Abstract
IMPORTANCE
Alzheimer disease (AD) pathology starts long before clinical symptoms manifest, and there is no therapy to treat, delay, or prevent the disease. A shared blood circulation between 2 mice (aka parabiosis) or repeated injections of young blood plasma (plasma from 2- to 3-month-old mice) into old mice has revealed benefits of young plasma on synaptic function and behavior. However, to our knowledge, the potential benefit of young blood has not been tested in preclinical models of neurodegeneration or AD.
OBJECTIVES
To determine whether young blood plasma ameliorates pathology and cognition in a mouse model for AD and could be a possible future treatment for the disease.
DESIGN, SETTING, AND PARTICIPANTS
In this preclinical study, mice that harbor a human mutant APP gene, which causes familial AD, were aged to develop AD-like disease including accumulation of amyloid plaques, loss of synaptic and neuronal proteins, and behavioral deficits. The initial parabiosis studies were done in 2010, and the final studies were conducted in 2014. Alzheimer disease model mice were then treated either by surgically connecting them with a young healthy mouse, thus providing a shared blood circulation through parabiosis, or through repeated injections of plasma from young mice.
MAIN OUTCOMES AND MEASURES
Neuropathological parameters and changes in hippocampal gene expression in response to the treatment were assessed. In addition, cognition was tested in AD model mice intravenously injected with young blood plasma.
RESULTS
Aged mutant amyloid precursor protein mice with established disease showed a near complete restoration in levels of synaptic and neuronal proteins after exposure to young blood in parabiosis (synaptophysin P = .02; calbindin P = .02) or following intravenous plasma administration (synaptophysin P < .001; calbindin P = .14). Amyloid plaques were not affected, but the beneficial effects in neurons in the hippocampus were accompanied by a reversal of abnormal extracellular receptor kinase signaling (P = .05), a kinase implicated in AD. Moreover, young plasma administration was associated with improved working memory (P = .01) and associative memory (P = .02) in amyloid precursor protein mice.
CONCLUSIONS AND RELEVANCE
Factors in young blood have the potential to ameliorate disease in a model of AD.
Alzheimer disease (AD) is a complex neurodegenerative disorder, characterized by progressive loss of memory and deterioration of higher cognitive functions. Alzheimer disease is neuropathologically defined by extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tangles, and research during the past decades has led to the understanding that the disease is multifactorial and starts long before clinical symptoms manifest. The earliest cognitive deficits may be the result of neural network disruptions, caused by loss or dysfunction of dendritic spines and synapses leading to aberrant network activity.1 Loss of synaptophysin immunoreactive presynaptic terminals and depletion of calcium-dependent proteins, such as calbindin, are characteristics that correlate well with cognitive decline in both human patients with AD and transgenic mouse models of the disease.2–4 Genetic mutations in the APP gene result in abnormal accumulation of Aβ and are sufficient to cause AD in humans, and amyloid precursor protein (APP) transgenic mice develop pathological and cognitive deficits that model aspects of the human disease.5 Currently, no effective therapy exists that treats, stops, or reverses AD. Given a rapidly aging population and advancing age as the primary nongenetic risk factor for AD, finding a cure or preventive measure is now more urgent than ever.
Several studies have shown that exposure to a young blood circulation through parabiosis or administration of young blood plasma (plasma from 2- to 3-month-old mice) reverses cognitive deficits observed with normal aging by increasing synaptic plasticity and hippocampal gene expression networks related to learning and memory and by improving vascular function.6–8 Heterochronic parabiosis is a surgical union of 2 organisms of different age that results in a shared blood circulation between, eg, 2 mice. The model thus allows us to study whether circulating factors from the young mouse can alter tissue function of the old parabiont or vice versa. Tissues from heterochronic pairs are compared with those of isochronic pairs, 2 organisms of the same age sharing a blood circulation, as a control.
To determine the effect of young circulatory factors on AD-like disease in mice, we used heterochronic parabiosis, in which we joined the circulatory systems of young, wildtype animals together with disease-laden human amyloid precursor protein APP transgenic mice harboring familial London and Swedish mutations (APP mice5) (Figure 1A). These APP mice have an accelerated pathology with a rapid appearance of Aβ plaques and synaptic degeneration,5 and they display a behavioral phenotype that resembles cognitive impairment of patients with AD.9 First, we analyzed changes in protein expression in the brains of these mice, and second, we used genome-wide microarray analysis to assess gene expression changes in the hippocampi of APP mice with established disease after heterochronic parabiosis. Finally, we tested cognitive performance of APP mice after exposure to young blood plasma.
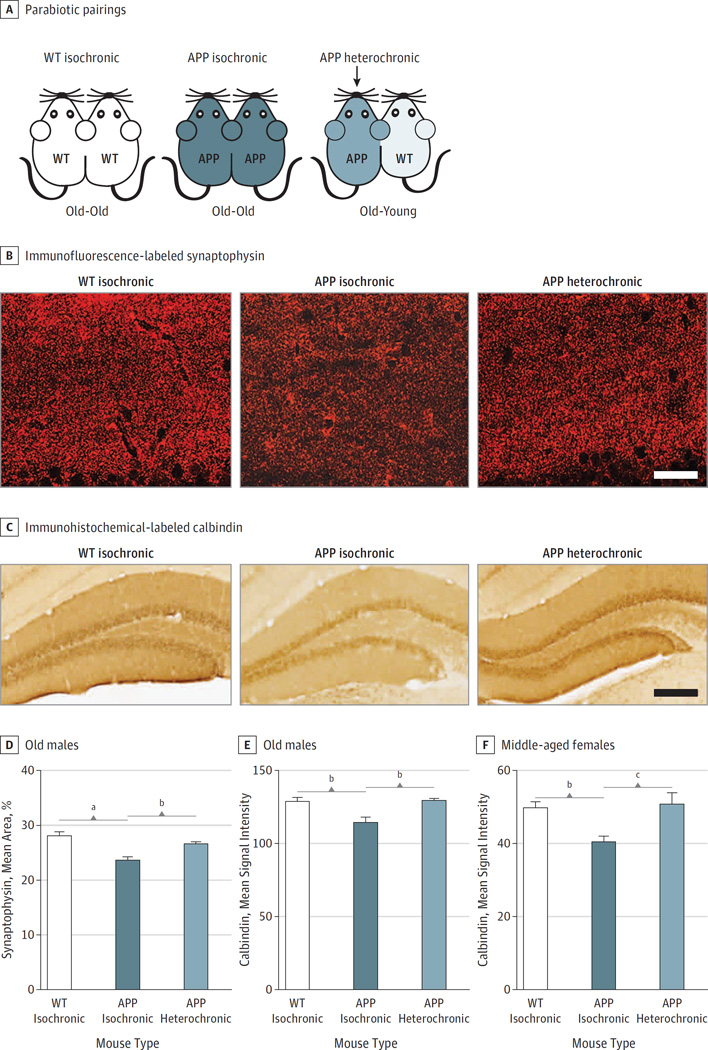
Figure 1. The Effect of Young Blood on Synaptic Activity Related Proteins inAmyloid Precursor Protein (APP) Mice.
A, Schematic depicting the 3 different parabiotic pairings: wildtype (WT) isochronic, APP isochronic, and APP heterochronic. Isochronic pairs are age-matched and the same age as the APP mouse from the heterochronic pair, which is connected to a young WT mouse (2–3 months old). One cohort consisted of old male mice (16–20 months old) and another of middle-aged female mice (10–12 months old). All pairs were surgically connected for 5 weeks.
B, Immunofluorescence-labeled synaptophysin in presynaptic terminals in the molecular layer of the DG of old male WT isochronic, APP isochronic, and APP heterochronic parabionts. C, Calbindin immunoreactivity in the DG of old male WT isochronic, APP isochronic, and APP heterochronic parabionts. Quantification of synaptophysin immunoreactivity (D), and calbindin immunoreactivity (E), in the molecular layer of the dentate gyrus of old male parabionts; WT isochronic (n = 6), APP isochronic (n = 6), and APP heterochronic (n = 4).
F, Quantification of calbindin immunoreactivity in the molecular layer of the dentate gyrus of middle-aged female parabionts; WT isochronic (n = 9), APP isochronic (n = 11), and APP heterochronic (n = 9). All data are shown as the mean (SEM).
a P < .001, 1-way analysis of variance, Tukey post hoc test (D–F). Scale bars: 25 µm in B, and 100 µm in C.
b P < .05.
c P < .01.
Methods
Mice
All animal procedures were conducted with approval of the animal care and use committee of the Veterans Administration Palo Alto Health Care System. The APP mice (mThy-1-hAPP751V171I, KM670/671NL; T41 line) have been described previously.5 The line was maintained on a C57BL/6 genetic background. For heterochronic parabiosis and plasma collection, young (2–3 months old) C57BL/6 mice were used (The Jackson Laboratory). Mice were anesthetized with chloral hydrate (Sigma-Aldrich) and transcardially perfused with phosphate-buffered saline (PBS). Brains were dissected, and 1 hemibrain was fixed for 48 hours in 4% paraformaldehyde and cryoprotected in 30% sucrose. Serial coronal sections (40 µm) were cut with a freezing microtome (Leica) and stored in cryoprotective medium. The hippocampus was dissected from the other hemibrain and stored in RNA later at −20°C.
Parabiosis
Parabiosis surgery followed previously described procedures.7 Mirror-image incisions were made at the left and right flanks through the skin and shorter incisions through the peritoneum. The peritoneal openings of the adjacent parabionts were sutured together. Elbow and knee joints from each parabiont were sutured together and the skin of each mouse was stapled (9-mm Autoclip, Clay Adams) to the skin of the adjacent parabiont. Each mouse received an intraperitoneal injection of saline and subcutaneous injections with Baytril antibiotic and Buprenex analgesic and was monitored during recovery. For overall health, several recovery characteristics were analyzed at various times after surgery including paired weights, nesting behavior, grooming, and stress response. Two cohorts were generated with wildtype isochronic old pairs, age-matched APP isochronic pairs, and heterochronic pairs with old APP mice connected to young mice (aged 2–3 months). One cohort consisted of old male mice (aged 16–20 months), and another of middle-aged female mice (aged 10–12 months). All pairs were surgically connected for 5 weeks.
Plasma Collection and Administration
Pooled mouse plasma was collected from young female mice (n = 120; aged 2–3 months) by intracardial bleed at time of sacrifice. Plasma was prepared from blood collected with edetic acid followed by centrifugation at 1000g. Plasma aliquots were stored at −80°C until use. Prior to administration, plasma was dialyzed using 3.5-kDa Slide-A-Lyzer Dialysis Cassettes (Thermo Scientific) in PBS to remove edetic acid. Adult female APP mice (n = 13; aged 10–12 months) and wildtype littermates (n = 13) were systemically treated with young plasma (150 µL/injection) via intravenous injections into the tail vein twice a week for 4 weeks.
Microarray Analysis
Hippocampi from 3 groups of parabionts (wildtype isochronic, APP isochronic, and APP heterochronic) were homogenized using a TissueLyserLT (Qiagen), and RNA was isolated using the Rneasy spin columns (Qiagen; Stanford Translational Applications Service Center). The quality of the RNA was determined on a Bioanalyzer (Agilent). We submitted 7 samples per group, but the RNA quality of 1 wildtype isochronic sample was too low for further processing. cDNA was synthesized, labeled, and hybridized to Affymetrix Mouse 430 2.0 arrays in biologic duplicates. The arrays were scanned using the Affymetrix GeneChip Scanner 3000 7G (Stanford Protein and Nucleic Acid Facility). Analysis was done in R (R Programming; The R Foundation). Raw data from the chips were normalized using robust multiarray average method.10 Genes with a mean log2 signal less than 6 in the wildtype group were considered as lowly expressed and were removed from the analysis. To reduce the probability of finding genes with unknown function or non-coding RNAs, all gene symbols with “Rik,” “Loc,” and “Mir,” were excluded from the analysis in addition to probes not associated with known genes. For comparisons between the 3 groups, significant analysis of microarrays (SAM; q < 0.05) followed by Tukey honestly significant difference (HSD; P < .05) were performed. To determine the intrinsic grouping in the gene expression profiles, hierarchical clustering has been applied using genes differentially expressed between groups according to SAM (P < .05). Specific differences between APP heterochronic and APP isochronic groups were analyzed using SAM (P < .05). Least absolute shrinkage and selection operator and elastic-net regularized generalized linear models (glmnet R package) were applied to select genes that best discriminate the APP isochronic and APP heterochronic groups.11 We used α = .6 as the elastic-net mixing parameter and α = 1 for least absolute shrinkage and selection operator penalty. The misclassification rate was used to select top candidates genes. Permutation-based validation of the genes selected by each glmnet model was performed (500 permutations). To get a list of candidate genes differentiating APP isochronic and APP heterochronic, a meta-ranking analysis was performed using the “RobustRankAggreg” R package.12 For each analysis, genes were ranked according to their significance. We used the following criteria: P values for SAM between APP heterochronic and APP isochronic groups and number of times that a gene is selected by the elastic-net and least absolute shrinkage and selection operator models across permutations. Absolute value of the log2 fold change between APP isochronic and APP heterochronic was also included in the meta-ranking analysis, but only for genes with P less than .05 in SAM.
For functional gene ontology analysis, the top 100 meta-ranked genes were subjected to the online gene ontology tool DAVID (http://david.abcc.ncifcrf.gov/) with the mouse genome as background list. Ingenuity Pathway Analysis software (Qiagen) was used to generate gene networks.
Y-Maze Test
Spontaneous alternation behavior was tested in a Y-maze as described previously.9 The Y-maze was made of solid white plastic and consisted of 2 symmetrical arms and 1 longer arm at 120° angles. At the beginning of trials, mice were placed in the longer arm and allowed to freely explore the 3 arms for 5 minutes. Arm entry was defined as having all 4 limbs inside an arm. The maze was cleaned with 70% ethanol between animals and before the first animal to eliminate traces of odor. The number of arm entries and the number of triads were recorded to calculate the alternation percentage (number of triads divided by the number of possible alternations multiplied by 100).
Fear Conditioning
Our paradigm followed a previously published protocol of a study with APP mice.9 On the training day, mice were placed in the chamber for a 3-minute baseline recording followed by 5 tone-shock pairings, separated by a 100-second interval. The foot shocks (0.5 mA, 2 seconds) were delivered 18 seconds following the tone (70 dB, 2 kHz, and 20 seconds). The next day, a novel context (different odor [peppermint], floor texture, chamber walls, and shape) was used for cued testing. After 3 minutes of baseline recording, 3 tones were presented to the mice. On the third day, mice were placed in the same context as the first day for 5 minutes with no shocks or tones to test contextual memory. Freezing was measured using a FreezeScan video tracking system and software (Cleversys Inc).
Smart-Homecage Monitoring
Mice were placed in a Smart Homecage (AfaSci Inc) for 1 hour. Infrared matrices detected activity, position, and locomotion of each mouse. Parameters of exploratory behavior, such as travel distance and rearing, were calculated automatically by CageScore software for different times.
Immunohistochemical and Fluorescent Immunostaining
Tissue processing and immunostaining were performed on free-floating sections (40 µm) following standard published techniques.13 Coronal brain sections were incubated overnight with either rabbit anticalbindin (1:15 000–1:100 000; Swant), biotinylated rabbit anti-3D6 (1:3500; Elan Pharmaceuticals), or mouse antisynaptophysin (1:1000) primary antibodies. Calbindin and 3D6 staining was revealed using the ABC kit (Vector) with diaminobenzidine (Sigma-Aldrich). Mean signal intensity was measured using National Institutes of Health ImageJ software. Synaptophysin was labeled with a fluorescent secondary antibody and quantified as previously reported.14
Tissue Aβ ELISA
Mouse tissue was subjected to serial Aβ extraction using radioimmunoprecipitation assay (50mM tris hydrochloride pH 7.4, 150mM sodium chloride, 1% Nonidet P40, 1mM edetic acid, and 0.25% deoxycholic acid) and guanidine buffer (5 M guanidine hydrochloride in 50 mM tris hydrochloride), all buffers containing Roche complete protease inhibitor cocktail at a concentration of 2× (Roche). Enzyme-linked immunosorbent assays were performed using Meso Scale technology (Meso Scale Discovery). Multiarray 96-well plates (Meso Scale Discovery) were coated with capture antibody 21D12 for total Aβ (Aβ12–28) and capture antibody 21F12 for Aβ42 (Aβ33–42). Plates were washed and diluted samples or Aβ1–40 and Aβ1–42 peptide standards were added. β-Amyloid was detected using biotinylated-3D6 antibody and SULFO-TAG streptavidin (Meso Scale Discovery). Plates were read on a Sector Imager 2400 (Meso Scale Discovery) and samples were normalized to Aβ standards. All Aβ antibodies were provided by Elan Pharmaceuticals.
Western Blot Analysis
Hippocampi from PBS- or plasma-treated mice were dissected after perfusion of the animals, snap frozen, cryohomogenized, and lysed in radioimmunoprecipitation assay lysis buffer with protease and phosphatase inhibitors. Protein concentrations were measured using the BCA Protein Assay Kit (Pierce). Tissue lysates were mixed with NuPage lithium dodecyl sulfate–loading buffer (Invitrogen), separated on 4%to 12% bis-tris gels (Invitrogen) using 3-(N-morpholino) propane-sulfonic acid sodium dodecyl sulfate running buffer (Invitrogen) and subsequently transferred onto a nitrocellulose membrane. Blots were blocked in 5% milk in TBS and incubated with mouse anti–extracellular receptor kinase (ERK) 1/2 (1:1000; 3A7; Cell Signaling Technology), rabbit anti-pERK1/2 (1:1000; Thr202/Tyr204; Cell Signaling Technology), and mouse anti–neuron-specific enolase (1:2000; 5E2; Thermo Scientific) in 5% milk in TBS with Tween. IRdye secondary antibodies and the Odyssey CLx (LI-COR Biosciences) were used to detect protein signals. Images were quantified using ImageJ software, version 1.44i.
Results
Effect of Young Blood on Synaptic Activity–Related Proteins in APP Mice
Synaptic and calcium-binding proteins are depleted early in AD2,4 and in mouse models of the disease.4,15 Similarly, 16- to 20-month-old APP male isochronic parabionts showed a significant reduction of synaptophysin immunoreactivity in presynaptic terminals in the molecular layer of the dentate gyrus compared with wild-type age-matched isochronic parabionts (Figure 1B and D). This deficit was significantly reversed in APP heterochronic parabionts that had been paired with young mice for 5 weeks (Figure 1B and D). Likewise, exposure to this young environment completely restored the loss of hippocampal calbindin observed in isochronic APP mice (Figure 1C and E). Female sex is a strong risk factor for AD,16 and in our model, female APP mice were associated with accelerated Aβ deposition at middle age (10–12 months old; eFigure 1 in the Supplement).15 We observed that the almost 20% loss in calbindin immunoreactivity in middle-aged female APP mice was also restored in age-matched female APP heterochronic parabionts (Figure 1F), suggesting that the effect of young blood is not sex specific. Interestingly, heterochronic parabiosis neither altered the levels of Aβ immunoreactivity nor guanidine and radioimmunoprecipitation assay–soluble Aβ in the hippocampi of APP mice with preexisting amyloidosis (eFigure 2 in the Supplement), suggesting that young blood could restore synaptic protein levels in spite of high levels of amyloid. Moreover, microglial activation as indicated by CD68 immunoreactivity was not significantly altered by exposure to young blood (eFigure 3 in the Supplement).
Heterochronic Parabiosis Modulates Genes Involved in Key Neuronal Signaling Pathways in the Hippocampus of APP Mice
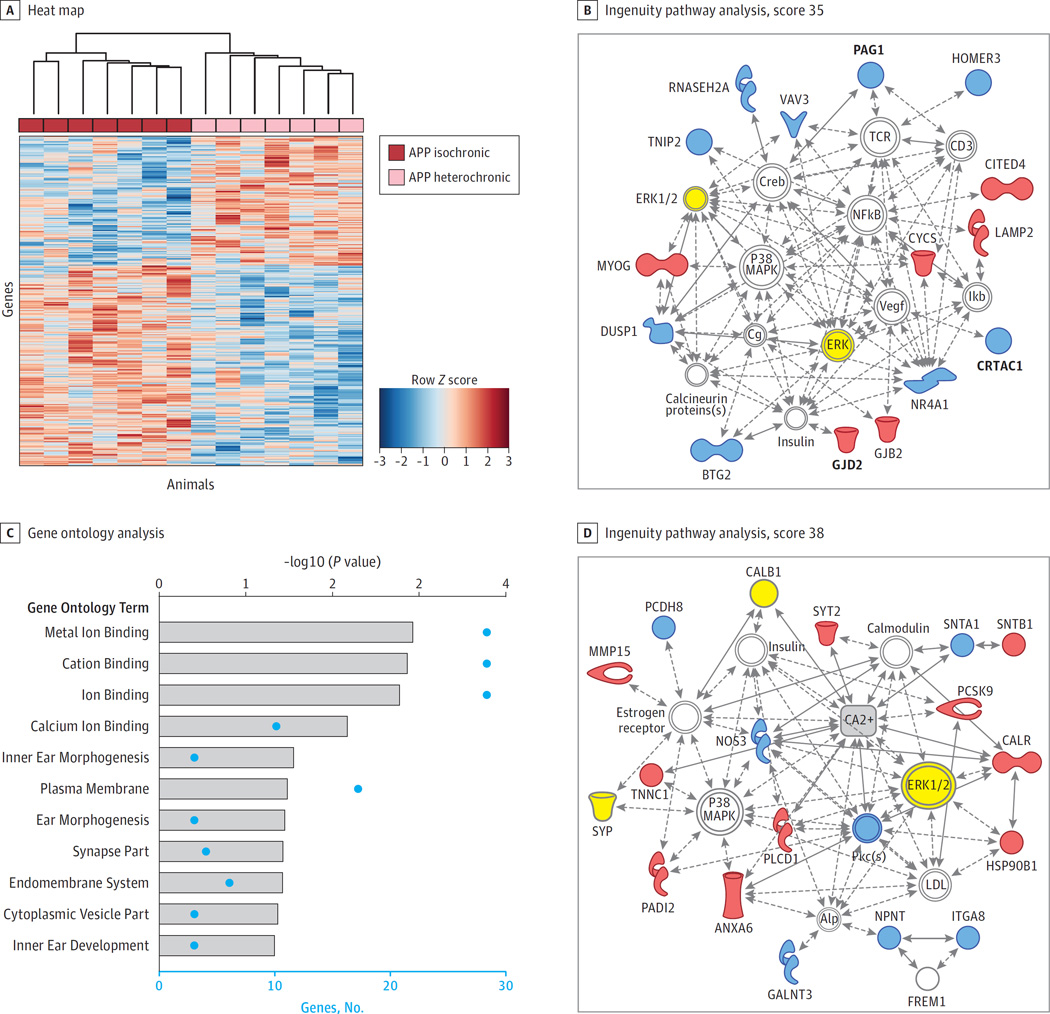
To better understand how systemic exposure to young blood affects the hippocampi of APP mice with established disease, a genome-wide microarray analysis was performed. Unsupervised hierarchical clustering based on differentially expressed genes segregated APP heterochronic parabionts from age-matched wildtype or APP isochronic mice (eFigure 4A in the Supplement) indicative of activation of a distinct gene expression network induced by a young systemic environment. Among the most significantly changed genes that showed restored gene expression in APP heterochronic parabionts (eFigure 4B in the Supplement) were γ-secretase–activating protein (GSAP) and the synaptic lipid raft adaptor protein PAG1, which have been postulated to modulate γ-secretase–dependent pathways.17,18 However, this analysis is biased toward the comparison between wildtype and APP hippocampi with major changes in genes related to the immune response, which was not significant for changes between APP isochronic and APP heterochronic parabionts (eFigure 5 in the Supplement). Instead, to find gene expression changes as a consequence of exposure to young blood, we focused on genes that were differentially expressed between APP isochronic and APP heterochronic parabionts. To find the most robust changes, we used a combination of factors including ranking by fold change, SAM, elastic-net, and least absolute shrinkage and selection operator algorithms,11 and generated a meta-ranking of genes. The top 100 meta-ranked genes can be found in the eTable in the Supplement. Ingenuity pathway analysis using the top 100 meta-ranked genes produced the strongest network around ERK, p38 MAPK, and NFkB signaling (Figure 2B). Gene ontology analysis of the top 100 meta-ranked genes showed a significant enrichment of genes encoding proteins involved in calcium ion binding (Figure 2C) and selection of the genes categorized as calcium ion binding by DAVID gene ontology analysis produced a network that was centered around ERK and p38 MAPK in addition to calcium (Figure 2D). Synaptophysin and calbindin, which were restored in APP heterochronic parabionts at the protein level (Figure 1), could be embedded in this calcium-binding network (Figure 2D). Calcium signaling is tightly linked to synaptic transmission, and dyshomeostasis in AD is thought to lead to synaptic dysfunction and subsequent cognitive deficits.4 Our findings corroborate those of others that ERK is abnormally activated in AD mouse models,19 similar to its activation in AD (Figure 3B and C).20,21 Thus, exposure to a young systemic environment targets these key pathways linked to AD.
Figure 2. Hippocampal Gene Expression Changes After Exposure to a Young Systemic Environment.
A whole-genome microarray analysis was performed on the hippocampi of middle-aged female wildtype (WT) isochronic (n = 6), amyloid precursor protein (APP) isochronic (n = 7), and APP heterochronic (n = 7) parabionts. A, Heat map generated by unsupervised hierarchical clustering with a data set of genes differentially expressed between hippocampi of APP isochronic and APP heterochronic parabionts according to significance analysis of microarrays (P < .05). B, The top signaling network generated by ingenuity pathway analysis (score 35) based on the top 100 meta-ranked genes that were differentially expressed in APP isochronic and APP heterochronic parabionts (eTable 1 in the Supplement). In bold are genes that were differentially expressed in APP isochronic and APP heterochronic parabionts when analyzed in a multiple comparison analysis. C, Gene ontology analysis of the top 100 meta-ranked genes was conducted using the the online gene ontology tool DAVID for gene ontology term annotation categories biological process, molecular function, and cellular component. This graph represents all gene ontology terms with P < .05 for enrichment in the top 100 meta-ranked genes. Besides P values (gray bars), the number of genes in each category is shown. D, The top network generated by ingenuity pathway analysis (score 38) based on all differentially expressed genes that were related to calcium ion binding according to DAVID gene ontology analysis. Calcium was added (gray rectangle) to show direct interaction of many genes with this compound. Inferred molecular interactions identified by ingenuity pathway analysis are gray, down regulated genes are blue, and up regulated genes are red. Molecules for which changes were confirmed on protein level are yellow.
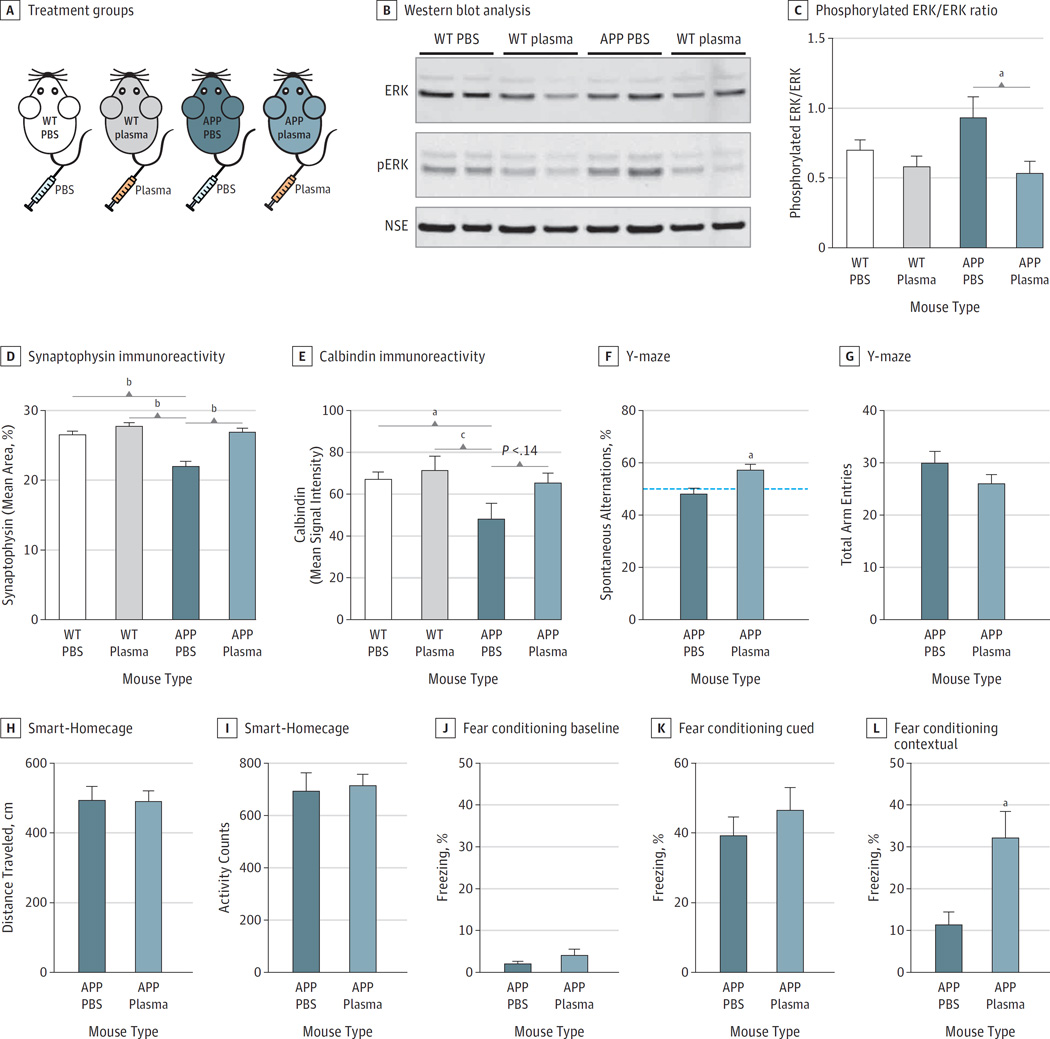
Figure 3. Effect of Administration of Young Blood Plasma on Memory Deficits in Amyloid Precursor Protein (APP) Mice.
A, Schematic depicting the 4 treatment groups; wildtype (WT) or APP mice treated with either phosphate-buffered saline (PBS) or young plasma. Western blot analysis of hippocampal lysates for extracellular receptor kinase (ERK) (44/42 kDa), phosphorylated ERK (44/42 kDa) and neuron-specific enolase as loading control (B) and quantification of the ratio of phosphorylated ERK/ERK (C) (n = 8 mice per group). D, Quantification of synaptophysin immunoreactivity in the molecular layer of the dentate gyrus of WT PBS (n = 14), WT plasma (n = 13), APP PBS (n = 11), and APP plasma (n = 13) mice. E, Quantification of calbindin immunoreactivity in the molecular layer of the dentate gyrus of WT PBS (n = 15), WT plasma (n = 13), APP PBS (n = 10), and APP plasma (n = 12) mice. F, Y-maze spontaneous alternations. Dotted line represents chance level (50%). G, The total number of arm entries.
H–I, Exploratory behavior monitored in a Smart-Homecage. APP mice treated with either PBS (APP PBS; n = 11) or plasma (n = 13) show no difference as measured by distance traveled, or total activity counts, over 5 minutes.
J–L, Assessment of associative memory by fear conditioning. J, APP PBS or APP plasma mice exhibited similar baseline freezing time during training.
K, Amygdala-dependent cued memory indicated by percentage freezing after being exposed to the conditioned tone and light in a different context 24 hours after training. L, Hippocampal-dependent learning and memory assessed by contextual fear conditioning indicated by percentage freezing in the first minute after being exposed to the same context 48 hours after training. One mouse was excluded from the APP PBS group owing to abnormal freezing behavior, corroborated by the ROUT method of identifying outliers. All data are shown as the mean (SEM).
a P < .001, 1-way analysis of variance, Tukey post hoc test (D–F). Scale bars: 25 µm in B, and 100 µm in C.
b P < .05.
c P < .01.
Effect of Young Blood Plasma on Abnormal ERK Activation, Synaptophysin, and Calbindin Expression
We have previously shown that the beneficial effects of heterochronic parabiosis on the aging brain can be recapitulated, in part, by systemic injection of plasma from young mice.6 To determine whether circulating factors within young blood were sufficient to restore abnormal gene and protein expression in APP mice, we intravenously injected middle-aged female APP mice (10–12 months old) with established disease and wildtype littermates with young plasma or PBS twice a week for 4 weeks (Figure 3A). We found that plasma treatment led to a prominent decrease in abnormal phosphorylation of hippocampal ERK back to wildtype levels (Figure 3B and C). Consistent with our findings in heterochronic parabiosis, the loss of synaptophysin observed in APP mice was completely rescued by young plasma in the hippocampus (Figure 3D) and neocortex (eFigure 6 in the Supplement). Calbindin immunoreactivity was also strongly reduced in APP mice compared with wildtype mice, and young plasma showed a trend to reverse this depletion (Figure 3E).
Effect of Administration of Young Blood Plasma on Memory Deficits in APP Mice
To determine whether young blood and the gene network changes associated with it could restore cognitive impairments in APP mice, we treated them with intravenous injections of young plasma and performed a number of behavioral tests. In the Y-maze spontaneous alternation test, which measures spatial working memory, PBS-treated APP mice performed at chance level, but plasma-treated APP mice performed significantly higher than the 50% chance level and made more spontaneous alternations (Figure 3F). Total number of arm entries was similar between the 2 groups (Figure 3G), indicating that the benefits of plasma were not owing to a change in locomotor activity. This was supported by the Smart-Homecage monitoring of exploratory behavior, which showed no difference in distance traveled (Figure 3H) or total activity counts (Figure 3I). Moreover, young plasma treatment improved hippocampus-dependent associative learning and memory, which was assessed by contextual fear conditioning. During the training phase, both groups exhibited similar baseline freezing (Figure 3J) and no difference was observed in amygdala-dependent cued memory (Figure 3K). However, APP mice receiving young plasma demonstrated increased freezing in the hippocampus-dependent contextual memory test (Figure 3L).
Discussion
Together, our findings demonstrate that blood from young adult mice contains soluble factors that are sufficient to ameliorate depletion of neuronal proteins that have served as sensitive markers for neurodegeneration in AD and APP transgenic mouse models2–4 and restore cognitive deficits observed in APP mice. Although our mouse model misses aspects of the disease, such as neurofibrillary tangle formation, we demonstrated therapeutic properties of young plasma by ameliorating aspects of the disease that are present in patients. Many clinical trials with drugs targeting Aβ have failed to slow the memory decline of patients with AD. Heterochronic parabiosis and young plasma administration was able to exert beneficial effects in APP mice without reducing Aβ burden.
Gene expression network analysis showed that young blood was associated with restored expression of many genes involved in key neuronal signaling pathways toward wildtype levels and reduced activation of ERK, a kinase previously linked to AD and neurodegeneration.20–22 Future studies will have to determine the importance of the gene expression changes and identify key factors responsible for these effects and elucidate their mechanisms of action. This could lead to the development of potential small molecule interventions. However, a group of soluble factors in young plasma targeting several pathways may be necessary for therapeutic benefits. Therefore, intravenous administration of plasma in humans, a low-risk procedure that is already offered as a therapy with limited complications, is feasible to test the efficacy of young plasma in patients with AD and possibly other forms of cognitive dysfunction and neurodegeneration. Indeed, clinical studies are ongoing to test young plasma in patients with AD.
This is a preclinical study done in an AD mouse model, which might have limited translation to the human disease. Young mouse plasma had beneficial effects on a number of disease parameters in this model, but it did not reverse all aspects of the mouse disease. For example, plasma treatment did not reduce amyloid levels in the mice tested with advanced pathology. We did not carry out pharmacological studies optimizing the frequency or duration of plasma administration or the volume given per injection. Last, it is possible that continuous administration of young plasma in an old recipient might have detrimental effects, although we have not observed any at this point.
Conclusions
This preclinical study shows encouraging beneficial effects of intravenous young plasma administration on molecular and functional outcomes in a mouse model of AD. Plasma did not reduce amyloidosis but appears to target molecular pathways involved in learning, memory, and inflammation. Although plasma administration is quite safe in humans and it would be possible to translate these findings to patients relatively quickly, pharmacological parameters and clinical end points will have to be established and safety in older patients needs to be demonstrated.
Supplementary Material
Key Points.
Question
What is the effect of young blood factors on Alzheimer-like disease in mice?
Findings
A mouse model of Alzheimer disease was exposed to young blood either through surgically facilitated shared blood circulation or intravenous plasma administration, which was associated with a partial restoration of molecular and behavioral disease-related deficits.
Meaning
Young blood plasma benefits mice that model Alzheimer disease and could be a new therapy for humans.
Acknowledgments
Dr Wyss-Coray is a cofounder and scientific advisor to Alkahest Inc. Drs Middeldorp, Villeda, Luo, and Wyss-Coray are Alkahest shareholders and are listed as inventors on a US patent application related to young plasma filed by Stanford University.
Funding/Support: This work was financially supported by Netherlands Organisation for Scientific Research Rubicon fellowship (Dr Middeldorp), Roche Postdoctoral Fellowship (Dr Lehallier), National Research Science Awards AG034045 and AG040877 (Dr Villeda, Dr Mosher), Alzheimer Nederland (Ms Miedema), an anonymous donor (Dr Wyss-Coray), Department of Veterans Affairs (Dr Wyss-Coray), and the National Institute on Aging (AG045034, Dr Wyss-Coray).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Middeldorp had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Concept and design: Middeldorp, Lehallier, Villeda, Wyss-Coray.
Acquisition, analysis, or interpretation of data: All Authors.
Drafting of the manuscript: Middeldorp, Wyss-Coray.
Critical revision of the manuscript for important intellectual content: All Authors.
Statistical analysis: Middeldorp, Lehallier.
Obtaining funding: Middeldorp, Wyss-Coray.
Administrative, technical, or material support: Middeldorp, Zhang, Luo, Stan, Mosher, Masliah, Wyss-Coray.
Study supervision: Middeldorp, Villeda, Wyss-Coray.
Conflict of Interest Disclosures: No other disclosures were reported.
Additional Contributions: We thank V. Mathur, PhD (Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, California), for advice and assistance with plasma collection and behavior, and R. Narasimhan, BS, R. Wabl, BS, and A. Nguyen, MSc (Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, California), for assistance with immunostainings. No compensation was received from funding sources for their work.
REFERENCES
- 1.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masliah E, Mallory M, Alford M, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56(1):127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- 3.Mucke L, Masliah E, Yu GQ, et al. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palop JJ, Jones B, Kekonius L, et al. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci U S A. 2003;100(16):9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta (1–42) J Neurosci Res. 2001;66(4):573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- 6.Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20(6):659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344(6184):630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faizi M, Bader PL, Saw N, et al. Thy1-hAPP (Lond/Swe+) mouse model of Alzheimer’s disease displays broad behavioral deficits in sensorimotor, cognitive and social function. Brain Behav. 2012;2(2):142–154. doi: 10.1002/brb3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 11.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 12.Kolde R, Laur S, Adler P, Vilo J. Robust rank aggregation for gene list integration and meta-analysis. Bioinformatics. 2012;28(4):573–580. doi: 10.1093/bioinformatics/btr709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo J, Elwood F, Britschgi M, et al. Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J Exp Med. 2013;210(1):157–172. doi: 10.1084/jem.20120412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15(3):331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickford F, Masliah E, Britschgi M, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J Clin Invest. 2008;118(6):2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. Alzheimers Dement. 2014;10(2):e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 17.He G, Luo W, Li P, et al. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010;467(7311):95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgakopoulos A, Xu J, Xu C, Mauger G, Barthet G, Robakis NK. Presenilin1/gamma-secretase promotes the EphB2-induced phosphorylation of ephrinB2 by regulating phosphoprotein associated with glycosphingolipid-enriched microdomains/Csk binding protein. FASEB J. 2011;25(10):3594–3604. doi: 10.1096/fj.11-187856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: in vitro and in vivo mechanisms related to Alzheimer’s disease. J Neurosci. 2001;21(12):4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster B, Hansen L, Adame A, et al. Astroglial activation of extracellular-regulated kinase in early stages of Alzheimer disease. J Neuropathol Exp Neurol. 2006;65(2):142–151. doi: 10.1097/01.jnen.0000199599.63204.6f. [DOI] [PubMed] [Google Scholar]
- 21.Perry G, Roder H, Nunomura A, et al. Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation. Neuroreport. 1999;10(11):2411–2415. doi: 10.1097/00001756-199908020-00035. [DOI] [PubMed] [Google Scholar]
- 22.Dineley KT, Weeber EJ, Atkins C, Adams JP, Anderson AE, Sweatt JD. Leitmotifs in the biochemistry of LTP induction: amplification, integration and coordination. J Neurochem. 2001;77(4):961–971. doi: 10.1046/j.1471-4159.2001.00321.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.