Supplemental Digital Content is Available in the Text.
Key Words: HIV, children, antiretroviral treatment, PMTCT, Africa
Abstract
Background:
Strategies to improve HIV diagnosis and linkage into care, antiretroviral treatment coverage, and treatment outcomes of mothers and children are urgently needed in sub-Saharan Africa.
Methods:
From December 2012, we implemented an intervention package to improve prevention of mother-to-child transmission (PMTCT) and pediatric HIV care in our rural Tanzanian clinic, consisting of: (1) creation of a PMTCT and pediatric unit integrated within the reproductive and child health clinic; (2) implementation of electronic medical records; (3) provider-initiated HIV testing and counseling in the hospital wards; and (4) early infant diagnosis test performed locally. To assess the impact of this strategy, clinical characteristics and outcomes were compared between the period before (2008–2012) and during/after the implementation (2013–2014).
Results:
After the intervention, the number of mothers and children enrolled into care almost doubled. Compared with the pre-intervention period (2008–2012), in 2013–2014, children presented lower CD4% (16 vs. 16.8, P = 0.08) and more advanced disease (World Health Organization stage 3/4 72% vs. 35%, P < 0.001). The antiretroviral treatment coverage rose from 80% to 98% (P < 0.001), the lost-to-follow-up rate decreased from 20% to 11% (P = 0.002), and mortality ascertainment improved. During 2013–2014, 261 HIV-exposed infants were enrolled, and the early mother-to-child transmission rate among mother–infant pairs accessing PMTCT was 2%.
Conclusions:
This strategy resulted in an increased number of mothers and children diagnosed and linked into care, a higher detection of children with AIDS, universal treatment coverage, lower loss to follow-up, and an early mother-to-child transmission rate below the threshold of elimination. This study documents a feasible and scalable model for family-centered HIV care in sub-Saharan Africa.
INTRODUCTION
Globally, 1.8 million children live with HIV,1 >90% of them residing in sub-Saharan Africa. Mother-to-child transmission (MTCT) accounts for >90% of the infections.2 Most of the newly infected children are found among those not reached by prevention of MTCT (PMTCT) programs, and offering them timely diagnosis is vital. Systems to promote HIV testing for children outside the PMTCT programs need to be enhanced.3–6
Antiretroviral treatment (ART) initiation and retention in care are essential for HIV-infected children.7–9 However, ART coverage among children in low- and middle-income countries was only 25% in 2012.2 Training health care providers in pediatric HIV/AIDS is needed to bridge this gap, ensure high-quality programs, and expand access to care and treatment among children.10–12 Moreover, fragmentation of health services hinders the access to key populations, and integration of services is recommended.13–16
In 2014, the Joint United Nations Programme on HIV/AIDS presented its “90-90-90 target” for 2020: (1) 90% of people living with HIV will know their HIV status, (2) 90% of people with diagnosed HIV infection will receive ART, and (3) 90% of people receiving ART will be virologically suppressed.17 To move toward this goal, special efforts need to be taken for the pediatric population given the challenges associated with timely diagnosis, ART coverage, and retention in care.
In Tanzania, in 2013, 73% of HIV-infected pregnant women received PMTCT services and the estimated MTCT rate was 16%,18 with 23,000 new pediatric infections.19 There are 250,000 children living with HIV, and ART coverage is dramatically lower than among adults, 26% vs. 68%.2
Previous data from our clinic revealed several gaps in the care pathway of HIV-infected mothers and their children.20 In response to these challenges, we adopted a package of interventions to improve the care of HIV-infected children, mothers, and their families attending our clinic.
The aim of this study was to evaluate the impact of this strategy on outcomes among both children and pregnant women over time.
METHODS
This is a prospective cohort study evaluating the clinical outcomes of children and pregnant women before and during the implementation of a bundle of measures to improve the quality of HIV care in rural Tanzania.
Study Setting and Population
The Chronic Diseases Clinic of Ifakara is a rural HIV clinic in the Kilombero district, southern Tanzania. It is part of the Saint Francis Referral Hospital and works in cooperation with the Ifakara Health Institute, the Swiss Tropical and Public Health Institute, and the Department of Infectious Diseases and Hospital Epidemiology of the University Hospitals of Basel and Bern, Switzerland. All patients diagnosed with HIV within the hospital or diagnosed at a peripheral health centre and transferred for further treatment are referred to the clinic to receive HIV care and treatment according to the National AIDS Control Program. Since 2004, all HIV-infected patients attending the clinic are asked for informed consent to be enrolled in the Kilombero and Ulanga Antiretroviral Cohort (KIULARCO). This cohort study obtained ethical approval from the Ifakara Health Institute ethical review board, the Tanzanian National Institute for Medical Research, the Tanzanian Commission for Science and Technology, and the Ethical Review Board of the Canton of Basel, Switzerland.
The cohort comprises >8000 patients and is the largest rural HIV cohort in Tanzania.21–23 PMTCT Option B+ was implemented in April 2014.24
In this study, we included all HIV-infected children (≤15 years) and pregnant women enrolled in the cohort between January 1, 2008 and December 31, 2014. For HIV-exposed infants, we included infants enrolled from January 1, 2013 until December 31, 2014, since no information was collected before this period.
Description of the Intervention
From December 2012, a bundle of measures to improve the PMTCT and Pediatric HIV services were introduced, namely: (1) creation of the One Stop Clinic of Ifakara: a PMTCT and pediatric unit integrated within the Reproductive and Child Health Clinic; (2) implementation of electronic medical records; (3) implementation of provider-initiated HIV testing and counseling (PITC) in the hospital wards; and (4) early infant HIV diagnosis (EID) test locally (See Table, Supplemental Digital Content 1, http://links.lww.com/QAI/A919).
Establishment of the One Stop Clinic of Ifakara
We created a unit to address the special needs of HIV-infected children and pregnant women. As in most HIV clinics in sub-Saharan Africa, before the intervention, infants, children, and pregnant women were not assigned to specific clinicians and could be seen by different clinicians at each visit. Also, there was no family-centered approach and different family members were required to visit the clinic on different days. Additionally, clinicians based at our clinic were previously not in charge of the HIV-infected patients admitted in the hospital wards.
To establish and implement the One Stop Clinic, we pursued 2 steps.
Step 1: Creation of a PMTCT and Pediatric HIV Team
From January to March 2013, 2 clinicians, a nurse, and a midwife were identified from the existing staff of the hospital. In July 2014, a counselor was newly hired and joined the team. All staff were provided specific training and appointed to take care of HIV-infected children and pregnant women and HIV-exposed infants in both the outpatient clinic and the hospital wards.
Step 2: Integration Within the Reproductive and Child Health Clinic
In April 2013, the care of HIV-infected children, pregnant women, HIV-exposed infants, and their families was transferred to the Reproductive and Child Health Clinic. We intended to maximize the opportunities to diagnose women and children, facilitate their linkage into care, and improve retention by offering a range of services in a single day and place (Fig. 1).
FIGURE 1.
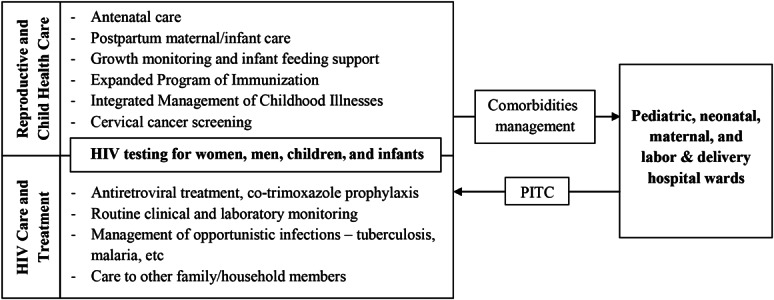
The One Stop Clinic of Ifakara integrates health services for HIV-infected children, pregnant women, HIV-exposed infants, and their families in a rural Tanzanian hospital.
Electronic Medical Records for HIV-Infected Patients
The Open Medical Record System (http://openmrs.org/) was adopted to implement an electronic data collection system at our clinic.25 In December 2012, different questionnaires were introduced to systematically and comprehensively collect clinical, laboratory, and drug prescription information and to minimize missing data and data entry mistakes. In May 2013, the clinic became paperless.
Provider-Initiated HIV Testing and Counseling
Provision of PITC had been recommended in Tanzania since 2007 but suboptimally implemented in most settings, including our hospital.26 In January 2014, our clinic joined efforts with the hospital administration to institutionalize PITC. Since then, all patients admitted in the pediatric and maternity wards are offered HIV testing through an opt-out strategy.
Early Infant Diagnosis Testing
The national guidelines recommend that HIV-exposed infants are tested for HIV at 4–6 weeks of life or thereafter as soon as enrolled. Proviral DNA polymerase chain reaction (PCR) is used for infants aged ≤9 months. For those aged 9–18 months, HIV rapid antibody tests are used for screening and, if positive, proviral DNA PCR is performed.24
Since March 2014, proviral HIV DNA PCR for EID is performed at the Ifakara Health Institute laboratory applying an in-house nested PCR protocol executed on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA) using GoTaq DNA Polymerase (Promega Corporation, Madison, WI). Previously, we had used the national EID circuit, which was often challenged by frequent stock outs of the kits to collect and store the samples and by the unreliable transport to 1 of the only 4 national referral laboratories.
Statistical Analysis
Baseline characteristics of children and pregnant women were summarized according to year with appropriate statistical measures. Baseline is defined as the time of enrollment into the cohort.
To assess the impact of the interventions listed above, both the baseline characteristics and the clinical outcomes were compared in children and pregnant women separately between the period before the intervention (2008–2012) and during/after the implementation of the intervention (2013–2014). Baseline and follow-up comparisons between both periods were made using χ2 and Wilcoxon rank-sum tests for categorical and continuous variables, respectively. Also, we compared the notified cases of tuberculosis and malnutrition between the time periods by estimating the overall Mantel–Haenszel rate ratio. Finally, we described the characteristics of the HIV-exposed infants enrolled and reported the early MTCT rate. Test statistics and 95% confidence intervals were presented. Data were analyzed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
Cohort Characteristics
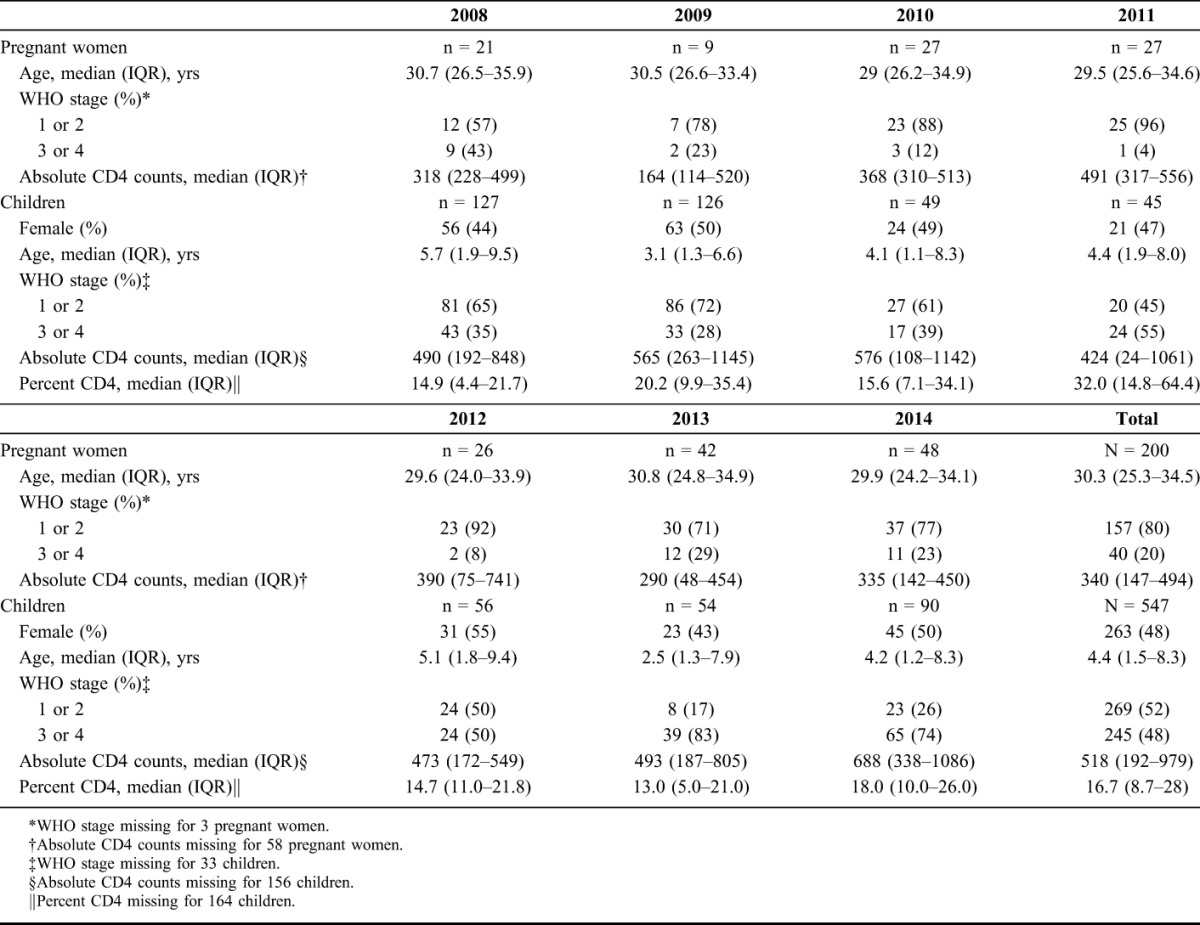
A total of 200 HIV-infected pregnant women and 547 HIV-infected children were enrolled during the study period. Table 1 summarizes the baseline characteristics of the patients per year of enrolment. Pregnant women were enrolled at a median age of 30.3 years [interquartile range (IQR) 25.3–34.5], 20% were classified as World Health Organization (WHO) stage 3/4, and median CD4 was 340 cells per microliter (IQR 147–494). Among children, 48% were female, with a median age of 4.4 years (IQR 1.5–8.3) and 48% presented with WHO stage 3/4. The median CD4 percent was 16.7% (IQR 8.7–28).
TABLE 1.
Baseline Characteristics of HIV-Infected Children and Pregnant Women Newly Enrolled in Care From 2008 to 2014
Outcomes of Pregnant Women
The median number of women enrolled per year increased from 26 (IQR 21–27) in 2008–2012 to 45 (IQR 42–48) in 2013–2014 (P = 0.05) (Fig. 2A). After the intervention, there was a trend toward a higher WHO stage 3/4 (26% in 2013–2014 vs. 15% in 2008–2012, P = 0.08) and lower CD4 counts (median 315 cells/µL versus 370 cells/µL, P = 0.06). ART coverage among those eligible was >98% during the whole study period. In 2013–2014, most women were initiated on a fixed-dose combination of tenofovir/lamivudine or emtricitabine/efavirenz as expected due to the changes of the national guidelines. There were no major differences in retention in care, although the number of those lost to follow-up declined after 2012 (26.5% vs. 30.9%) (Table 2).
FIGURE 2.
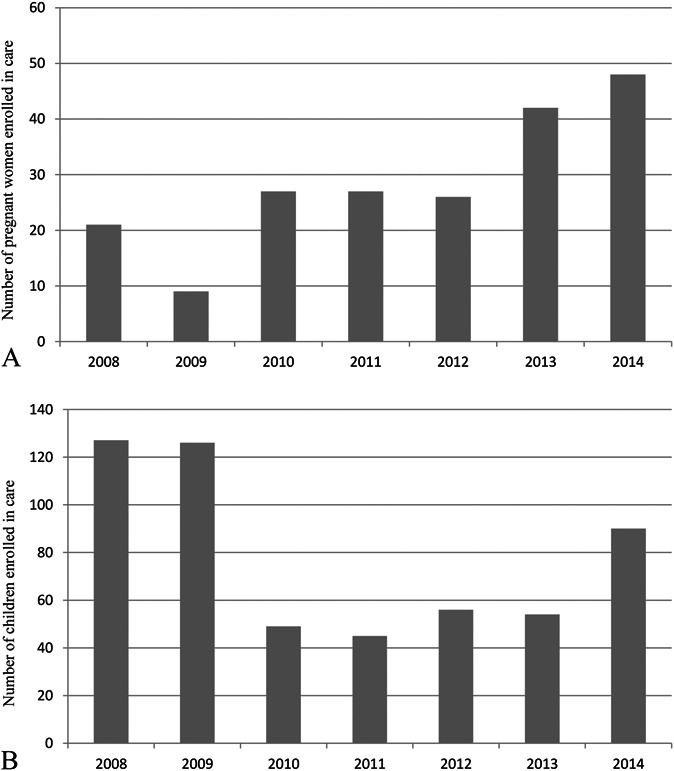
Number of newly enrolled pregnant women (A) and children (B) per year.
Table 2.
Comparison of the Clinical Characteristics, Antiretroviral Treatment Coverage and Regimens, and Retention in Care of Patients Enrolled Before and After the Intervention
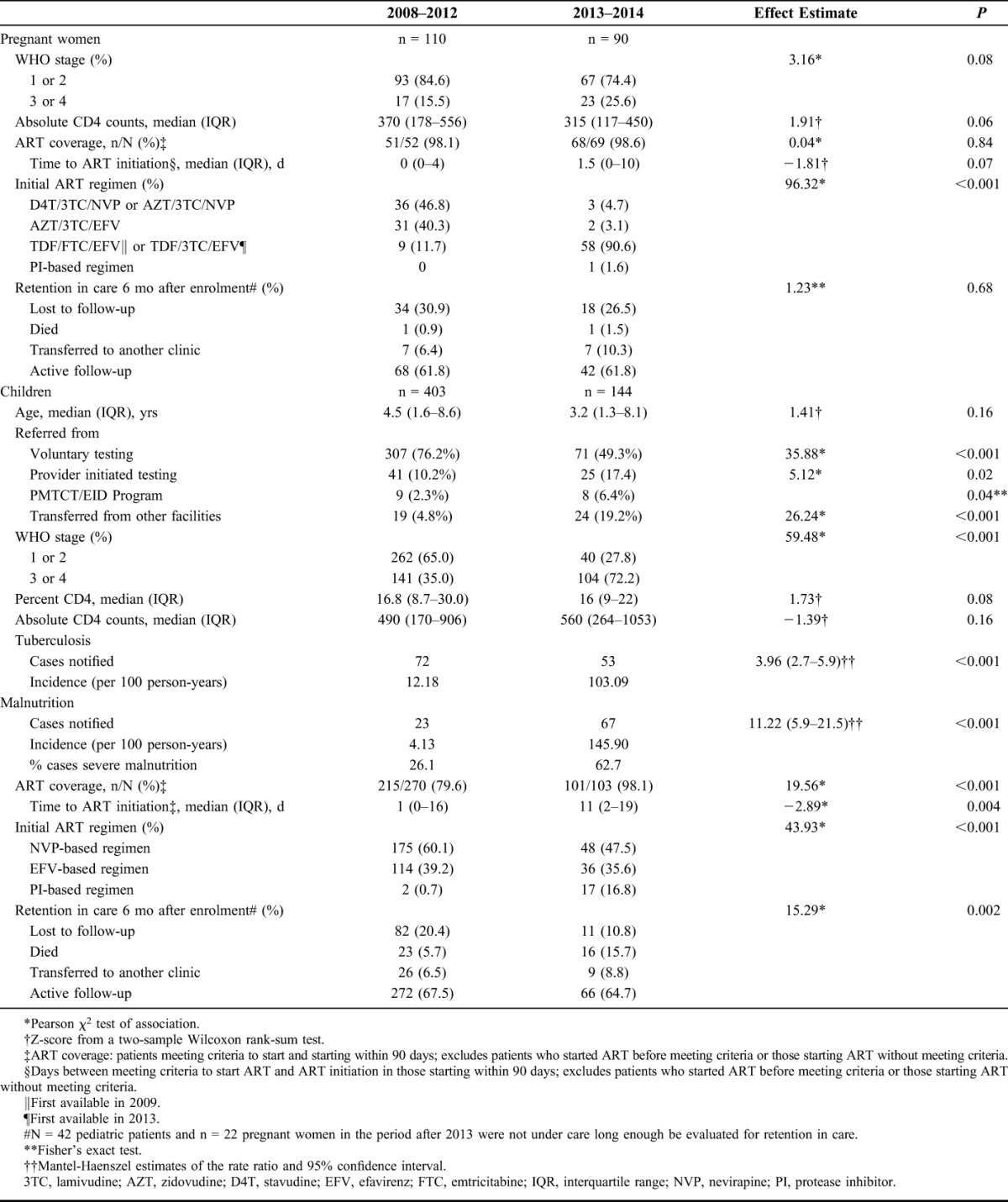
Outcomes of Children
The number of children enrolled peaked in 2008 and 2009, following the national trends,27 and increased sharply in 2014 in opposition to the tendency in the country (Fig. 2B). As shown in Table 2, after the intervention, the proportion of children diagnosed through PITC and PMTCT/EID program, and transferred from other facilities increased significantly. In 2013–2014, children were enrolled with more advanced disease (WHO stage 3/4 72.2% vs. 35%, P < 0.001) and immunosuppression (median CD4 percent 16 vs. 16.8, P = 0.08). In total, 125 cases of tuberculosis were notified during the study period, 53 of them during 2013–2014, representing an 8.5-fold increase of the incidence of tuberculosis ascertainment, from 12.2 in 2008–2012 to 103.1 per 100 person-years in 2013–2014. The thorough nutritional assessment was reflected in the 35.6-fold increased incidence of malnutrition ascertainment, from 4.1 in 2008–2012 to 145.9 per 100 person-years in 2013–2014. After the intervention, ART coverage increased from 79.6% to 98.1% (P < 0.001). The time between meeting criteria to start treatment and ART initiation increased from 1 to 11 days (P = 0.004). Children prescribed protease inhibitor-based regimens increased from 0.7% to 16.8% (P < 0.001) as a result of the adoption of the 2010 WHO ART guidelines by Tanzania in 2012 combined with the increased diagnosis of children through the PMTCT/EID program. The loss to follow-up and documented mortality rates varied largely during the 2 periods. Although the lost-to-follow-up rate decreased from 20.4% to 10.8%, the documented mortality increased from 5.7% to 15.7%. However, remarkably all deaths in 2013–2014 happened among children diagnosed through PITC: 12/16 in the inpatient wards, 2/16 in the tuberculosis clinic, and 2/16 in the outpatient department. Fifteen (15/16) children were malnourished, 6/16 had tuberculosis, and all died within 90 days after diagnosis, the majority (10/16) within 6 weeks.
Characteristics of the HIV-Exposed Infants and Early Mother-to-Child Transmission
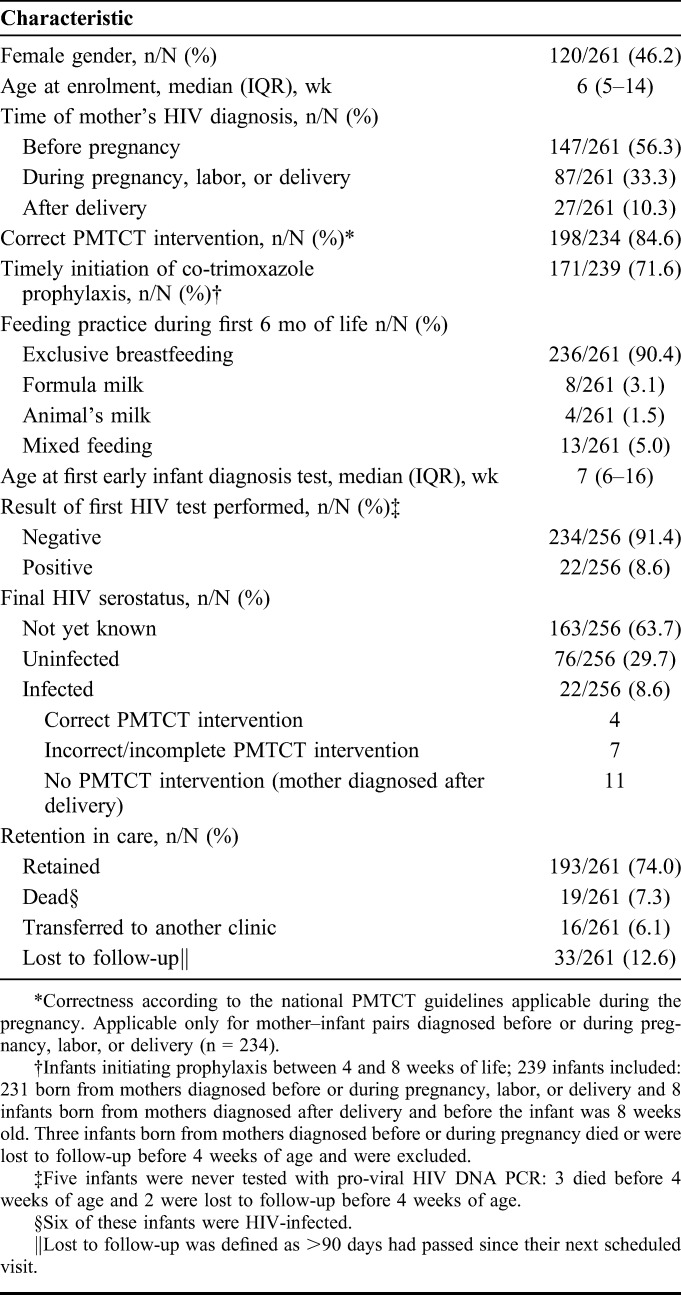
Since January 2013, all HIV-exposed infants aged <18 months were enrolled in our clinic. From January 1, 2013 to December 31, 2014, 261 infants were enrolled. Table 3 summarizes the characteristics of these infants. Fifty-six percent (157/261) of the mothers were diagnosed before pregnancy, 33.3% (87/261) during pregnancy or delivery, and 10.3% (27/261) after delivery. Among the mothers diagnosed before or during pregnancy, 15.4% (36/234) did not receive a correct PMTCT intervention, all recruited before the integration of services. The overall early MTCT rate, defined as the rate of positivity of the first HIV test performed, was 8.6%. Yet, it varied largely depending on the timing of the HIV diagnosis of the mother and the correctness of PMTCT (See Figure, Supplemental Digital Content 2, http://links.lww.com/QAI/A920). The MTCT rate of mother–infant pairs diagnosed before or during pregnancy was 4.8% (11/231), being as low as 2% (4/195) for the 84.6% (195/234) of pairs who received a correct PMTCT intervention. However, the rate was 44% (11/25) for pairs diagnosed after delivery. Noteworthy, many infants in this last group were found to be HIV-exposed through PITC, when admitted with symptoms. By the time of analysis, none of the breastfeeding infants with an initial negative test had seroconverted.
TABLE 3.
Characteristics of the 261 HIV-Exposed Infants Enrolled During 2013–2014
After EID was implemented in Ifakara in March 2014, the turnaround time for results—time between sample collection and delivery of results to caregivers—decreased from 7 months in 2013 to 13 and 35 days for positive and negative results, respectively.
DISCUSSION
The implementation of the One Stop Clinic of Ifakara combined with the improved efficiency of a paperless clinic and the rollout of PITC and EID resulted in: (1) an increased number of pregnant women and children diagnosed and linked into care; (2) an increased detection of children with AIDS; (3) universal ART coverage; (4) lower loss to follow-up and better ascertainment of mortality; and (5) a proof that elimination of MTCT can be achieved in rural Tanzania.
The intervention resulted in an increased number of pregnant women and children diagnosed and linked into care. This is an effect of the active case-finding approach of our strategy and the presence of a PMTCT and pediatric HIV team, which is well-integrated and coordinated with the outpatient clinic and the hospital wards. The opt-out approach for HIV testing during antenatal care visits has been in place in our hospital since 2008. However, in 2010–2011 only 25% of newly diagnosed women were linked to HIV services.20 Through the One Stop Clinic, the number of women diagnosed and linked into care increased substantially, with the most likely impact of preventing new pediatric infections.
Diagnosing children outside the PMTCT programs remains challenging.28–30 Measures to identify HIV-infected children must be implemented in parallel to the efforts to achieve universal PMTCT and the elimination of MTCT. In our study, the increased number of children enrolled in 2014 was mainly attributable to PITC but also to the enrollment of children diagnosed in other facilities and admitted with severe disease and the implementation of EID locally. Also, it is possible that as a result of an increased awareness among the staff, there was an expansion of family-centered testing using parents as index cases. Children enrolled after 2012 presented with more advance disease and immunosuppression, reflecting the high yield of infants and children enrolled from the inpatient wards31–33 and probably a better clinical assessment by a specially trained team.11 The incidence of HIV has decreased over the past years in Tanzania.2 Thus, we presume that before the intervention, some children were admitted and eventually died without being diagnosed with HIV and therefore enrolled in our cohort. The improvement of EID is expected to increase the identification of asymptomatic HIV-infected infants. However, given the low MTCT among mother–infant pairs who received an appropriate PMTCT intervention, the few infants diagnosed during the routine follow-up did not have an impact on the proportion of children with advanced disease at diagnosis. On the contrary, the implementation of local HIV DNA PCR testing allowed us to confidentially diagnose children aged <18 months admitted in the hospital with advance disease. Diagnosing and offering health-restoring care and treatment to children with AIDS is one of the major achievements of our strategy. However, it highlights the high percentage of late presenters in sub-Saharan Africa34 and the need to expand the case-finding approaches to places where large number of infants and children congregate, such as immunization clinics, schools, and the community, to increase early diagnosis and improve outcomes.28
ART coverage among eligible children increased from 79.6% to 98.1%. This achievement is especially remarkable since, to date, similar coverage rates in Africa have only been reported by urban programs.35,36 For children meeting the criteria, the time to initiation of drugs increased from 1 to 11 days, likely reflecting a better ascertainment of opportunistic infections and improved pre-ART counseling to children and caregivers.
After initiated on ART, children need to be retained, adhere to treatment, and get the support needed to face the challenges of growing with HIV.37–39 Integration of PMTCT and pediatric HIV services with reproductive and child care, and family-centered approaches have proven effective to improve retention.10,40,41 In our cohort, the lost-to-follow-up rate decreased from 20.4% to 10.8%. However, we could not fully assess the contribution of mortality to these cases.42 The increased documented mortality after the intervention can be explained by the increased number of children with AIDS and a better ascertainment of death. Known risk factors for mortality are low CD4 percent, WHO stage 3/4 disease, severe malnutrition, and tuberculosis,43–45 all common among children registered in 2013–2014. High fatality rates have been reported in pediatric African cohorts. In Malawi, the 12-month mortality after diagnosis through PITC was 20%,46 same as for children with tuberculosis-HIV co-infection.47 In Zambia, mortality of malnourished children was 46%, with HIV-infected children being 80% more likely to die.48 Although the lost-to-follow-up rate decreased in our clinic, the reported early mortality emphasizes once more the need for strategies to timely diagnose children who fell through the cracks of the PMTCT programs. Moreover, the availability of pediatric antiretroviral formulations is likely to lead to further improvements in adherence and retention.49
The MTCT rate for mother–infant pairs reached by PMTCT in our clinic achieved the goal of virtual elimination (<5%). These low transmission rates combined with the finding that all mothers who attended after the establishment of the One Stop Clinic received a complete PMTCT intervention confirm the programmatic benefits of integrating services. The dramatic reduction of the turnaround time of EID results leverages the decentralization of this test to facilitate the timely initiation of life-saving treatment for infected infants.8
This study has some limitations. First, although we believe that given the comprehensive nature of the intervention, its positive impact has a good chance to be maintained over time, a longer follow-up will be needed to measure the long-term impact of the strategy on the clinical outcomes and retention in care. Second, it is challenging to distinguish the impact of the specific interventions, as they were implemented as a bundle. Third, tuberculosis might have been overdiagnosed, since, despite having Xpert MTB/RIF (Cepheid, Sunnyvale, CA) in our clinic,21 confirming childhood tuberculosis is challenging, and most diagnoses were based on symptoms and radiological findings. Fourth, it is possible that some cases ascertained as lost to follow-up had died. Finally, given the short follow-up time, we could not assess the final MTCT rate. Strengths of our study include the comprehensive clinical data and the prospective character of the evaluation.
In conclusion, the creation of the One Stop Clinic of Ifakara combined with a comprehensive electronic data collection system and the implementation of PITC and EID resulted in an increased number of mothers and children diagnosed and linked into care, a higher detection of children with AIDS, universal ART coverage, better retention in care and ascertainment of mortality, and an early MTCT rate below the elimination threshold. This strategy may provide a feasible and scalable model for delivering high-quality family-centered HIV care in Tanzania and achieve the 90-90-90 target.
Supplementary Material
ACKNOWLEDGMENTS
The KIULARCO Study Group: Aschola Asantiel, Adolphina Chale, Diana Faini, Ingrid Felger, Gideon Francis, Hansjakob Furrer, Speciosa Hwaya, Aneth Kalinjuma, Bryson Kasuga, Namvua Kimera, Yassin Kisunga, Thomas Klimkait, Antonia Luhombero, Leticia Mbwile, Mengi Mkulila, Julius Mkumbo, MargarethMkusa, Dorcas K. Mnzava, Germana Mossad, Dolores Mpundunga, Daimon Msami, Athumani Mtandanguo, Selerine Myeya, Sanula Nahota, Regina Ndaki, Agatha Ngulukila, George Sikalengo, Fiona Vanobberghen, and Maja Weisser. The authors thank all the children and families who attended the One Stop Clinic and the staff from the Saint Francis Referral Hospital in Ifakara.
Footnotes
The Chronic Diseases Clinic of Ifakara and its Pediatric and PMTCT unit, the One Stop Clinic, are funded through the Ministry of Health and Social Welfare of Tanzania; the Swiss Tropical and Public Health Institute; the Ifakara Health Institute; the Government of the Canton of Basel; USAID through the local implementer TUNAJALI-Deloitte; and the Merck for Mothers Global Giving Program.
Presented in part at the 15th European AIDS Conference, October 21–24, 2105, Barcelona, Spain.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.UNAIDS. Children, and HIV: Fact Sheet. Geneva, Switzerland: UNAIDS; 2016. Available at: http://www.unaids.org/sites/default/files/media_asset/FactSheet_Children_en.pdf. Accessed August 3, 2016. [Google Scholar]
- 2.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic. Geneva, Switzerland: UNAIDS; 2013. Available at: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. Accessed May 21, 2015. [Google Scholar]
- 3.Fergusson P, Tomkins A. HIV prevalence and mortality among children undergoing treatment for severe acute malnutrition in sub-saharan Africa: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103:541–548. [DOI] [PubMed] [Google Scholar]
- 4.Cohen D, Lungu M, van Oosterhout JJ. HIV testing coverage of family members of adult antiretroviral therapy patients in Malawi. AIDS Care. 2010;22:1346–1349. [DOI] [PubMed] [Google Scholar]
- 5.Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis. 2009;48:108–114. [DOI] [PubMed] [Google Scholar]
- 6.Kranzer K, Meghji J, Bandason T, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med. 2014;11:e1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. [DOI] [PubMed] [Google Scholar]
- 8.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382:1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essajee SM, Arpadi SM, Dziuban EJ, et al. Pediatric treatment 2.0: ensuring a holistic response to caring for HIV-exposed and infected children. AIDS. 2013;27(suppl 2):S215–S224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kline MW, Ferris MG, Jones DC, et al. The pediatric AIDS corps: responding to the African HIV/AIDS health professional resource crisis. Pediatrics. 2009;123:134–136. [DOI] [PubMed] [Google Scholar]
- 12.Green A, de Azevedo V, Patten G, et al. Clinical mentorship of nurse initiated antiretroviral therapy in Khayelitsha, South Africa: a quality of care assessment. PLoS One. 2014;9:e98389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindegren ML, Kennedy CE, Bain-Brickley D, et al. Integration of HIV/AIDS services with maternal, neonatal and child health, nutrition, and family planning services. Cochrane Database Syst Rev. 2012;9:CD010119. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Cyamatare FR, Niyigena P, et al. Clinical outcomes of a comprehensive integrated program for HIV-exposed infants: a 3-year experience promoting HIV-free survival in rural Rwanda. J Acquir Immune Defic Syndr. 2013;62:e109–114. [DOI] [PubMed] [Google Scholar]
- 15.Tonwe-Gold B, Ekouevi DK, Bosse CA, et al. Implementing family-focused HIV care and treatment: the first 2 years' experience of the mother-to-child transmission-plus program in Abidjan, Cote d'Ivoire. Trop Med Int Health. 2009;14:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Global Health Sector Strategy on HIV/AIDS 2011–2015. Geneva, Switzerland: World Health Organization; 2011. Available at: http://apps.who.int/iris/bitstream/10665/112790/1/9789241507295_eng.pdf. Accessed October 1, 2015. [Google Scholar]
- 17.UNAIDS. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva, Switzerland: UNAIDS; 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. Accessed October 1, 2015. [Google Scholar]
- 18.UNAIDS. Progress Report on the Global Plan Towards the Elimination of New HIV Infection Among Children by 2015 and Keeping Their Mothers Alive. Geneva, Switzerland: UNAIDS; 2014. Available at: http://www.unaids.org/sites/default/files/documents/JC2681_2014-Global-Plan-progress_en.pdf. Accessed October 10, 2015. [Google Scholar]
- 19.National AIDS Control Program. Global AIDS response country report. Dar es Salaam, Tanzania: Ministry of Health and Social Welfare, United Republic of Tanzania; 2014. Available at: http://www.unaids.org/sites/default/files/country/documents/TZA_narrative_report_2014.pdf. Accessed 1 October, 2015. [Google Scholar]
- 20.Gamell A, Letang E, Jullu B, et al. Uptake of guidelines on prevention of mother-to-child transmission of HIV in rural Tanzania: time for change. Swiss Med Wkly. 2013;143:w13775. [DOI] [PubMed] [Google Scholar]
- 21.Haraka F, Glass TR, Sikalengo G, et al. A bundle of services increased ascertainment of tuberculosis among HIV-infected Individuals enrolled in a HIV cohort in rural sub-saharan Africa. PLoS One. 2015;10:e0123275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letang E, Muller MC, Ntamatungiro AJ, et al. Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis. 2015;2:ofv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanobberghen F, Letang E, Gamell A, et al. A Decade of HIV Care and Treatment in Rural Tanzania: Trends in Treatment, Opportunistic Infections and Laboratory Abnormalities Among HIV-positive Adults. 15th European AIDS Conference; Barcelona, Spain, 2015. Abstract PE21/5.
- 24.National Guidelines for Comprehensive Care Services for Prevention of Mother-to-Child Transmission of HIV and Keeping Mothers Alive. Dar es Salaam, Tanzania: Ministry of Health and Social Welfare, United Republic of Tanzania; 2013. Available at: http://pmtct.or.tz/wp-content/uploads/2013/10/tz_guidelines_ccs_optionb_all.pdf. Accessed October 1, 2015. [Google Scholar]
- 25.Wolfe BA, Mamlin BW, Biondich PG, et al. The OpenMRS system: collaborating toward an open source EMR for developing countries. AMIA Annu Symp Proc. 2006:1146. [PMC free article] [PubMed] [Google Scholar]
- 26.National AIDS Control Program. Guidelines for HIV Testing and Counselling in Clinical Settings. Dar es Salaam, Tanzania: Ministry of Health and Social Welfare, United Republic of Tanzania; 2007. Available at: http://ihi.eprints.org/823/1/MoHSW.pdf_(53).pdf. Accessed January 20, 2016. [Google Scholar]
- 27.National AIDS Control Program. Implementation of HIV/AIDS Care and Treatment Services in Tanzania—Report Number 3. Dar es Salaam, Tanzania: Ministry of Health and Social Welfare, United Republic of Tanzania; 2013. Available at: www.nacp.go.tz/site/download/bookreport3.pdf. Accessed October 3, 2015. [Google Scholar]
- 28.Ahmed S, Kim MH, Sugandhi N, et al. Beyond early infant diagnosis: case finding strategies for identification of HIV-infected infants and children. AIDS. 2013;27(suppl 2):S235–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamla D, Mbori-Ngacha D, Newman M, et al. Evidence from the field: missed opportunities for identifying and linking HIV-infected children for early initiation of ART. AIDS. 2013;27(suppl 2):S139–S146. [DOI] [PubMed] [Google Scholar]
- 30.Horwood C, Voce A, Vermaak K, et al. Routine checks for HIV in children attending primary health care facilities in South Africa: attitudes of nurses and child caregivers. Soc Sci Med. 2010;70:313–320. [DOI] [PubMed] [Google Scholar]
- 31.Kankasa C, Carter RJ, Briggs N, et al. Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune defic Syndr. 2009;51:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutanga JN, Raymond J, Towle MS, et al. Institutionalizing provider-initiated HIV testing and counselling for children: an observational case study from Zambia. PLoS One. 2012;7:e29656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCollum ED, Preidis GA, Kabue MM, et al. Task shifting routine inpatient pediatric HIV testing improves program outcomes in urban Malawi: a retrospective observational study. PLoS One. 2010;5:e9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahuerta M, Wu Y, Hoffman S, et al. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006–2011: findings from four sub-saharan African countries. Clin Infect Dis. 2014;58:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leyenaar JK, Novosad PM, Ferrer KT, et al. Early clinical outcomes in children enrolled in human immunodeficiency virus infection care and treatment in Lesotho. Pediatr Infect Dis J. 2010;29:340–345. [DOI] [PubMed] [Google Scholar]
- 36.Anaky MF, Duvignac J, Wemin L, et al. Scaling up antiretroviral therapy for HIV-infected children in Cote d'Ivoire: determinants of survival and loss to programme. Bull World Health Organ. 2010;88:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kellerman SE, Sugandhi N. Pediatric AIDS in the elimination agenda. PLoS Med. 2013;10:e1001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernays S, Jarrett P, Kranzer K, et al. Children growing up with HIV infection: the responsibility of success. Lancet. 2014;383:1355–1357. [DOI] [PubMed] [Google Scholar]
- 39.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, et al. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14:627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myer L, Akugizibwe P. Impact of HIV treatment scale-up on women's reproductive health care and reproductive rights in Southern Africa. J Acquir Immune Defic Syndr. 2009;52(suppl 1):S52–S53. [DOI] [PubMed] [Google Scholar]
- 41.Rochat TJ, Bland R, Coovadia H, et al. Towards a family-centered approach to HIV treatment and care for HIV-exposed children, their mothers and their families in poorly resourced settings. Future Virol. 2011;6:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geng EH, Odeny TA, Lyamuya RE, et al. Estimation of mortality among HIV-infected people on antiretroviral therapy treatment in east Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV. 2015;2:e107–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutcliffe CG, van Dijk JH, Bolton C, et al. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–489. [DOI] [PubMed] [Google Scholar]
- 44.Bong CN, Yu JK, Chiang HC, et al. Risk factors for early mortality in children on adult fixed-dose combination antiretroviral treatment in a central hospital in Malawi. AIDS. 2007;21:1805–1810. [DOI] [PubMed] [Google Scholar]
- 45.Eley B, Davies MA, Apolles P, et al. Antiretroviral treatment for children. S Afr Med J. 2006;96:988–993. [PubMed] [Google Scholar]
- 46.McCollum ED, Preidis GA, Golitko CL, et al. Routine inpatient human immunodeficiency virus testing system increases access to pediatric human immunodeficiency virus care in sub-Saharan Africa. Pediatr Infect Dis J. 2011;30:e75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buck WC, Olson D, Kabue MM, et al. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis co-infection. Int J Tuberc Lung Dis. 2013;17:1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munthali T, Jacobs C, Sitali L, et al. Mortality and morbidity patterns in under-five children with severe acute malnutrition (SAM) in Zambia: a five-year retrospective review of hospital-based records (2009-2013). Arch Public Health. 2015;73:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Refocusing Research and Development of Paediatric HIV Treatments on the Needs of Children in Resource-Poor Settings: A Paediatric HIV Round table with Industry & Joint Call to Action on Paediatric HIV. Geneva, Switzerland: DNDi; 2013. Available at: http://www.dndi.org/images/stories/diseases_portfolio/DNDi_Paediatric_HIV_roundtable.pdf. Accessed October 1, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


