberculosis: epidemiology and characteristics of tuberculosis cases originating outside the European Union and European Economic Area, 2007 to 2013. Euro Surveill 2016; 21: [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: World Health Organization; 2011. [Google Scholar]
- 7.World Health Organization. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 8.Briggs MA, Emerson C, Modi S, Taylor NK, Date A. Use of isoniazid preventive therapy for tuberculosis prophylaxis among people living with HIV/AIDS: a review of the literature. J Acquir Immune Defic Syndr 2015; 68 suppl 3:S297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid A, Grant AD, White RG, Dye C, Vynnycky E, Fielding K, et al. Accelerating progress towards tuberculosis elimination: the need for combination treatment and prevention. Int J Tuberc Lung Dis 2015; 19:5–9. [DOI] [PubMed] [Google Scholar]
- 10.Commission Decision of 22 December 1999 on the communicable diseases to be progressively covered by the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. Off J Eur Communities 2000; L28:50–53. [Google Scholar]
- 11.Regulation (EC) No 851/2004 of the European Parliament and Council of 21 April 2004 establishing a European centre for disease prevention and control. Off J Eur Union 2004; L142:1–11. [Google Scholar]
- 12.European Centre for Disease Prevention and Control, World Health Organization Regional Office for Europe. HIV/AIDS surveillance in Europe 2013. Stockholm: European Centre for Disease Prevention and Control; 2014. [Google Scholar]
- 13.Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. Off J Eur Communities 2008; L159:46. [Google Scholar]
- 14.United States Department of Health and Human Services, CDC. Reported tuberculosis in the United States, 2014. Atlanta: CDC; 2015. [Google Scholar]
- 15.Zenner D, Abubakar I, Conti S, Gupta RK, Yin Z, Kall M, et al. Impact of TB on the survival of people living with HIV infection in England, Wales and Northern Ireland. Thorax 2015; 70:566–573. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Global tuberculosis report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 17.Monge S, Diez M, Pulido F, Iribarren JA, Campins AA, Arazo P, et al. Tuberculosis in a cohort of HIV-positive patients: epidemiology, clinical practice and treatment outcomes. Int J Tuberc Lung Dis 2014; 18:700–708. [DOI] [PubMed] [Google Scholar]
- 18.Karo B, Haas W, Kollan C, Gunsenheimer-Bartmeyer B, Hamouda O, Fiebig L, et al. Tuberculosis among people living with HIV/AIDS in the German ClinSurv HIV cohort: long-term incidence and risk factors. BMC Infect Dis 2014; 14:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taarnhoj GA, Engsig FN, Ravn P, Johansen IS, Larsen CS, Roge B, et al. Incidence, risk factors and mortality of tuberculosis in Danish HIV patients 1995–2007. BMC Pulm Med 2011; 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haar CH, Cobelens FG, Kalisvaart NA, Van der Have JJ, Van Gerven PJ, Van Deutekom H. HIV prevalence among tuberculosis patients in The Netherlands, 1993–2001: trends and risk factors. Int J Tuberc Lung Dis 2006; 10:768–774. [PubMed] [Google Scholar]
- 21.Camoni L, Regine V, Boros S, Salfa MC, Raimondo M, Suligoi B. AIDS patients with tuberculosis: characteristics and trend of cases reported to the National AIDS Registry in Italy – 1993–2010. Eur J Public Health 2013; 23:658–663. [DOI] [PubMed] [Google Scholar]
- 22.Post FA, Grint D, Werlinrud AM, Panteleev A, Riekstina V, Malashenkov EA, et al. Multidrug-resistant tuberculosis in HIV positive patients in eastern Europe. J Infect 2014; 68:259–263. [DOI] [PubMed] [Google Scholar]
- 23.Kruk A, Bannister W, Podlekareva DN, Chentsova NP, Rakhmanova AG, Horban A, et al. Tuberculosis among HIV-positive patients across Europe: changes over time and risk factors. AIDS 2011; 25:1505–1513. [DOI] [PubMed] [Google Scholar]
- 24.del Amo J, Moreno S, Bucher HC, Furrer H, Logan R, Sterne J, et al. Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clin Infect Dis 2012; 54:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad Khan F, Minion J, Al-Motairi A, Benedetti A, Harries AD, Menzies D. An updated systematic review and meta-analysis on the treatment of active tuberculosis in patients with HIV infection. Clin Infect Dis 2012; 55:1154–1163. [DOI] [PubMed] [Google Scholar]
- 26.Alemie GA, Gebreselassie F. Common types of tuberculosis and co-infection with HIV at private health institutions in Ethiopia: a cross sectional study. BMC Public Health 2014; 14:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naing C, Mak JW, Maung M, Wong SF, Kassim AI. Meta-analysis: the association between HIV infection and extrapulmonary tuberculosis. Lung 2013; 191:27–34. [DOI] [PubMed] [Google Scholar]
- 28.Solovic I, Jonsson J, Korzeniewska-Kosela M, Chiotan DI, Pace-Asciak A, Slump E, et al. Challenges in diagnosing extrapulmonary tuberculosis in the European Union, 2011. Euro Surveill 2013; 18: pii=20432. [PubMed] [Google Scholar]
- 29.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008; 8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 2009; 9:153–161. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. Guidelines on the management of latent tuberculosis infection. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 32.European AIDS Clinical Society. EACS guidelines version 8.0. Brussels: European AIDS Clinical Society; 2015. [Google Scholar]
- 33.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; 1:CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Pino I, Sambeat MA, Lacalle-Remigio JR, Domingo P, Group VCS. Incidence of tuberculosis in HIV-infected patients in Spain: the impact of treatment for LTBI. Int J Tuberc Lung Dis 2013; 17:1545–1551. [DOI] [PubMed] [Google Scholar]
- 35.Elzi L, Schlegel M, Weber R, Hirschel B, Cavassini M, Schmid P, et al. Reducing tuberculosis incidence by tuberculin skin testing, preventive treatment, and antiretroviral therapy in an area of low tuberculosis transmission. Clin Infect Dis 2007; 44:94–102. [DOI] [PubMed] [Google Scholar]
- 36.Mansfeld M, Skrahina A, Shepherd L, Schultze A, Panteleev AM, Miller RF, et al. Major differences in organization and availability of healthcare and medicines for HIV/TB coinfected patients across Europe. HIV Med 2015; 16:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Treatment of tuberculosis: guidelines. Geneva: World Health Organization; 2009. [Google Scholar]
- 38.Pozniak AL, Coyne KM, Miller RF, Lipman MC, Freedman AR, Ormerod LP, et al. British HIV Association guidelines for the treatment of TB/HIV coinfection 2011. HIV Med 2011; 12:517–524. [DOI] [PubMed] [Google Scholar]
- 39.TB CARE I. International Standards for Tuberculosis Care. 3rd edThe Hague: TB CARE I; 2014. [Google Scholar]
- 40.Kruijshaar ME, Pimpin L, Abubakar I, Rice B, Delpech V, Drumright LN, et al. The burden of TB-HIV in the EU: how much do we know? A survey of surveillance practices and results. Eur Respir J 2011; 38:1374–1381. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009; 58:1–207.quiz CE201-204. [PubMed] [Google Scholar]
- 42.EuroHIV. Completeness of AIDS case reporting in Europe. HIV/AIDS surveillance in Europe, quarterly report 1996. Paris: Institut de médecine et d’épidémiologie africaines; 1996. [Google Scholar]
- 43.Antonucci G, Girardi E, Raviglione MC, Ippolito G. Risk factors for tuberculosis in HIV-infected persons. A prospective cohort study. The Gruppo Italiano di Studio Tubercolosi e AIDS (GISTA). JAMA 1995; 274:143–148. [DOI] [PubMed] [Google Scholar]
- 44.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 2009; 23:1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aldridge RW, Shaji K, Hayward AC, Abubakar I. Accuracy of probabilistic linkage using the enhanced matching system for public health and epidemiological studies. PLoS One 2015; 10:e0136179. [DOI] [PMC free article] [PubMed] [Google Scholar]


