Abstract
Although systemic diseases take the biggest toll on human health and well-being, increasingly, a failing brain is the arbiter of a death preceded by a gradual loss of the essence of being. Ageing, which is fundamental to neurodegeneration and dementia, affects every organ in the body and seems to be encoded partly in a blood-based signature. Indeed, factors in the circulation have been shown to modulate ageing and to rejuvenate numerous organs, including the brain. The discovery of such factors, the identification of their origins and a deeper understanding of their functions is ushering in a new era in ageing and dementia research.
According to a 2015 United Nations report on world population ageing1, the number of people aged 60 and older worldwide is projected to more than double in the next 35 years, reaching almost 2.1 billion people. Most of this growth will come from developing regions of the world, although the oldest old, who are more than 80 years of age, are the fastest growing segment of the population in developed regions. Despite these improvements in life expectancy, Alzheimer's disease (AD) and related neurodegenerative conditions have arguably become the most dreaded maladies of older people. The observation that almost all aged brains show characteristic changes that are linked to neurodegeneration raises the question of whether these hallmarks represent lesser aspects of brain ageing that do not considerably affect function or whether they are the harbingers of neurodegenerative diseases (Figure 1). Immune cells and secreted communication factors, which are responsible for tissue homeostasis in general, probably play important parts in brain ageing and neurodegeneration. However, comprehending or controlling the immune response in ageing has been a challenge. In the ageing organism, the brain seems to be susceptible to both cell-intrinsic and local signals, as well as to cues from the systemic environment. Animal models suggest that cues that are present in the circulatory system can either accelerate or slow aspects of brain ageing and cognitive function. This Review will synthesize present knowledge on brain ageing and neurodegeneration and discuss the prospect of stalling or even reversing these processes through circulatory factors.
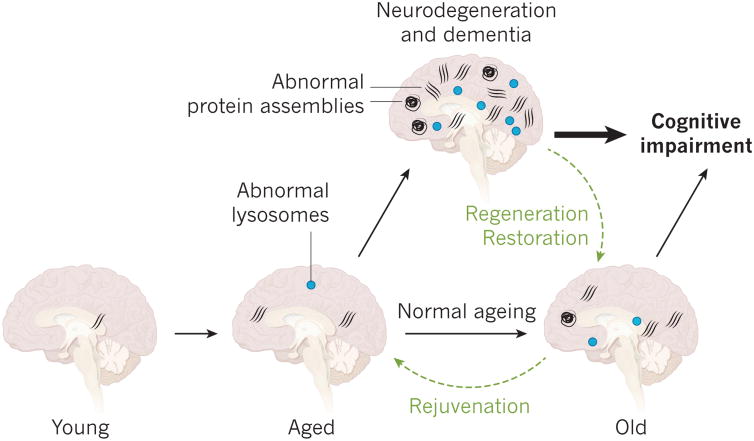
Figure 1. Ageing, neurodegeneration and brain rejuvenation.
As the brain ages, abnormal protein assemblies and inclusion bodies take hold and abnormal lysosomes are observed more frequently. It is unclear whether these defects promote ageing and neurodegeneration or whether they are innocent bystanders. Aged brains become highly prone to neurodegenerative diseases in which the same lesions amass as those that are found in old brains in smaller numbers. The relationship between such lesions and cognitive impairment is often blurred and normal aging and neurodegeneration and dementia can overlap. The concept of rejuvenation posits that old brains are malleable and that aspects of the ageing process can be reversed to a younger stage. If this can be achieved, it might also be possible to slow or reverse neurodegeneration and cognitive impairment.
Overlap between aging and neurodegeneration
Population-based autopsy studies of the brains of aged people who had not been diagnosed with a neurological disease consistently report the presence of amyloid plaq0ues, neurofibrillary tangles, Lewy bodies, inclusions of TAR DNA-binding protein 43 (TDP-43), synaptic dystrophy, the loss of neurons and the loss of brain volume in most of the brains2. These features vary greatly between individuals, with particular lesions dominating a particular brain or restricted to specific regions. It is unknown what causes such lesions and whether they are the precursors to neurodegeneration and disease or simply the products of brain ageing. As well as classic protein deposits, other subcellular structures that consist of cross-linked proteins, carbohydrates or lipids accumulate in ageing brains, either in the extracellular space (for example, corpora amylacea) or inside glial cells or neuronal cells (for example, stress granules, lipofuscin, Marinesco bodies and Hirano bodies). Although most of these structures are characterized poorly and their importance in neurodegeneration is unclear, it is probable that they take a toll on normal brain function3.
The presence of age-related protein abnormalities and inclusion bodies in the ageing brain points to defects in proteostasis, an idea that is supported by mounting evidence from experiments. According to one such hypothesis, in normal ageing, macromolecules become oxidized and can no longer be degraded by lysosomes4. This leads to the further production of lysosomal enzymes that are also unable to digest the cellular material. A well-known deposit that results from lysosomal inefficiency is lipofuscin, which is an accepted marker of ageing for postmitotic cells4. Similarly, the increase in damaged proteins and dying cells that accompanies ageing can overwhelm phagocytic processes and lead to an accumulation of material in lysosomes. Indeed, myelin debris have been demonstrated to accumulate in ageing microglia, in which it forms insoluble, lipofuscin-like lysosomal inclusions5. With ageing, and even more so with neurodegeneration, the brain shows increased levels of many lysosomal proteins and enzymes, and neurons and other cell types show abnormal endosomes, lysosomes and autophagosomes6-8. Whether these abnormalities contribute to or are the result of ageing must still be elucidated. However, the genetic manipulation of autophagy-related pathways in transgenic mice that overexpress amyloid precursor protein (also known as amyloid-β A4 protein) or the protein tau results in prominent changes in the accumulation of these proteins or the progression of disease9-11. Furthermore, stress granules, which consist of RNA and protein and can form in response to cellular stress, might have an important role in amyotrophic lateral sclerosis and frontotemporal dementia12. Stress granules are also associated with aggregates of tau in the brains of people with AD and in the brains of mice that overexpress mutated tau protein, and the overexpression of stress-granule protein TIA1 seems to stimulate a tauopathy13. These studies underline the importance of protein homeostasis in brain function and suggest that ageing and neurodegeneration could result partly from a loss of proteostasis. Although cause and effect must again be established, it is probable that the stabilization of protein homeostasis would benefit the ageing brain.
Our limited knowledge about the relevance of protein abnormalities in the brain is demonstrated by a striking discrepancy between the clinical manifestations of dementia and its associated physical characteristics in the brain, particularly in the oldest old. For example, in a study of a large series of brains from cognitively unimpaired aged people, almost all had abnormal accumulation of tau, roughly half had deposits of amyloid-β or TDP43 and one-fifth had deposits of α-synuclein2, although the regional distribution of these lesions should be considered when assessing their relevance to neurodegeneration. The brains of people who were aged 90 and older were found to weigh 11 % less than those of individuals in their fifties2, which indicates that more than 150 g of brain tissue had vanished in the older brains. This disappearance could be due to the loss of neurons or glial cells, myelin, fluid or other factors, and it will be important to determine whether it is related to neurodegeneration or is simply a part of normal brain ageing. Similarly, in a population-based sample of nonagenerians and centenarians without dementia, almost half fulfilled the neuropathological criteria of AD or had a mix of numerous pathologies14. Yet of the nonagenerians and centenarians who had been clinically diagnosed with dementia, 12% were free of pathological features, 23% could be considered to have AD and 45% had mixed dementia14. These observations are supported by studies of cerebrospinal fluid biomarkers for amyloid-β and tau, as well as positron-emission tomography imaging tracers in people, which show that around 30% of cognitively unimpaired elderly individuals are positive for these otherwise highly predictive markers of disease. Such individuals could be at a preclinical stage of AD, a stage of the disease that seems to be gaining clinical acceptance15. Around one quarter of cognitively healthy elderly people or people with mild cognitive impairment have pathological levels of tau in their brains in the absence of amyloid-β, a condition called suspected non-AD pathophysiology (SNAP)16, 17. Most such individuals do not express the apolipoprotein ε4 (APOE4) isoform, which is consistent with the observation that APOE4 promotes the accumulation of amyloid-β and that the APOE locus is linked to longevity.
In summary, clinical diagnoses often do not correlate with relevant pathological features in the brain, and there are few people above the age of 80 whose brains lack these features. The processes that characterize neurodegenerative diseases and, in particular, AD take place in most old brains; however, some people might have compensatory mechanisms that enable them to cope with these processes and to maintain normal cognition.
Causes of brain ageing and neurodegeneration
Given that neurodegenerative diseases in the elderly are common and that disease-free brains, especially in the oldest old, are rare, it is possible that normal brain ageing forms a continuum with neurodegeneration and disease, and that stochastic factors, framed by a person's genetics and environment, determine the type of neurodegenerative disease that will dominate their brain eventually (Figure 2). It is therefore tempting to view neurodegenerative diseases as expressions of accelerated ageing. However, this simplification is unhelpful because it does not accurately capture the underlying mechanisms that tie neurodegeneration to ageing, and all age-related diseases could essentially be described as forms of accelerated ageing. Instead, our understanding of how age contributes to disease is more likely to be advanced by dissecting how environmental factors and genes intersect in a particular disease with distinct hallmarks of ageing and by identifying the importance of these processes in the disease (Figure 2). For example, lesions associated with a disease rather than ageing are often more region specific and cognitive changes with age seem to be distinct from those observed in AD18.
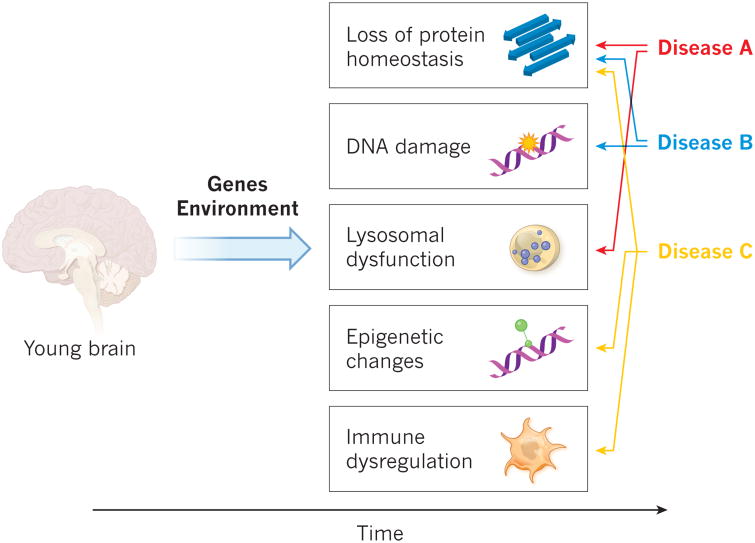
Figure 2. Cell-specific and pathway-specific acceleration of ageing.
Ageing can be dissected into individual processes, including a loss of protein homeostasis that leads to the development of aggregates and inclusion bodies, DNA damage, lysosomal dysfunction, epigenetic changes and immune dysregulation. The genetic predisposition of an individual, together with his or her exposure to the environment, determine the incidence and prevalence of the lesions that result from such processes, probably in a cell-specific manner. Various diseases might develop in accordance with the spatiotemporal distribution of the lesions.
Twin studies show that the heritability of the human lifespan is 20–30% and that the genetic contribution increases with age19–21. The lower heritability at younger ages is probably caused by a greater number of accidental deaths at such ages22. Environmental factors therefore account for at least 70% of variation in lifespan and an increasing number of studies show that lifestyle, diet, exposure to toxins, including drugs of abuse, can have profound effects on healthspan, longevity and the development of neurodegenerative diseases, although the molecular pathways that underpin effects are mostly unknown. (The epidemiology of longevity is reviewed comprehensively elsewhere23.) Further insights into the links between ageing and neurodegeneration are being generated from genetic studies that explore not only longevity and exceptional lifespan but also the genetics of disease-free ageing24,25 and the integration of genetics with other omics approaches26.
Exceptional longevity is linked consistently to the TOMM40–APOE– APOC1 locus, and other strong links are observed at genes such as FOXO3 and IL6 (refs 21, 23 and 27). In a large meta analysis of centenarian cohorts, many of the single nucleotide polymorphisms (SNPs) linked to longevity with the greatest significance were linked negatively to AD and coronary heart disease28. Interestingly, healthy ageing—that is, ageing without developing a disease—does not seem to be linked to longevity genes; instead, it might be associated with the absence of risk factors for AD and cardiovascular disease24. In the same study, analysis of the genes of people aged 80 years or older who had not been affected by chronic diseases revealed links to SNPs that are involved in cognitive performance, which offers the possibility that brain health and cognition might be surrogates for or even determinants of healthy ageing.
Another approach to deciphering the mechanistic contribution of ageing to neurodegeneration examines the pace of ageing in a cell- and pathway-specific fashion that focuses on gene expression, DNA methylation and other epigenetic DNA modifications (Figure 2) that change dramatically with age. For example, a comparison of age-related changes in gene expression in the brains of people with AD and those without the disease revealed that AD is characterized by signatures of accelerated ageing in a neuronal-stress gene expression module, which includes genes that are involved in protein folding and metabolism, and in an inflammation module, which is defined by genes involved in cytokines and microglia29. A strong positive correlation between ageing in various regions of the brain and methylation was observed in several hundred human brains30, a finding consistent with the epigenetic clock—a generalized DNA methylation pattern that seems to characterize most tissues31. Analysis of this pattern in Parkinson's disease showed that DNA methylation in blood cells is consistent with accelerated ageing32. A correlation of the pathology of AD with DNA methylation across the genome in almost 1,000 autopsied brains in two independent studies identified methylation sites close to the genes ANK1, CDH23, RHBDF2 and RPL13 that were linked to the disease33-35. Remarkably, all of these genes except for CDH23 have biological links to the AD-associated gene PTK2B. A combination of transcriptome and epigenome analyses in brains affected by AD and in a mouse model of AD-related neurodegeneration enabled the discovery of a downregulation of genes and regulatory regions involved in synaptic plasticity as well as a concomitant increase in the expression of genes involved in immune response and regulatory regions36—most notably SPI1, which encodes PU.1, a transcription factor with importance for the development of the myeloid lineage, including microglia37.
Genetic and epigenetic studies can therefore help to uncover the molecular pathways that link ageing with neurodegeneration. More refined omics studies, conducted with single cells isolated from defined brain regions, will probably deepen our insights and enable us to identify new targets to delay aspects of ageing in a disease-specific fashion.
The circulatory proteome of organismal ageing
Through plastic surgery, people can look years younger than their wrinkled hands, and although stretching the skin might not change its intrinsic age, it poses the question of whether all of a person's organs age at a similar pace. Ageing has been categorized into nine separate processes or hallmarks38, seven of which consist of molecular, mostly cell-intrinsic, changes such as telomere shortening, mitochondrial dysfunction or DNA damage. The other ‘integrative’ hallmarks include stem-cell ageing and dysfunction of intercellular communication38. The molecular or pharmacological manipulation of several of these processes has been shown to affect lifespan in mammals.
From an organismal perspective, intercellular communication is of particular interest as it could provide insights into the ageing process as well as help to identify biomarkers of ageing. Cellular communication occurs at numerous levels, from cells to tissues, across an organism and is accomplished by a myriad of molecules, including secreted proteins, lipids and metabolites, which must be tightly controlled. This network of communication factors changes as an organism develops, ages or is affected by disease. It is possible that age-related changes in cellular communication are simply adaptations to ageing. However, such changes might also contribute to ageing, either locally or distantly, and as a consequence, a particular organ or cell type might modulate or even control ageing at the organismal level (Figure 3).
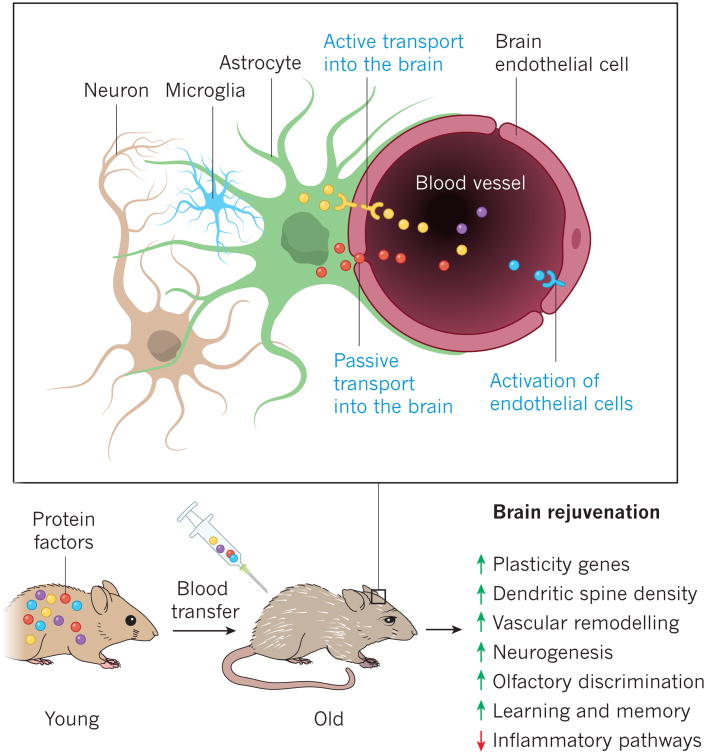
Figure 3.
Technologies for studying the proteome, the lipidome and the metabolome can be used to characterize age-related changes, and an increasing number of studies are describing changes in the blood that occur with normal brain ageing or with neurodegeneration, under the hypothesis that such changes mirror, in part, changes in the brain. As there are few studies of blood-based lipids or metabolites that regulate brain function, I will focus instead on studies that quantify secreted signaling proteins involved in intercellular communication—a subset of the proteome that has been dubbed the communicome39. The most comprehensive study of the cellular communicome of ageing quantified around 1,100 proteins using aptamer-based assays of the blood of about 800 people40. The protein most strongly correlated with ageing, chordin-like protein 1, is an antagonist of bone morphogenetic protein 4 and might therefore be involved in neural stem-cell fate and angiogenesis. Other proteins with links to ageing include pleiotrophin, which is a neurotrophic and mitogenic factor, the metalloproteinase inhibitor TIMP1 and the cysteine-proteinase inhibitor cystatin-C, all of which were also associated strongly with ageing in human cerebrospinal fluid41. Of the 281 proteins detected in cerebrospinal fluid, 81 were correlated significantly with age in 90 cognitively unimpaired people aged between 21 and 85 (ref. 41), which indicates that the brain is exposed to very different environments depending on the expression of these communication factors. Using antibody-based multiplex assays, several studies have measured tens to hundreds of known communication factors in the blood plasma of people with various stages of AD, reporting protein signatures that characterize the prodromal stages of the disease42 or the progression from early to late-stage AD39, 43, 44. Other studies have described protein signatures that correlate with APOE genotypes45 or with cerebrospinal-fluid levels of amyloid-β and tau in people with AD46, 47. The aptamer platform, which is more encompassing and precise than alternative methods, was also used to measure 1,001 proteins in almost 700 people with no cognitive impairment, mild cognitive impairment or AD: 14% of proteins showed a significant association with AD and 13 proteins could be used to classify AD with 70% accuracy48. By combining plasma proteomic data from healthy individuals, AD and frontotemporal dementia with existing brain gene-expression data and data from genome-wide association studies (GWAS), the most prominent changes in AD were found to relate to TGF–BMP–GDF signaling, the activation of complement and apoptosis, and GDF-3 was linked to neurogenesis and AD49. Although common factors, including APOE, complement, CCL5, clusterin and ICAM1, have been identified in these studies, it will be crucial to replicate them independently and to establish the in vivo biological importance of newly discovered proteins. If validated, such proteins or their combinations could become useful markers for brain ageing or neurodegeneration, as well as potential therapeutic targets.
To address this challenge, several communication factors were measured in the plasma of both young and aged mice, as well as in mice exposed to the blood of young or aged mice through heterochronic parabiosis, using an antibody-based multiplex assay50. (Parabiosis is established by surgically joining two mice at their flanks, leading to the formation of a vascular anastomosis and a shared circulatory system.) One of the factors that correlated most strongly with ageing and the effects of parabiosis on hippocampal neurogenesis was eotaxin (also known as CCL11), a small chemokine with a role in allergies and certain types of parasitic infections. Indeed, systemic administration of recombinant CCL11 to young mice was sufficient to reduce neurogenesis and to impair cognition (Table 1). In line with these potentially detrimental effects on the brain and cognition, the level of CCL11 increases in the choroid plexus during ageing51 and in fat deposits with obesity, and it decreases after exercise in people who are obese52, 53. Similarly, β-2-microglobulin (B2M), a component of major histocompatibility complex class I (MHC I) molecules, was found to be a pro-ageing factor that can impair cognition and neurogenesis in young mice and is necessary to maintain these functions in old mice54 (Table 1). Together with studies that link MHC I molecules with synaptic plasticity and brain repair55, these findings further implicate the MHC I locus in brain ageing and neurodegeneration. Importantly, these studies also indicate that proteins in the blood circulation involved in intercellular communication are both correlated with and able to modulate brain ageing, and they demonstrate the feasibility of using plasma proteomics to discover factors of relevance to brain ageing and cognitive function.
Table 1. Effects of systemically administered ageing and rejuvenation factors on healthy brains.
| Factors | Organism or model | Effect on the brain | References |
|---|---|---|---|
| Blood or plasma from old mouse | Young adult mouse | In the young brain: reduction in neurogenesis; increase in microglial reactivity; reduction in learning and memory. | 50 |
| CCL11 | Young adult mouse | In the young brain: reduction in neurogenesis; increase in microglial reactivity; reduction in learning and memory. | 50 |
| B2M | Young adult mouse | In the young brain: reduction in neurogenesis; increase in microglial reactivity; reduction in learning and memory. | 54 |
| Blood or plasma from young mouse | Aged mouse | Increase in neurogenesis; reduction in microglial reactivity; improvement in learning and memory; improvement in olfactory discrimination. | 80, 81 |
| IGF1 | Young adult rat | Increase in neurogenesis. | 91 |
| IGF2 | Young adult mouse | Increase in retention and persistence of working, short-term and long-term memory. | 92 |
| GHRH | Healthy older people; people with mild cognitive impairment | A 20-week treatment improved executive functions in both groups of people. | 94 |
| GnRH | Aged mouse | Increase in neurogenesis; improvement in memory. | 95 |
| GDF-11 | Aged mouse | Increase in neurogenesis; increase in cerebrovascular integrity. | 81 |
Abbr: IGF, insulin-like growth factor; GHRH, growth hormone-releasing hormone; GnRH, gonadotropin-releasing hormone; GDF-11, growth and differentiation factor 11.
Abnormal intercellular communication and inflammation
Interestingly, the immune factors CCL11 and B2M, as well as the chemokines CCL2, CCL12 and CCL19 and haptoglobin, which are linked to negative effects on neurogenesis during parabiosis50, might be part of a low-grade inflammation that is linked to ageing, known as inflammageing56. Inflammatory factors in the ageing brain could originate from microglia and astrocytes as they become senescent and adopt a senescence-associated secretory phenotype57. Some ageing astrocytes express increased levels of cytokines, intermediate filament proteins and intracellular protein aggregates, which is consistent with the phenotype58. As discussed previously, this senescent phenotype could result from epigenetic changes that activate immune-response genes targeted by, for example, the transcription factor PU.1 (ref. 36). Alternatively, microglia that change their gene-expression repertoire dramatically with age in a brain-region-specific manner59 might become reactive and inflamed as a result of impaired phagocytosis and protein dyshomeostasis. (A detailed discussion of microglia in brain ageing is presented elsewhere60.)
A role for inflammatory factors in autosomal dominant forms of neurodegeneration was suggested by the observation that numerous SNPs in the chemokine cluster that contains the gene CCL11 were linked to a 10-year difference in the age of onset of clinical AD symptoms in 72 people carrying a highly penetrant presenilin 1 mutation61. Indeed, inflammation has long been associated with neurodegeneration62, 63, and the use of non-steroidal anti-inflammatory drugs for several years before the onset of clinical symptoms is associated with a reduced risk of AD64-66. However, the same drugs do not seem to benefit people with Parkinson's disease67. It is unclear exactly how inflammation contributes to AD but it might involve both local and systemic mechanisms. In support of a detrimental role for systemic inflammation in the early stages of AD, the number of systemic inflammatory events (such as urinary tract infections) correlate positively with the progression and severity of AD68, 69. Genome-wide transcriptome studies of numerous brain regions in more than 1,600 brains provide further evidence of a role for immune mechanisms in ageing and AD; they also show that the expression of genes involved in inflammation increases considerably with normal ageing and precedes the development of AD29. Another bioinformatics-based study used signaling pathway and network analysis to conclude that the gene TYROBP (also known as DAP12), restricted mainly to microglia in the brain, is deregulated in AD70. DAP12 is an adaptor for several receptor molecules, including complement receptor 3, an important phagocytic receptor expressed by microglia, and TREM2 (ref. 71). The most direct evidence that altered immune function has a role in AD emerged from genetic studies that showed that rare polymorphisms in the myeloid-lineage gene TREM2 increase the risk of developing AD several fold72, 73. GWAS also identified further polymorphisms in genes involved in immune responses that modify the risk of developing AD74-76. In the brain, most of these genes, including TREM2, are expressed predominantly or exclusively by microglia. Dysfunction of microglia would probably impair the capacity of these cells to uptake and degrade amyloid-β and could therefore directly promote or even initiate AD. Antibodies that bind amyloid-β to facilitate its clearance by microglia are being tested in the clinic at present77.
Together, the genetic, transcriptomic and proteomic evidence suggests that changes in inflammation and intercellular communication represent chief aspects of normal brain ageing and neurodegeneration. However, it is unclear whether inflammatory pathways simply drive ageing and disease or whether aspects of the inflammatory response fulfil reparative and regenerative functions.
Brain rejuvenation and the manipulation of ageing
The concept of organismal and systemic ageing has been tested radically using heterochronic parabiosis78, which enables the exchange of blood, including its cells and factors, between young and old organisms. This surgically and conceptually simple model can therefore be used to investigate whether a youthful intercellular communicome can inhibit or reverse age-related abnormalities in an old mouse or whether an aged, and possibly dysfunctional, communicome can promote ageing in a young mouse. According to studies from nine independent laboratories, stem-cell activity is increased and other indices of ageing are delayed or reversed in several tissues of aged mice that share a circulatory system with young mice. These studies included: initial observations of effects on muscle and the liver78; reports of effects on the brain by four separate laboratories50,79-81 (Table 1); and observations of rejuvenating effects in the pancreas82, the heart83, bone84 and muscle85 (for detailed reviews, see refs 86 and 87). By contrast, the ageing thymus does not seem to benefit from parabiosis; however, the injection of young epithelial cells enabled thymic regrowth88. Perhaps most remarkable, with respect to the brain, is that the repeated intravenous administration of plasma, the soluble fraction of blood, from young mice (performed systematically for the first time in 2011, to study ageing factors50), was sufficient to improve cognitive function in old mice in several behavioural test80 (Figure 3). These functional changes were accompanied by molecular, subcellular, cellular and electrophysiological correlates, which suggests that factors in young blood have the capacity both to regulate brain function and to improve it to levels found in younger mice (Table 1). Parabiosis of amyloid-precursor protein transgenic mice with young mice, or the intravenous administration of plasma from young mice, also reversed the loss of synaptophysin and calbindin (an indicator of cognitive decline both in people with AD and in transgenic mouse models of the disease), normalized MAPK–ERK signaling and improved their working memory89.
A deficiency in growth hormone or insulin–insulin-like growth factor I signaling has been linked to deficits in memory, and the activation of growth hormone and insulin-like growth factor I signaling has been associated with improved brain function after injury or under conditions in which these proteins are lacking (reviewed in ref. 90). Few studies have treated healthy aged animals or people with growth factors and even fewer have demonstrated beneficial effects of such treatment (Table 1). For example, although insulin-like growth factor I increases neurogenesis91 and insulin-like growth factor II increases memory92 in young rodents, these factors have not been tested in aged animals. And in healthy older women or women with mild cognitive impairment, treatment with insulin-like growth factor I for 1 year showed no effect on bone density, bone strength, mood or memory93. By contrast, systemic administration of growth hormone-releasing hormone (GHRH), which triggers the hypophyseal release of growth hormone and increases the levels of circulating insulin-like growth factor I, among other factors, resulted in improved cognition in healthy elderly people or in people with mild cognitive impairment94. On the basis of the observation that the hypothalamus might have a role in regulating organismal ageing, systemic treatment of aged mice with gonadotropin-releasing hormone I (GnRH I) was found to increase neurogenesis and to improve cognitive function95.
In a search for factors that decrease with ageing and that might be responsible for the beneficial effects of heterochronic parabiosis on the heart, GDF-11 was identified as a potential heart-rejuvenation factor83. Subsequently, GDF-11 was found to increase neurogenesis, to improve olfaction and to exert beneficial effects on the brain vasculature81 (Table 1), as well as on aged muscle85. Other studies were unable to repeat these effects on systemic tissues96, and mass-spectrometry-based assays for GDF-11 and the related protein myostatin (GDF-8) observed no decrease in GDF-11 levels in human plasma with ageing and also found that GDF-11 levels are associated with frailty in people with cardiovascular disease97. Further studies will need to determine whether particular forms of GDF-11 (for example, mature, immature or post-translationally modified GDF-11) can explain these discrepancies and, most importantly, whether systemic administration of GDF-11 might be beneficial for human brains.
Overall, parabiosis with young mice or the transfer of young plasma seems to be capable of restoring brain function in old mice to more youthful levels. GHRH, GnRH I and GDF-11 are putative brain-rejuvenation factors and it is probable that other age-related proteins with detrimental or beneficial effects on the brain will be discovered. So far, it is unknown how the plasma from young animals or the factors listed in Table 1 exert their effects. It is possible that some of these proteins enter the brain actively or passively through the blood–brain barrier or at sites that lack a functional barrier, including the circumventricular organs and, perhaps, the neurogenic niches (Figure 3). Other proteins might modulate vascular function by interacting with endothelial cells and modulating the neurovascular unit81. In the future, studies will have to determine these modes of action and explore their potential for use as therapeutic approaches.
Outlook
In humans, the old brain shows the classic hallmarks of ageing and is particularly susceptible to abnormal protein accumulation and impairments in the phagolysosomal system, which leads to fluid boundaries between ageing and neurodegenerative diseases. Consequently, many old people have pathological abnormalities of the brain that do not necessarily correlate with their cognitive abilities. This has important implications for the treatment of those with clinical symptoms as well as for designing clinical trials to target protein abnormalities in a specific manner. Given the crucial functions that immune responses and inflammation have in brain ageing and neurodegeneration, it will be essential to discern beneficial attempts to maintain or repair damage from maladaptive ones. Clearly, the term neuroinflammation fails to capture the age- or disease-related changes in this sophisticated interplay between the surveillance, identification, targeting and execution functions of immunity and should probably be avoided. When studying age-related neurodegenerative diseases in animal models, it is important to consider ageing; those that have been genetically engineered to develop disease during adolescence and before midlife are unlikely to be influenced sufficiently by ageing and are therefore not very informative about age-related factors in sporadic neurodegeneration.
The increasing number of studies that show systemic effects on the brain, including those of young plasma or heterochronic parabiosis, as well as the effects of the microbiome, should remind neuroscientists that neurons do not function in isolation; instead, they are part of a sophisticated network that includes glial cells, vascular cells and peripheral cells.
So far, there is no published evidence that young blood or plasma has beneficial effects on an ageing human body, and the observation that young plasma can modulate brain ageing in mice presents more questions and opportunities than answers. Only a handful of proteins, which might represent factors involved in ageing or rejuvenation, have been shown to mimic the effects of plasma. However, many more proteins or other types of molecules are likely to exist, some of which might have direct therapeutic applications. Basic research will address the exciting questions that surround the origins of these factors, how they signal to the brain and why they change with age. Ultimately, it is hoped that by using such knowledge to alter basic processes involved in ageing, it will become feasible to counter the cellular abnormalities that lead to neurodegeneration.
Acknowledgments
I would like to thank T. Montine at Stanford University for his critical reading of the manuscript. This work was supported by the US Department of Veterans Affairs and the US National Institute on Aging (AG045034).
Footnotes
Disclosures: T.W.-C. is scientific adviser to and founder of Alkahest Inc., a company developing blood-based treatments to increase health span.
References
- 1.United Nations Department of Economic and Social Affairs Population Division. World Population Ageing 2015. Report ST/ESA/SER.A/390 http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf (United Nations, 2015)
- 2.Elobeid A, Libard S, Leino M, Popova SN, Alafuzoff I. Altered proteins in the aging brain. J Neuropathol Exp Neurol. 2016;75:316–325. doi: 10.1093/jnen/nlw002. A comprehensive study and review of the literature describing the prevalence of protein aggregates in cognitively unimpaired aged brains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mrak RE, Griffin ST, Graham DI. Aging-associated changes in human brain. J Neuropathol Exp Neurol. 1997;56:1269–1275. doi: 10.1097/00005072-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 5.Safaiyan S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nature Neurosci. 2016;19:995–998. doi: 10.1038/nn.4325. An elegant demonstration of how age-related myelin breakdown results in the accumulation of microglial lipofuscin and cell dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nixon RA, Cataldo AM, Mathews PM. The endosomal-lysosomal system of neurons in Alzheimer's disease pathogenesis: a review. Neurochem Res. 2000;25:1161–1172. doi: 10.1023/a:1007675508413. [DOI] [PubMed] [Google Scholar]
- 7.Nixon RA, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 8.Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nature Rev Neurosci. 2015;16:345–357. doi: 10.1038/nrn3961. [DOI] [PubMed] [Google Scholar]
- 9.Pickford F, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson P, et al. Aβ secretion and plaque formation depend on autophagy. Cell Rep. 2013;5:61–69. doi: 10.1016/j.celrep.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Caccamo A, et al. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer's disease and other tauopathies. Aging Cell. 2013;12:370–380. doi: 10.1111/acel.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ash PEA, Vanderweyde TE, Youmans KL, Apicco DJ, Wolozin B. Pathological stress granules in Alzheimer's disease. Brain Res. 2014;1584:52–58. doi: 10.1016/j.brainres.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderweyde T, et al. Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies. J Neurosci. 2012;32:8270–8283. doi: 10.1523/JNEUROSCI.1592-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawas CH, et al. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85:535–542. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois B, et al. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR, et al. Suspected non-Alzheimer disease pathophysiology—concept and controversy. Nature Rev Neurol. 2016;12:117–124. doi: 10.1038/nrneurol.2015.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landau SM, Horng A, Fero A, Jagust WJ. Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology. 2016;86:1377–1385. doi: 10.1212/WNL.0000000000002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herskind AM, et al. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- 20.vB Hjelmborg J, et al. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 21.Shadyab AH, LaCroix AZ. Genetic factors associated with longevity: a review of recent findings. Ageing Res Rev. 2015;19:1–7. doi: 10.1016/j.arr.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Pilling LC, et al. Human longevity is influenced by many genetic variants: evidence from 75,000 UK Biobank participants. Aging. 2016;8:547–560. doi: 10.18632/aging.100930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman AB, Murabito JM. The epidemiology of longevity and exceptional survival. Epidemiol Rev. 2013;35:181–197. doi: 10.1093/epirev/mxs013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erikson GA, et al. Whole-genome sequencing of a healthy aging cohort. Cell. 2016;165:1002–1011. doi: 10.1016/j.cell.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matteini AM, et al. GWAS analysis of handgrip and lower body strength in older adults in the CHARGE consortium. Aging Cell. 2016;15:792–800. doi: 10.1111/acel.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putin E, et al. Deep biomarkers of human aging: application of deep neural networks to biomarker development. Aging. 2016;8:1021–1033. doi: 10.18632/aging.100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng Y, et al. Novel loci and pathways significantly associated with longevity. Sci Rep. 2016;6:21243. doi: 10.1038/srep21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebastiani P, et al. Meta-analysis of genetic variants associated with human exceptional longevity. Aging. 2013;5:653–661. doi: 10.18632/aging.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Podtelezhnikov AA, et al. Molecular insights into the pathogenesis of Alzheimer's disease and its relationship to normal aging. PLoS ONE. 2011;6:e29610. doi: 10.1371/journal.pone.0029610. The first large-scale analysis of transcriptional brain networks in ageing people and those with AD, and the discovery of an accelerated ageing profile in AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez DG, et al. Distinct DNA methylation changes highly correlated with chronological age in the human brain. Hum Mol Genet. 2011;20:1164–1172. doi: 10.1093/hmg/ddq561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. erratum 16, 96 (2015). Description of an ‘epigenetic clock’ that correlates with tissue ageing and shows acceleration in cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horvath S, Ritz BR. Increased epigenetic age and granulocyte counts in the blood of Parkinson's disease patients. Aging. 2015;7:1130–1142. doi: 10.18632/aging.100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lunnon K, et al. Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer's disease. Nature Neurosci. 2014;17:1164–1170. doi: 10.1038/nn.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Jager PL, et al. Alzheimer's disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nature Neurosci. 2014;17:1156–1163. doi: 10.1038/nn.3786. A large-scale genome-wide DNA methylation study of a neurodegenerative disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lord J, Cruchaga C. The epigenetic landscape of Alzheimer's disease. Nature Neurosci. 2014;17:1138–1140. doi: 10.1038/nn.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gjoneska E, et al. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer's disease. Nature. 2015;518:365–369. doi: 10.1038/nature14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satoh JI, Asahina N, Kitano S, Kino Y. A comprehensive profile of ChIP-Seq-based PU.1/Spi1 target genes in microglia. Gene Regul Syst Bio. 2014;8:127–139. doi: 10.4137/GRSB.S19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray S, et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nature Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 40.Menni C, et al. Circulating proteomic signatures of chronological age. J Gerontol A Biol. 2015;70:809–816. doi: 10.1093/gerona/glu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baird GS, et al. Age-dependent changes in the cerebrospinal fluid proteome by slow off-rate modified aptamer array. Am J Pathol. 2012;180:446–456. doi: 10.1016/j.ajpath.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu WT, et al. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology. 2012;79:897–905. doi: 10.1212/WNL.0b013e318266fa70. erratum 79, 1935 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnstone D, Milward EA, Berretta R, Moscato P. Multivariate protein signatures of pre-clinical Alzheimer's disease in the Alzheimer's Disease Neuroimaging Initiative (ADNI) Plasma Proteome Dataset. PLoS ONE. 2012;7:e34341. doi: 10.1371/journal.pone.0034341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hye A, et al. Plasma proteins predict conversion to dementia from prodromal disease. Alzheimers Dement. 2014;10:799–807. doi: 10.1016/j.jalz.2014.05.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soares HD, et al. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch Neurol. 2012;69:1310–1317. doi: 10.1001/archneurol.2012.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Britschgi M, et al. Modeling of pathological traits in Alzheimer's disease based on systemic extracellular signaling proteome. Mol Cell Proteomics. 2011;10:M111.008862. doi: 10.1074/mcp.M111.008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiddle SJ, et al. Plasma based markers of [11C] PiB-PET brain amyloid burden. PLoS ONE. 2012;7:e44260. doi: 10.1371/journal.pone.0044260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sattlecker M, et al. Alzheimer's disease biomarker discovery using SOMAscan multiplexed protein technology. Alzheimers Dement. 2014;10:724–734. doi: 10.1016/j.jalz.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 49.Jaeger PA, et al. Network-driven plasma proteomics expose molecular changes in the Alzheimer's brain. Mol Neurodegener. 2016;11:31. doi: 10.1186/s13024-016-0095-2. erratum 11, 42 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. The first demonstration of the effects of circulatory blood factors on brain ageing and cognitive function and the first systematic treatment of ageing with plasma injections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baruch K, et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad of Sci USA. 2013;110:2264–2269. doi: 10.1073/pnas.1211270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasudevan AR. Eotaxin and obesity. J Clin Endocrinol Metab. 2006;91:256–261. doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
- 53.Choi KM, et al. Effect of exercise training on plasma visfatin and eotaxin levels. Eur J Endocrinol. 2007;157:437–442. doi: 10.1530/EJE-07-0127. [DOI] [PubMed] [Google Scholar]
- 54.Smith LK, et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nature Med. 2015;21:932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 57.Coppé JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salminen A, et al. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- 59.Grabert K, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nature Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosher KI, Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer's disease. Biochem Pharmacol. 2014;88:594–604. doi: 10.1016/j.bcp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lalli MA, et al. Whole-genome sequencing suggests a chemokine gene cluster that modifies age at onset in familial Alzheimer's disease. Mol Psychiatry. 2015;20:1294–1300. doi: 10.1038/mp.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heneka MT, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGeer PL, McGeer E, Rogers J, Sibley J. Anti-inflammatory drugs and Alzheimer disease. Lancet. 1990;335:1037. doi: 10.1016/0140-6736(90)91101-f. [DOI] [PubMed] [Google Scholar]
- 65.Côté S, et al. Nonsteroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2012;8:219–226. doi: 10.1016/j.jalz.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manthripragada AD, et al. Non-steroidal anti-inflammatory drug use and the risk of Parkinson's disease. Neuroepidemiology. 2011;36:155–161. doi: 10.1159/000325653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmes C, Cunningham C, Zotova E, Culliford D, Perry VH. Proinflammatory cytokines, sickness behavior, and Alzheimer disease. Neurology. 2011;77:212–218. doi: 10.1212/WNL.0b013e318225ae07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holmes C, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang B, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell. 2013;153:707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linnartz B, Neumann H. Microglial activatory (immunoreceptor tyrosine-based activation motif)- and inhibitory (immunoreceptor tyrosine-based inhibition motif)-signaling receptors for recognition of the neuronal glycocalyx. Glia. 2013;61:37–46. doi: 10.1002/glia.22359. [DOI] [PubMed] [Google Scholar]
- 72.Jonsson T, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guerreiro R, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nature Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hollingworth P, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nature Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambert JC, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nature Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spencer B, Masliah E. Immunotherapy for Alzheimer's disease: past, present and future. Front Aging Neurosci. 2014;6:114. doi: 10.3389/fnagi.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. This landmark study provides the first molecular evidence that heterochronic parabiosis can rejuvenate and reverse stem cell ageing in numerous tissues. [DOI] [PubMed] [Google Scholar]
- 79.Ruckh JM, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Villeda SA, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nature Med. 2014;20:659–663. doi: 10.1038/nm.3569. The first report to show that systemic administration of plasma from young mice can reverse cognitive deficits in aged mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katsimpardi L, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salpeter SJ, et al. Systemic regulation of the age-related decline of pancreatic β-cell replication. Diabetes. 2013;62:2843–2848. doi: 10.2337/db13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loffredo FS, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baht GS, et al. Exposure to a youthful circulaton rejuvenates bone repair through modulation of β-catenin. Nature Commun. 2015;6:7131. doi: 10.1038/ncomms8131. erratum 6, 7761 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sinha M, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–530. doi: 10.1111/acel.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castellano JM, Kirby ED, Wyss-Coray T. Blood-borne revitalization of the aged brain. JAMA Neurol. 2015;72:1191–1194. doi: 10.1001/jamaneurol.2015.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim MJ, Miller CM, Shadrach JL, Wagers AJ, Serwold T. Young, proliferative thymic epithelial cells engraft and function in aging thymuses. J Immunol. 2015;194:4784–4795. doi: 10.4049/jimmunol.1403158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Middeldorp J, et al. Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.3185. http://dx.doi.org/10.1001/jamaneurol.2016.3185. [DOI] [PMC free article] [PubMed]
- 90.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stern SA, Kohtz AS, Pollonini G, Alberini CM. Enhancement of memories by systemic administration of insulin-like growth factor II. Neuropsychopharmacol. 2014;39:2179–2190. doi: 10.1038/npp.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friedlander AL, et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86:1496–1503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- 94.Baker LD, et al. Effects of growth hormone-releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults. Arch Neurol. 2012;69:1420–1429. doi: 10.1001/archneurol.2012.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang G, et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. Provides genetic evidence that the hypothalamus controls age-related inflammatory changes in the periphery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walker RG, et al. Biochemistry and biology of GDF11 and myostatin: similarities, differences, and questions for future investigation. Circ Res. 2016;118:1125–1141. doi: 10.1161/CIRCRESAHA.116.308391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schafer MJ, et al. Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23:1207–1215. doi: 10.1016/j.cmet.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]