Abstract
Objectives:
HIV infection is known to worsen the outcome of cervical human papillomavirus (HPV) infection and may do so differentially by HPV type.
Design:
Twenty-one studies were included in a meta-analysis of invasive cervical cancers (ICC) among women infected with HIV in Africa.
Method:
Type-specific HPV DNA prevalence was compared with data from a similar meta-analysis of HIV-negative ICC using prevalence ratios (PR).
Results:
HPV detection was similar in 770 HIV-positive (91.2%) and 3846 HIV-negative (89.6%) ICC, but HIV-positive ICC harbored significantly more multiple HPV infections (PR = 1.75, 95% confidence intervals: 1.18 to 2.58), which were significantly more prevalent in ICC tested from cells than from biopsies. HPV16 was the most frequently detected type in HIV-positive ICC (42.5%), followed by HPV18 (22.2%), HPV45 (14.4%), and HPV35 (7.1%). Nevertheless, HIV-positive ICC were significantly less frequently infected with HPV16 than HIV-negative ICC (PR = 0.88, 95% confidence intervals: 0.79 to 0.99). Other high-risk types were significantly more prevalent in HIV-positive ICC, but only for HPV18 was there a significantly higher prevalence of both single and multiple infections in HIV-positive ICC. Increases for other high-risk types were primarily accounted for by multiple infections. The proportion of HPV-positive ICC estimated attributable to HPV16/18 (71.8% in HIV positive, 73.4% in HIV negative) or HPV16/18/31/33/45/52/58 (88.8%, 89.5%) was not affected by HIV.
Conclusions:
HIV alters the relative carcinogenicity of HPV types, but prophylactic HPV16/18 vaccines may nevertheless prevent a similar proportion of ICC, irrespective of HIV infection.
Key Words: human papillomavirus, human immunodeficiency virus, cervical cancer, epidemiology, Africa
INTRODUCTION
The 13 human papillomavirus (HPV) types classified as carcinogenic, or probably carcinogenic to humans (group 1/2A carcinogens),1 hereafter referred to as high-risk (HR) types, differ greatly in their carcinogenic potential.2 HPV16 is the most potent,1 causing more than half of all invasive cervical cancers (ICC) worldwide,3 followed by HPV18 and 45,2 accounting for about 15% and 5%, respectively.3 In Africa, a region of high burden of HPV infection4 and ICC,5 the attributable fraction for HPV16 seems slightly lower, and that of HPV45 slightly higher, compared with other regions.3
HIV is an important enhancer of HPV carcinogenesis, particularly in Africa where most women infected with HIV (WHIV) live. HIV-related immunodeficiency (as measured by CD4+ counts) is associated with increased prevalence,6 cumulative incidence,7 and persistence8 of HPV infection, cytological abnormalities,9 cervical intraepithelial neoplasia (CIN) 2 and 3,10 and ICC.11 Indeed, WHIV have an important excess risk of ICC in comparison with the general female population.12
There is also evidence that the unfavorable impact of HIV-related immunodeficiency on HPV natural history is different by HR HPV type. First, in 2003, it was shown that the detection of HPV16 in WHIV was less affected by changes in immunodeficiency levels than other HR HPV types.6 In 2006, a meta-analysis confirmed that HPV16 was underrepresented relative to other HR HPV types among HIV-positive women, in all grades of cervical diagnoses up to high-grade precancerous lesions.13 However, it is not known if this increased attributable fraction for non-HPV16 types in precancerous lesions is also relevant to HIV-infected ICC, because of the very limited information at that time.13
Since then, in the era of improved survival of WHIV, thanks to widespread access to combination antiretroviral therapy, our group and others have generated much relevant data on HIV-infected ICC in Africa. We aimed, therefore, to collate all data on HPV-type distribution in ICC diagnosed in WHIV and to compare it with similar meta-analytical data in the absence of HIV infection. These data are relevant to understanding the HPV type–specific impact of immunodeficiency and to estimate the fraction of ICC in this HR group that may be preventable by vaccines targeting different HPV types. We also tried to overcome the difficulty in estimating the attributable fraction of HR HPV types for ICC in WHIV that derives from the higher frequency of multiple infections in comparison with the general population.13,14
METHODS
In 2006, our group published a systematic review of HPV type–specific prevalence in WHIV across the full spectrum of cervical diagnoses (but which included only 14 ICC cases) published between January 1, 1989, and October 31, 2005.13 For the present report, we extended and updated the initial MEDLINE search up to June 2015, using the terms “human immunodeficiency virus” and “human papillomavirus.” Additional relevant studies were identified in the reference lists of selected articles and in abstract books of relevant conferences. Eligible studies had to report HPV type–specific prevalence among HIV-positive ICC, detected using polymerase chain reaction (PCR)–based assays. If publications did not present type-specific HPV prevalence in the required format, data requests were made to authors.
Given the final geographical representation of the eligible studies, analyses were restricted to the 770 ICC cases identified in 21 studies from 12 countries in Africa (Kenya, South Africa, Ghana, Nigeria, Uganda, Botswana, Senegal, Mozambique, Zambia, Ivory Coast, Tanzania, and Guinea; see study details in Table 1). The sparse data from ICC cases reported from outside Africa (14 cases in Spain,36 5 in India,37 and 5 in the United States6,20,38) are not reported here.
TABLE 1.
List of Key Characteristics for Included Studies
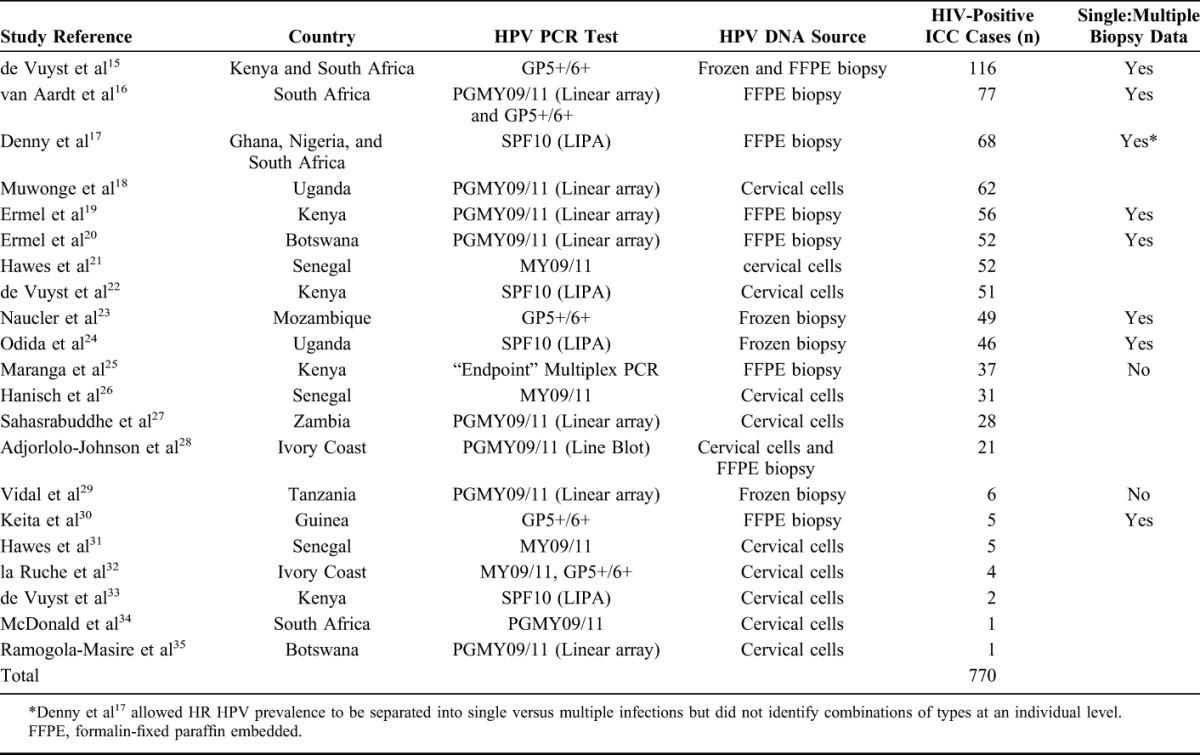
Data were extracted for 25 HPV types judged to be HR (group 1/2A) or possibly HR (group 2B) by a working group on the evaluation of carcinogenic risks to humans.1 Prevalence was estimated only among those studies that both genotyped and reported the HPV type in question, and thus, denominators can vary by type.
Type-specific HPV prevalence in HIV-positive ICC from Africa was compared with that in ICC from Africa reported in a previous meta-analysis,2 updated with recently published studies. Of note, none of the ICC cases included in this comparison group were known to be HIV-positive (72% were ICC diagnosed from the general population and were of unknown HIV status, whereas 28% were known HIV-negative ICC) and are hereafter referred to as HIV-negative ICC, for brevity sake.
Prevalence of HPV types in ICC were compared [between HIV-positive versus HIV-negative ICC and in HIV-positive ICC by type of sample (cervical cytology samples, hereafter referred to as “cells”, versus tumor biopsies)], using prevalence ratios (PR) calculated using generalized linear models, with 95% confidence intervals (CI) calculated assuming the nonindependence of cases within the same study using cluster-correlated robust variance estimates.39
For a subset of 8 studies (Table 1), which tested for all 13 HR HPV types from tumor biopsies and for which relevant data on HR HPV infection at an individual level were obtained, it was possible to describe the prevalence of HR HPV types in single (absence of any other HR HPV type) versus multiple (presence of another HR HPV type) infections. These studies were used to compare the prevalence of single and multiple infections between HIV-positive and known HIV-negative ICC cases by the use of study-adjusted PR and to estimate the attributable fraction of ICC due to HPV16/18 and HPV16/18/31/33/45/52/58. Of note, analyses of single/multiple infections were restricted to HPV-positive ICC, under the assumption that most HPV-negative ICC are false negative, and have the same underlying HPV-type distribution as HPV-positive ICC.
RESULTS
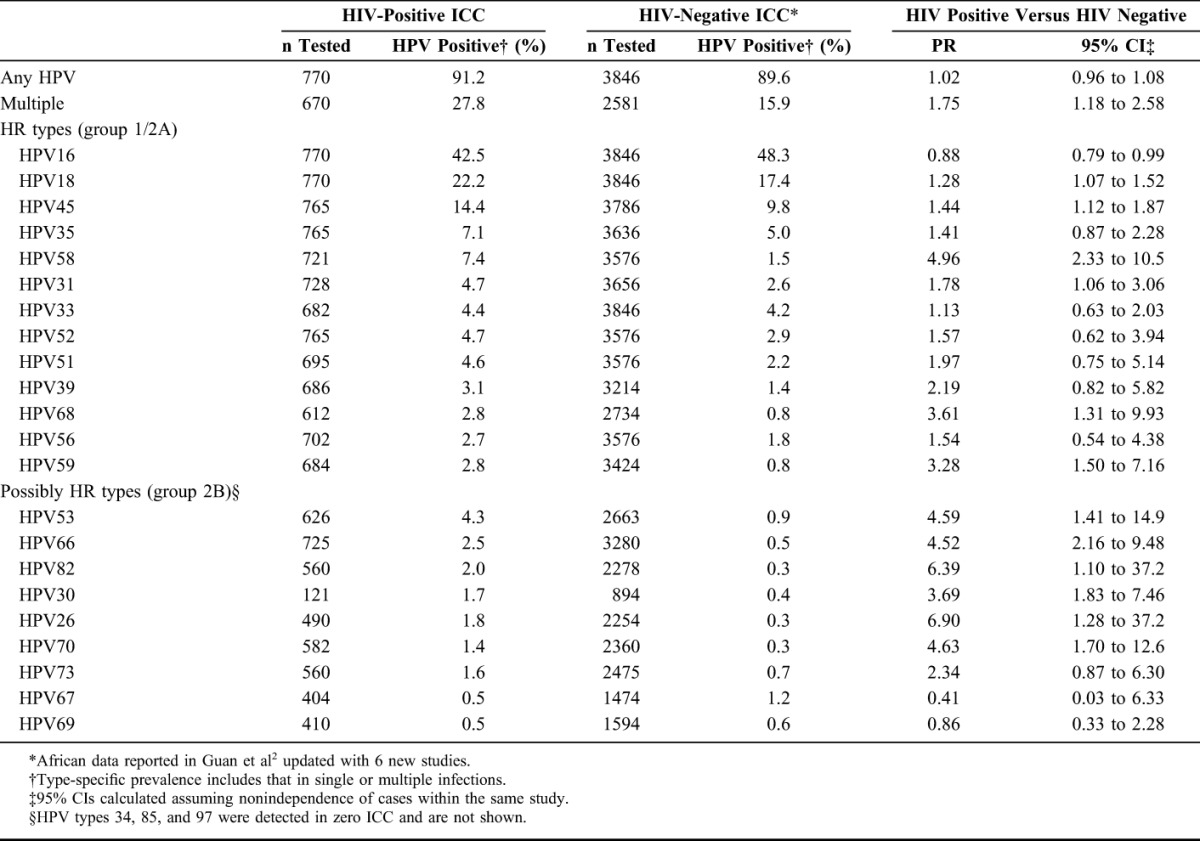
Twenty-one studies from Africa met the inclusion criteria, including a total of 770 HIV-positive ICC. Details on each of the included studies are presented in Table 1. Ten studies, including 512 ICC, tested for HPV DNA from biopsies; 10 studies, including 237 ICC, tested for HPV DNA from cervical cells; and 1 study, including 21 ICC, tested from cervical cells and/or tumor biopsies. HPV type–specific prevalence for HR and possibly HR types among all HIV-positive ICC is shown in Table 2 and compared with corresponding prevalence among 3846 HIV-negative African ICC, in decreasing order of prevalence in HIV-positive ICC. Detection of any HPV type was similar in HIV-positive (91.2%) and HIV-negative (89.6%) ICC, but HIV-positive ICC were more likely to be infected with multiple HPV types (27.8%) than HIV-negative ICC (15.9%) (PR = 1.75, 95% CI: 1.18 to 2.58). HPV16 was the most frequently detected HPV type in HIV-positive ICC (42.5%), followed by HPV18 (22.2%) and HPV45 (14.4%). Nevertheless, HIV-positive ICC were significantly less likely to be infected with HPV16 than HIV-negative ICC (PR = 0.88, 95% CI: 0.79 to 0.99). In contrast, all other HR types were more frequently detected in HIV-positive than HIV-negative ICC (Table 2), most significantly so, with the largest difference seen for HPV58 (PR = 4.96, 95% CI: 2.33 to 10.5). Almost all possibly HR HPV types were also more frequently detected in HIV-positive compared with HIV-negative ICC (Table 2).
TABLE 2.
Comparison of HPV Prevalence in ICC in Africa by HIV Status
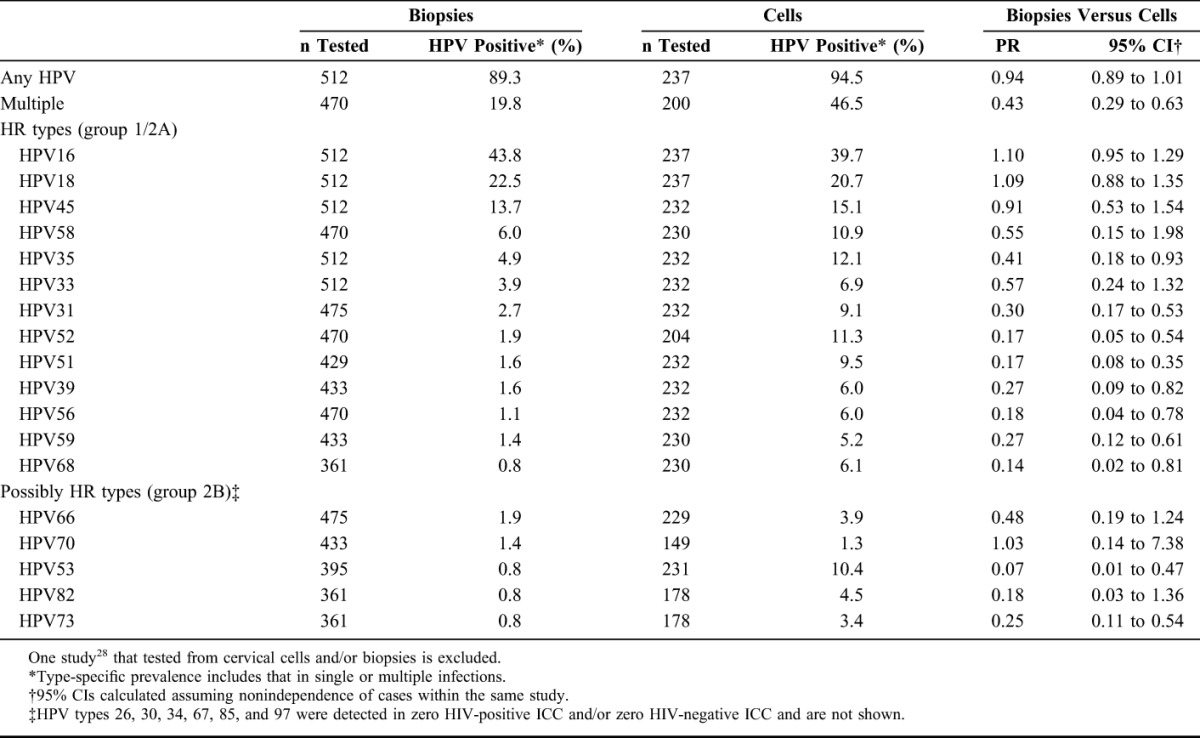
Comparisons of HPV prevalence in ICC tested from cells versus biopsies are shown in Table 3, in decreasing order of detection in biopsies, for HR and possibly HR types. The difference in detection of any HPV infection in HIV-positive ICC tested from biopsies (89.3%) compared with cells (94.5%) was of borderline statistical significance (PR = 0.94, 95% CI: 0.89 to 1.00), but the detection of multiple infections was significantly lower in biopsies (19.8% versus 46.5%) (PR = 0.43, 95% CI: 0.29 to 0.63). Detection of HPV16, 18, and 45 were similar in cells and biopsies, but most other HR types were significantly less frequently detected in biopsies than cells, with the greatest decrease seen for HPV51, HPV52, and HPV68. Large decreases in detection in biopsies versus cells were also seen for the possibly HR types HPV53, HPV73, and HPV82.
TABLE 3.
HPV Prevalence in HIV-Positive ICC in Africa, by HPV DNA Source (Exfoliated Cells Versus Biopsies)
In a subset of 8 studies testing HPV from biopsies of 469 HIV-positive and 1076 known HIV-negative ICC, it was possible to distinguish whether individual HR HPV types were found in the absence (single) or presence (multiple) of another HR type. After restriction to HPV-positive ICC (424 HIV positive and 946 HIV-negative), data are shown by HR type in Table 4, in decreasing order of prevalence of single infection in HIV-positive ICC. Detection of HPV16 single infection was significantly lower (PR = 0.84, 95% CI: 0.73 to 0.95) and HPV16 multiple infections higher (1.32, 95% CI: 0.90 to 1.93) in HIV-positive versus HIV-negative ICC. For HPV18, detection of both single (1.32, 95% CI: 1.03 to 1.71) and multiple (1.83, 95% CI: 1.12 to 2.99) infections were significantly higher in HIV-positive than HIV-negative ICC. Conversely, for most other HR types (also for possibly HR types—data not shown), multiple infections were more frequent in HIV-positive than HIV-negative ICC, but there were no significant differences in single infections. The most extreme case was HPV33 for which there was a strong lack of single infection (PR = 0.06, 95% CI: 0.01 to 0.42) and a strong excess of multiple infections (PR = 8.29, 95% CI: 2.77 to 24.81).
TABLE 4.
Prevalence of Single and Multiple Infections of HR HPV Types in HPV-Positive ICC in Africa, by HIV Status
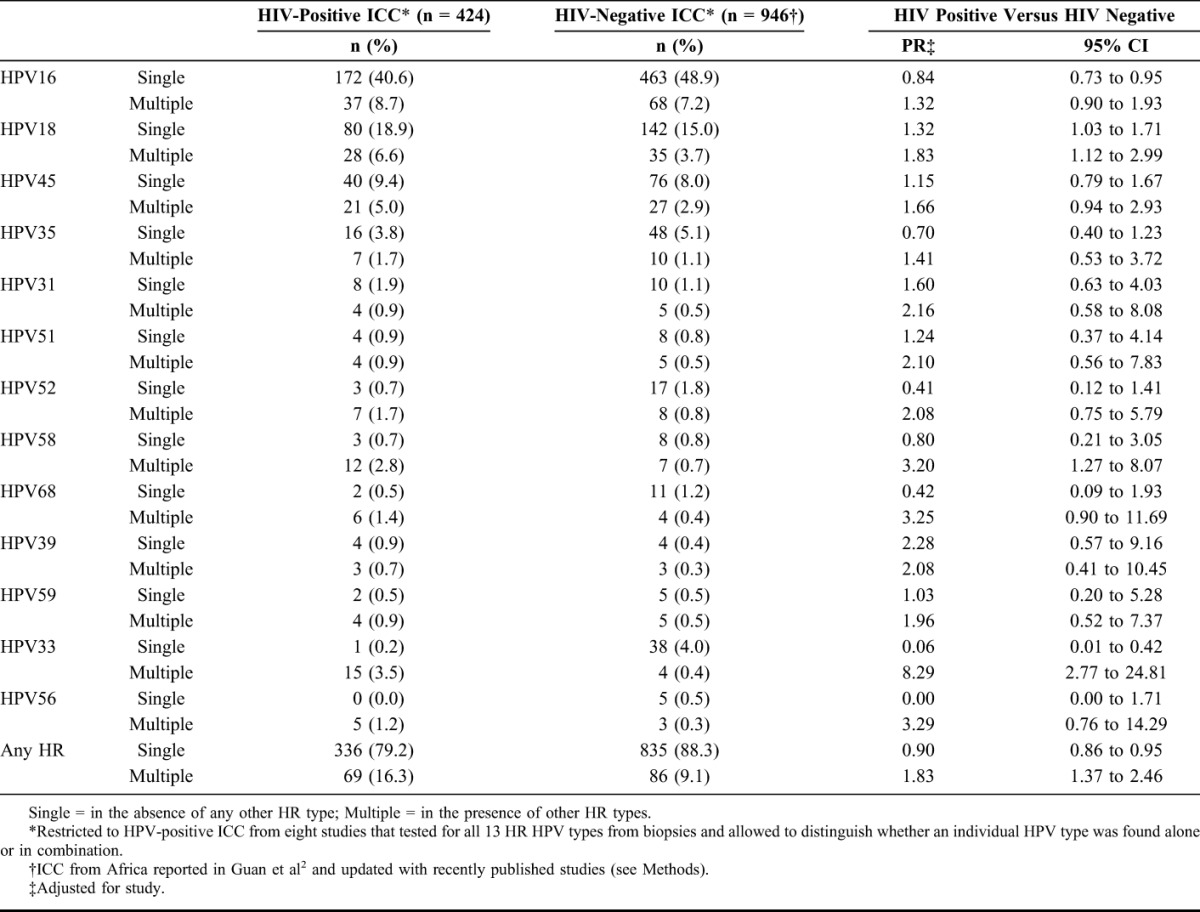
Table 5 presents prevalence of HR HPV types in different combinations, with the aim to estimate the fraction of ICC attributable to HPV16/18 and HPV16/18/31/33/45/52/58 in Africa, by HIV status. Overall detection of HPV16/18 in HPV-positive ICC was not significantly different between HIV-positive (71.8%) and HIV-negative (73.4%) ICC (PR = 0.99, 95% CI: 0.92 to 1.07). However, HPV16/18 infection alone was lower (61.7% versus 67.3%) and that of HPV16/18 infection in combination with another HR was higher (10.1% versus 6.1%, respectively). Table 5 also shows that the frequency of detection of HPV16/18/31/33/45/52/58 was not different between HIV-positive (88.8%) and HIV-negative (89.5%) ICC (PR = 1.00, 95% CI: 0.95 to 1.04), with a similar shift toward detection of HPV16/18/31/33/45/52/58 in combination with other HR types in HIV-positive ICC (5.9% versus 2.5%).
TABLE 5.
Prevalence of Combinations of HR HPV Types in HPV-Positive ICC in Africa by HIV Status
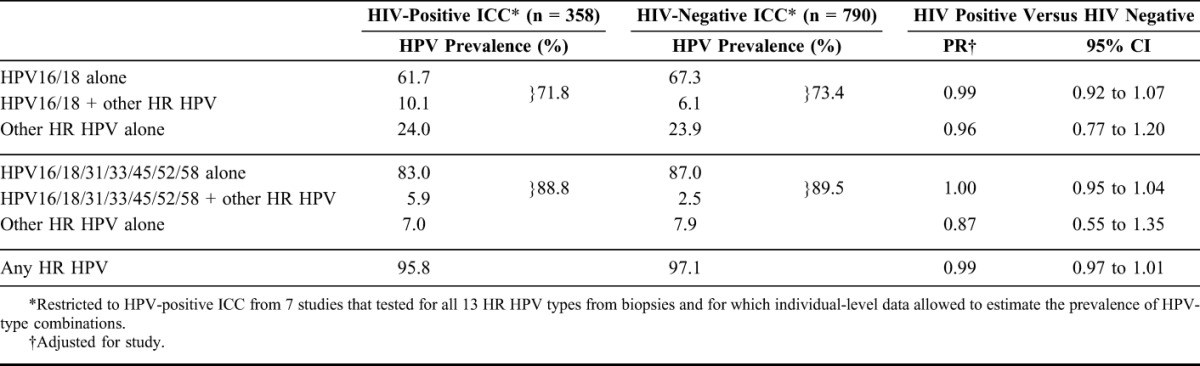
The detection of other HR HPV types alone was not significantly different between HIV-positive and HIV-negative ICC, when estimated neither in the absence of HPV16/18 (24.0% versus 23.9%; PR = 0.96, 95% CI: 0.77 to 1.20) nor in the absence of HPV16/18/31/33/45/52/58 (7.0% versus 7.9%; PR = 0.87, 95% CI: 0.55 to 1.35). There was no difference in the detection of HR HPV between HIV-positive and HIV-negative ICC (95.8% versus 97.1%; PR = 0.99, 95% CI: 0.97 to 1.01).
Among the 27 ICC in HIV-positive women that were reported as adeno- or adenosquamous carcinomas, HPV16, 18, 45, and 35 were detected in 6, 10, 6, and 2 cases, respectively. By comparison, among 75 HIV-negative adeno- or adenosquamous carcinomas, HPV16, 18, 45, and 35 were detected in 16, 29, 14, and 0 cases, respectively.
DISCUSSION
This meta-analysis of ICC from Africa is the first to assess type-specific HPV prevalence in a large number of ICC diagnosed among WHIV and to allow a comparison with similar data in the general population. Our main finding is that the proportion of ICC attributable to HPV16 is somewhat lower in HIV-positive than HIV-negative women. This is a confirmation of the relative shift from HPV16 to other HR types that has already been observed for WHIV with normal cytology and cervical intraepithelial lesions,13 even if the difference in HPV16 proportion in ICC is less than that seen among normal cytology and cervical intraepithelial lesions.13 Indeed, the importance of HPV16 increases with severity of lesion,2 so that HPV16 remains by far the most common HR HPV type, in both HIV-positive and HIV-negative ICC.
The detection of all HR types, even HPV16, is higher in WHIV in comparison with HIV-negative women. However, the prevalence of HPV16 in WHIV is known to be less affected by decreases in CD4+ cell counts than other HR HPV types.6 One possible explanation of HPV16's relative insensitivity to immune status is that, through its evolution, it has created better mechanisms to avoid host immune surveillance relative to other HPV types. Hence, relatively speaking, other HR types profit from HIV-related immunodeficiency to a greater degree than does HPV16.
Underrepresentation in HIV-positive ICC was seen uniquely for HPV16. All other HR HPV types were significantly overrepresented. Nevertheless, type-specific attribution of the non-HPV16 fraction of HIV-positive ICC is difficult because of the large proportion of HIV-positive ICC that are infected with multiple HR HPV types.
Testing HPV from biopsies instead of cells greatly reduced the detection of multiple HPV infections in ICC cases and helps to tackle the problem of HPV-type attribution. This difference is consistent with our previous findings among 468 WHIV in Kenya, in which cells contained 2-fold more multiple infections than biopsies among WHIV with biopsy-proven CIN2/3,14 and studies of HIV-negative women with cervical abnormalities.40,41 This is not surprising as exfoliated cells come from a wide genital area, including the cervix, vagina, and vulvar opening, whereas biopsies are concentrated at the site of the tumor. Interestingly, the prevalence of HPV16 and 18 were unaffected by the type of sample in our meta-analysis, suggesting a stronger causal relationship with ICC than most other HR types, that were significantly more likely to be present in cells than biopsies. This agrees, for HPV16 at least, with a study that used laser capture microdissection to ultrarefine HPV-type attribution to a single lesion in HIV-negative women.41 Among 257 CIN2/3, HPV16 was identified as the causal type in almost all CIN2/3 cases with HPV16 in cytology, whereas a large proportion of other HPV types found in cervical smears were not detected at the tissue level.41
The second approach we used to address the attribution problem was by characterizing each HR HPV in terms of single and multiple infections, in a subset of studies that best allowed such a distinction (and restricted to HPV-positive ICC from studies in which HPV DNA was tested from biopsies only). This approach suggested that the non-HPV16 fraction in HIV-positive ICC could in part be accounted for by an increased fraction attributable to HPV18. A significant excess of HPV18 detection in HIV-positive ICC was seen for both single and multiple HPV18 infections. Some of the individual studies included in this meta-analysis had already reported significantly increased detection of HPV1815,17 in HIV-positive ICC. For all other HR types and possibly HR types, their increased detection in HIV-positive ICC was mainly driven by multiple infections, so that current data are insufficient to support their increased attributable fraction in HIV-positive ICC.
With respect to the potential impact of HPV16/18 vaccines in WHIV, the decrease in the HPV16 attributable fraction in HIV-positive ICC seemed to be compensated by an increase in HPV18 fraction, so that the proportion of ICC attributable to HPV16/18 was similar in HIV-positive and HIV-negative ICC. However, this interpretation relies on the assumption that ICC that are positive for HPV16/18 and other HR types (ie, 10.1% in HIV-positive versus 6.1% in HIV-negative ICC) are causally related to HPV16/18. This assumption is supported by vast knowledge on the special carcinogenic potency of HPV16 and 181 and has been incorporated into “hierarchical” algorithms of attributing HPV types to cervical disease in previous studies.42 Similarly, the fraction theoretically preventable by an HPV16/18/31/33/45/52/58 vaccine (∼90%) seems unaffected by HIV status, with a large part of the remaining fraction in African ICC being attributable to HPV35, regardless of HIV status.
Restriction of the HIV-negative comparison to African ICC only was supported by the finding that the fraction of HIV-negative ICC infected with HPV16/18 in Africa remains slightly lower than in other world regions3 and that of HPV45 and 35 higher.3 Although only PCR-based protocols were accepted for inclusion in this meta-analysis, even they are known to not amplify all individual types,43 most notably in multiple-type infections, with the same sensitivity. We attempted to attenuate this problem by accounting for interstudy variation when calculating PR. Additional adjustment for PCR primers and/or HPV DNA source (cells versus biopsies) did not materially affect our findings (data not shown).
Important limitations of our meta-analysis are the lack of information on the time of acquisition of HIV infection before ICC diagnosis and the history of immunodeficiency (ie, CD4+ cell counts and eventual reconstitution with antiretroviral therapy). Indeed, in some HIV-positive ICC reported here, HIV-related immunodeficiency may have intervened after the beginning of HPV-driven carcinogenic transformation (a process that typically takes decades). Furthermore, the comparison group of ICC from the general population may have included some HIV-positive ICC cases. Nevertheless, a large majority of these cases were diagnosed before widespread combination antiretroviral therapy access in Africa, at which time few WHIV were living long enough to be diagnosed with ICC. This would have a dilution effect of biasing associations toward the null, so that true differences may be greater than those reported here.
In conclusion, HIV-related immunodeficiency seems to alter the relative carcinogenicity of HR HPV types, so that a lower fraction of ICC is caused by HPV16. However, the attributable fraction for HPV18 is concomitantly higher, so that current prophylactic vaccines against HPV16 and 18, which have shown good immunogenicity in HIV-infected populations,44 may still prevent a similar proportion of ICC, irrespective of HIV status. Attributable fractions by HIV status are also similar when considering possible cross-protection of HPV16/18 vaccines against other HR types45,46 or the 9-valent vaccine that is also efficacious against HPV31/33/45/52/58.47
ACKNOWLEDGMENTS
This meta-analysis is indebted to those authors who provided data on HIV-positive ICC in a format that was unavailable in the original publication of their studies, namely Drs. Stephen Hawes and Rachel Hanisch (University of Washington, Seattle, WA); Drs. Matthys Cornelis van Aardt and Greta Dreyer (University of Pretoria, South Africa); Dr. Catherine Muwonge (MRC/UVRI Uganda Research Unit on AIDS, Entebbe, Uganda); Drs. Elisabete Weiderpass, Pontus Naucler, and Joakim Dillner (Karolinska Institutet, Stockholm, Sweden); Dr. Ian Hampson (University of Manchester Institute of Cancer Sciences, Manchester, United Kingdom); Dr. Charles Lacey (University of York, York, United Kingdom); Drs. Darron Brown and Aaron Ermel (Indiana University); and Dr. Elizabeth Unger (Centers for Disease Control and Prevention, Atlanta, GA), and also the many authors who provided African data for our previously published meta-analysis on HIV-negative ICC.2
Footnotes
Supported by grants from the Fondation de France (Grant number: 00039621) and the Bill & Melinda Gates Foundation, USA (Grant number: OPP1053353). The work of ST was undertaken during the tenure of a Postdoctoral Fellowship from the International Agency for Research on Cancer, partially supported by the European Commission FP7 Marie Curie Actions—People—Co-funding of regional, national, and international programmes (COFUND).
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.IARC. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100B:1–475. Available from: http://monographs.iarc.fr/ENG/Monographs/vol100F/index.php. Accessed February 4, 2016. [PMC free article] [PubMed] [Google Scholar]
- 2.Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–2359. [DOI] [PubMed] [Google Scholar]
- 3.Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–935. [DOI] [PubMed] [Google Scholar]
- 4.Bruni L, Diaz M, Castellsagué X, et al. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–1799. [DOI] [PubMed] [Google Scholar]
- 5.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. [DOI] [PubMed] [Google Scholar]
- 6.Strickler HD, Palefsky JM, Shah KV, et al. Human papillomavirus type 16 and immune status in human immunodeficiency virus-seropositive women. J Natl Cancer Inst. 2003;95:1062–1071. [DOI] [PubMed] [Google Scholar]
- 7.Massad LS, Xie X, Burk R, et al. Long-term cumulative detection of human papillomavirus among HIV seropositive women. AIDS. 2014;28:2601–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowhani-Rahbar A, Hawes SE, Sow PS, et al. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J Infect Dis. 2007;196:887–894. [DOI] [PubMed] [Google Scholar]
- 9.Harris TG, Burk RD, Palefsky JM, et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA. 2005;293:1471–1476. [DOI] [PubMed] [Google Scholar]
- 10.de Vuyst H, Mugo NR, Chung MH, et al. Prevalence and determinants of human papillomavirus infection and cervical lesions in HIV-positive women in Kenya. Br J Cancer. 2012;107:1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham AG, Strickler HD, D'Souza G. Invasive cervical cancer risk among HIV-infected women is a function of CD4 count and screening. J Acquir Immune Defic Syndr. 2013;63:e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosupressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. [DOI] [PubMed] [Google Scholar]
- 13.Clifford GM, Goncalves MA, Franceschi S, et al. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS. 2006;20:2337–2344. [DOI] [PubMed] [Google Scholar]
- 14.de Vuyst H, Chung MH, Baussano I, et al. Comparison of HPV DNA testing in cervical exfoliated cells and tissue biopsies among HIV-positive women in Kenya. Int J Cancer. 2013;133:1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vuyst H, Ndirangu G, Moodley M, et al. Prevalence of human papillomavirus in women with invasive cervical carcinoma by HIV status in Kenya and South Africa. Int J Cancer. 2012;131:949–955. [DOI] [PubMed] [Google Scholar]
- 16.van Aardt MC, Dreyer G, Pienaar HF, et al. Unique human papillomavirus-type distribution in South African women with invasive cervical cancer and the effect of human immunodeficiency virus infection. Int J Gynecol Cancer. 2015;25:919–925. [DOI] [PubMed] [Google Scholar]
- 17.Denny L, Adewole I, Anorlu R, et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer. 2014;134:1389–1398. [DOI] [PubMed] [Google Scholar]
- 18.Muwonge C, Musana O, Othieno E, et al. The spectrum of HPV genotypes in cervical cancer in Uganda. Poster presented at: 26th International Papillomavirus Conference & Clinical and Public Health Workshops; July 3–8, 2010, Montreal, Canada, P-620. 2010. [Google Scholar]
- 19.Ermel A, Ramogola-Masire D, Macharia B, et al. Ongogenic HPV types in invasive cervical cancers from women living in the United States, Kenya, or Botswana. Poster presented at: 30th International Papillomavirus Conference & Clinical and Public Health Workshops; September 16–21, 2015, Lisbon, Portugal, P200. 2015. [Google Scholar]
- 20.Ermel A, Ramogola-Masire D, Zetola N, et al. Invasive cervical cancers from women living in the United States or Botswana: differences in human papillomavirus type distribution. Infect Agent Cancer. 2014;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawes S, Feng Q, Toure P, et al. Human papillomavirus types among HIV positive and negative women with invasive squamous cell cervical cancer in Senegal, West Africa [Oral presentation]. 24th International Papillomavirus Conference & Clinical Workshop, Beijing, China, November 3–9, 2007, 7C-04. 2007. [Google Scholar]
- 22.de Vuyst H, Gichangi P, Estambale B, et al. Human papillomavirus types in women with invasive cervical carcinoma by HIV status in Kenya. Int J Cancer. 2008;122:244–246. [DOI] [PubMed] [Google Scholar]
- 23.Naucler P, Mabota da Costa F, da Costa JL, et al. Human papillomavirus type-specific risk of cervical cancer in a population with high human immunodeficiency virus prevalence: case-control study. J Gen Virol. 2011;92(pt 12):2784–2791. [DOI] [PubMed] [Google Scholar]
- 24.Odida M, Sandin S, Mirembe F, et al. HPV types, HIV and invasive cervical carcinoma risk in Kampala, Uganda: a case-control study. Infect Agent Cancer. 2011;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maranga IO, Hampson L, Oliver AW, et al. HIV infection alters the spectrum of HPV subtypes found in cervical smears and carcinomas from Kenyan women. Open Virol J. 2013;7:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanisch RA, Sow PS, Toure M, et al. Influence of HIV-1 and/or HIV-2 infection and CD4 count on cervical HPV DNA detection in women from Senegal, West Africa. J Clin Virol. 2013;58:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, et al. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer. 2007;96:1480–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adjorlolo-Johnson G, Unger ER, Boni-Ouattara E, et al. Assessing the relationship between HIV infection and cervical cancer in Cote d'Ivoire: a case-control study. BMC Infect Dis. 2010;10:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidal AC, Murphy SK, Hernandez BY, et al. Distribution of HPV genotypes in cervical intraepithelial lesions and cervical cancer in Tanzanian women. Infect Agent Cancer. 2011;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keita N, Clifford GM, Koulibaly M, et al. HPV infection in women with and without cervical cancer in Conakry, Guinea. Br J Cancer. 2009;101:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawes SE, Critchlow CW, Faye Niang MA, et al. Increased risk of high-grade cervical squamous intraepithelial lesions and invasive cervical cancer among African women with human immunodeficiency virus type 1 and 2 infections. J Infect Dis. 2003;188:555–563. [DOI] [PubMed] [Google Scholar]
- 32.la Ruche G, You B, Mensah-Ado I, et al. Human papillomavirus and human immunodeficiency virus infections: relation with cervical dysplasia-neoplasia in African women. Int J Cancer. 1998;76:480–486. [DOI] [PubMed] [Google Scholar]
- 33.de Vuyst H, Steyaert S, Van Renterghem L, et al. Distribution of human papillomavirus in a family planning population in Nairobi, Kenya. Sex Transm Dis. 2003;30:137–142. [DOI] [PubMed] [Google Scholar]
- 34.McDonald AC, Tergas AI, Kuhn L, et al. Distribution of human papillomavirus genotypes among HIV-positive and HIV-negative women in Cape Town, South Africa. Front Oncol. 2014;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramogola-Masire D, McGrath CM, Barnhart KT, et al. Subtype distribution of human papillomavirus in HIV-infected women with cervical intraepithelial neoplasia stages 2 and 3 in Botswana. Int J Gynecol Pathol. 2011;30:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darwich L, Canadas MP, Sirera G, et al. Human papillomavirus genotype distribution and human papillomavirus 16 and human papillomavirus 18 genomic integration in invasive and in situ cervical carcinoma in human immunodeficiency virus-infected women. Int J Gynecol Cancer. 2011;21:1486–1490. [DOI] [PubMed] [Google Scholar]
- 37.Joshi S, Sankaranarayanan R, Muwonge R, et al. Screening of cervical neoplasia in HIV-infected women in India. AIDS. 2013;27:607–615. [DOI] [PubMed] [Google Scholar]
- 38.Jamieson DJ, Duerr A, Burk R, et al. Characterization of genital human papillomavirus infection in women who have or who are at risk of having HIV infection. Am J Obstet Gynecol. 2002;186:21–27. [DOI] [PubMed] [Google Scholar]
- 39.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. [DOI] [PubMed] [Google Scholar]
- 40.Gravitt PE, van Doorn LJ, Quint W, et al. Human papillomavirus (HPV) genotyping using paired exfoliated cervicovaginal cells and paraffin-embedded tissues to highlight difficulties in attributing HPV types to specific lesions. J Clin Microbiol. 2007;45:3245–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Marel J, Berkhof J, Ordi J, et al. Attributing oncogenic human papillomavirus genotypes to high-grade cervical neoplasia: which type causes the lesion? Am J Surg Pathol. 2015;39:496–504. [DOI] [PubMed] [Google Scholar]
- 42.Wentzensen N, Schiffman M, Dunn T, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. 2009;125:2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klug SJ, Molijn A, Schopp B, et al. Comparison of the performance of different HPV genotyping methods for detecting genital HPV types. J Med Virol. 2008;80:1264–1274. [DOI] [PubMed] [Google Scholar]
- 44.Toft L, Storgaard M, Muller M, et al. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind clinical trial. J Infect Dis. 2014;209:1165–1173. [DOI] [PubMed] [Google Scholar]
- 45.Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum Vaccin. 2011;7:1359–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. [DOI] [PubMed] [Google Scholar]
- 47.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723. [DOI] [PubMed] [Google Scholar]


