Abstract
Objective:
To evaluate the safety and pharmacokinetics of MIV-150 and zinc acetate in a carrageenan gel (PC-1005). Acceptability, adherence, and pharmacodynamics were also explored.
Design:
A 3-day open-label safety run-in (n = 5) preceded a placebo-controlled, double-blind trial in healthy, HIV-negative, abstinent women randomized (4:1) to vaginally apply 4 mL of PC-1005 or placebo once daily for 14 days.
Methods:
Assessments included physical examinations, safety labs, colposcopy, biopsies, cervicovaginal lavages (CVLs), and behavioral questionnaires. MIV-150 (plasma, CVL, tissue), zinc (plasma, CVL), and carrageenan (CVL) concentrations were determined with LC-MS/MS, ICP-MS, and ELISA, respectively. CVL antiviral activity was measured using cell-based assays. Safety, acceptability, and adherence were analyzed descriptively. Pharmacokinetic parameters were calculated using noncompartmental techniques and actual sampling times. CVL antiviral EC50 values were calculated using a dose–response inhibition analysis.
Results:
Participants (n = 20) ranged from 19–44 years old; 52% were black or African American. Among those completing the trial (13/17, PC-1005; 3/3, placebo), 11/17 reported liking the gel overall; 7 recommended reducing the volume. Adverse events, which were primarily mild and/or unrelated, were comparable between groups. Low systemic MIV-150 levels were observed, without accumulation. Plasma zinc levels were unchanged from baseline. Seven of seven CVLs collected 4-hour postdose demonstrated antiviral (HIV, human papillomavirus) activity. High baseline CVL anti–herpes-simplex virus type-2 (HSV-2) activity precluded assessment of postdose activity.
Conclusions:
PC-1005 used vaginally for 14 days was well tolerated. Low systemic levels of MIV-150 were observed. Plasma zinc levels were unchanged. Postdose CVLs had anti-HIV and anti–human papillomavirus activity. These data warrant further development of PC-1005 for HIV and sexually transmitted infection prevention.
Key Words: microbicide, multipurpose prevention technology, phase 1 trial, MIV-150, ARV-based prevention, vaginal gel
INTRODUCTION
Background
Globally, AIDS is the leading cause of death among women 15–44 years old.1 Young women and girls aged 15–24 are disproportionately affected by HIV/AIDS, particularly in sub-Saharan Africa. In some countries, HIV prevalence among 15–24-year-old females is more than 7 times that of their male counterparts.2 In addition to HIV, nearly 300 million women are infected with the human papillomavirus (HPV),3 and 267 million women are living with herpes-simplex virus type-2 (HSV-2).4 Both viruses increase the risk of HIV acquisition and cause significant morbidity.5 For over 2 decades, scientists have been working to develop vaginal microbicides to expand the limited options women currently have to protect themselves against HIV and other sexually transmitted infections (STIs).6 Of the 7 candidate microbicide gels to complete advanced-stage clinical trials,7 only the antiretroviral (ARV)-based 1% tenofovir gel was shown to reduce the risk of HIV acquisition in women (CAPRISA 004).8 However, 2 subsequent phase 3 trials found no reduction in HIV risk.9,10
In response to the suboptimal adherence that likely contributed to the lack of effectiveness in most phase 3 microbicide gel trials,11,12 product developers began pursuing other drug delivery platforms, such as intravaginal rings (IVRs). A one-month IVR containing dapivirine, a nonnucleoside reverse transcriptase inhibitor (NNRTI), is furthest in development, with 2 phase 3 efficacy trials recently completed.13,14 Despite adherence being an obstacle in many microbicide trials, including the 2 dapivirine IVR trials, data indicate that there are women who can achieve high adherence.15 In the CAPRISA 004 trial and in both dapivirine IVR trials, there was a direct relationship between higher adherence and higher effectiveness rates among participants randomized to the active products.8,13 Although other delivery platforms are clearly needed, gel formulations could be used both vaginally and rectally and may be preferred by some women. Given that those at risk of HIV practice vaginal and/or anal intercourse,16–18 a gel designed for dual compartment use is likely to improve adherence. This article reports on the first-in-human study of MIV-150 and zinc acetate (ZA) coformulated in a carrageenan (CG) gel (PC-1005).
PC-1005
The MIV-150, ZA, and CG combination is a promising multipurpose prevention technology (MPT) being developed as a dual compartment gel to prevent HIV, HSV-2, and HPV. MIV-150 is a potent, third-generation NNRTI that was originally developed as an orally administered HIV treatment. However, because oral bioavailability targets were not achieved in phase 1 studies, development of MIV-150 for oral therapy was discontinued.19 ZA is generally recognized as safe (GRAS) by the United States Food and Drug Administration,20 is used in many over-the-counter pharmaceuticals,21 and has been shown to have activity against HIV and other viruses.22–25 CG is also a GRAS substance used in many consumer products and foods,20 with excellent rheological properties. CG is the most potent inhibitor of HPV known,26 potentiates the anti-HSV-2 activity of ZA,27,28 and has been found safe for vaginal use in multiple studies.29–34 PC-1005 is active in vivo against vaginal or rectal challenge with HIV, simian-human immunodeficiency virus-reverse transcriptase, HSV-2, and HPV24,35,36; ex vivo against simian-human immunodeficiency virus-reverse transcriptase in explants37,38 and remains active in the presence of semen.27,28
In this first-in-human trial of PC-1005, the primary objectives were to evaluate the safety of PC-1005 and determine the plasma pharmacokinetics (PK) of MIV-150 in women who applied the gel vaginally once daily for up to 14 days. Secondary objectives were to assess acceptability and adherence. Exploratory objectives were to determine zinc (Zn2+) PK in plasma; measure MIV-150 concentrations in cervical tissue; and assess MIV-150, Zn2+, and CG pharmacodynamics (PD) in cervicovaginal lavages (CVLs).
METHODS
Trial Design
A randomized, placebo-controlled, double-blind, phase 1 trial of PC-1005, sponsored by the Population Council (New York, NY), was conducted at the University of Alabama, Birmingham (UAB). An open-label (OL), single-arm, safety study preceded the trial (randomized period). Women aged 19–49, who provided written informed consent, were screened based on medical history, physical examination, pelvic examination, colposcopy, and laboratory testing, including for HIV and STIs. Healthy, HIV-negative, nonpregnant women currently using an effective, nonvaginal contraceptive method, with a body mass index of 18–32 kg/m2, and who agreed to remain sexually abstinent from the time they were screened until they exited the study were eligible. Women who tested positive for STIs other than asymptomatic HSV-2, bacterial vaginosis or candidiasis, were ineligible.
Participants enrolled in the 6-day OL period (n = 5), who were not subsequently eligible for the randomized period, were requested to self-administer 4 g of PC-1005 vaginally, under clinical supervision, once daily at the same time (±5 minutes) for 3 consecutive days (days 1–3) to collect preliminary safety data and to identify the most appropriate PK time points for the randomized period. Participants stayed overnight for 24-h PK blood draws on day 1 into day 2 and day 3 into day 4, and attended outpatient visits on days 5 and 6. Safety was assessed after each dose and at study exit (day 6).
Participants enrolled in the randomized period were to be assigned (4:1) to apply PC-1005 or placebo (hydroxyethylcellulose) gel vaginally once daily for 14 consecutive days (days 1–14). Participants were simultaneously stratified (1:1) to a final PK/PD specimen collection time point either 4 hours (day 14) or 24 hours (day 15) after the last dose. Treatment randomization occurred in blocks of 5. At enrollment, a study nurse opened the next consecutive presealed randomization envelope indicating the randomization number and time point. Participants and staff at the Council and UAB were blinded to the product assignment, but not time point, through the end of data collection.
In the randomized period, doses 1, 8, and 14 (days 1, 8, and 14) were applied under direct observation in the clinic, and the remaining 11 doses were self-administered at home. Participants were given instructions to take home that illustrated how to insert the gel properly, emphasized the importance of adhering to the dosing regimen, and reinforced the need for abstinence. Participants were instructed to save all used applicators, to document the date and time each home applicator was inserted on individual plastic bags provided by the study staff, and to return all applicators at the next study visit. Clinical and laboratory safety assessments occurred at all visits. Drug concentrations were assessed after doses 1, 8, and 14, and PD was assessed before the first and after the last dose. The first dose (day 1) was scheduled at least 7 days after enrollment, and as soon as possible after the participant's last menstrual period. Participants assigned to the 24-hour PK/PD time point after dose 14 stayed overnight at the clinic (until day 15), whereas all other visits were outpatient.
The protocol and amendments were approved by the Population Council (Protocol Number PC-558) and UAB Institutional Review Boards. The trial adhered to International Conference on Harmonisation and US FDA Guidelines for Good Clinical Practices and was registered at ClinicalTrials.gov (NCT02033109).
Investigational Products
PC-1005 and hydroxyethylcellulose39 gels were manufactured and packaged in identical individually wrapped, prefilled, single-use, metered dose applicators (HTI Plastics; Lincoln, NE) at the Population Council's Good Manufacturing Practices facility (Center for Biomedical Research, New York, NY). Each participant was assigned a unique, prepackaged kit containing all the applicators needed for study participation: 4 applicators for the OL period (1 dose per day for 3 days, plus 1 extra as a backup) and 15 applicators for the randomized period (1 dose per day for 14 days, plus 1 extra).
Safety Assessments
Safety assessments included physical examinations, vital signs, electrocardiograms (OL only), and laboratory assessments at all visits. Pelvic examinations were performed at enrollment, and before the first and after the last dose. Colposcopy (with cervical and vaginal biopsies in the randomized period) was performed before the first and after the last dose. In the randomized period, nurses also called participants on days 5 and 11 to ensure they were not experiencing any problems. Primary outcomes included abnormal clinical or laboratory findings, and treatment emergent adverse events (TEAEs), defined as AEs occurring during or after the first dose, and on or before the third (during the OL period) or seventh (during the randomized period) day after the last dose.
Pharmacokinetics
In the OL period, blood was drawn to determine plasma drug concentrations of MIV-150 and Zn2+ predose; 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 16, 18, 20, and 24 hours after doses 1 and 3 (days 1 and 3); and 48 and 72 hours after dose 3 (days 5 and 6, respectively). In the randomized period, blood draws for MIV-150 plasma PK occurred predose; 0.5, 1, 2, 3, 4, 6, 8, and 12 hours postdose (days 1 and 8); and up to 4 hours (day 14) or 24 hours (day 15) postdose 14, per the stratification scheme. Concentrations of MIV-150 in plasma were determined using liquid chromatography with tandem mass spectrometry (LC-MS/MS) as published, with modifications.36 Concentrations of Zn2+ in plasma were determined using inductively coupled plasma-mass spectrometry (ICP-MS), the standard method used by Quest laboratories (Tucker, GA).
Acceptability and Adherence (Randomized Period Only)
On day 14, participants self-administered a written acceptability questionnaire in which they rated the gel overall, as well as specific characteristics (color, odor, and volume), as recommended by Morrow and Ruiz.40 Adherence was calculated as the proportion of home doses inserted compared with home doses planned (maximum of 11). An applicator was considered “used” if it was returned opened and if a participant reported using it during an interviewer-administered quantitative questionnaire (days 8 and 14).
PD (Randomized Period Only)
Before dose 1 (day 1) and after dose 14 (4 or 24 hours, per the stratification scheme), CVLs were collected (using 10 mL of sterile normal saline) to measure concentrations and antiviral activity of MIV-150, Zn2+, and CG in vaginal fluid. To measure MIV-150 concentration in tissue, one 3 × 5-mm pinch biopsy was collected from the ectocervix of each participant after dose 14 (4 or 24 hours postdose). Concentrations of CG (CVL) and MIV-150 (CVL, tissue) were determined using ELISA28 and LC-MS/MS,36 respectively. Zn2+ concentrations (CVL) were determined by atomic emission spectroscopy (Alliance Analytical Lab; Coopersville, MI). Antiviral activity in CVL was assessed using a TZM-bl assay with an HIV-1ADA-M laboratory strain,28 a colorimetric assay using an HSV-2 G strain,41 and a luciferase assay using HPV16 pseudovirus.28 Assays were performed on serial dilutions to establish a dose–response curve.
Data Analysis
Safety, acceptability, and adherence data were analyzed descriptively using SAS Version 9.1.3 (SAS Institute, Cary, NC). Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA, Version 16.0), and graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0 (clarification, August 2009) and the Female Genital Table for Use in Microbicide Studies.42 The number and percentage of participants with each AE were summarized. Plasma PK parameters were calculated from concentration–time data using noncompartmental techniques and actual sampling times using Phoenix WinNonlin Version 6.3 (Pharsight Corp., St. Louis, MO). A minimum of 3 quantifiable PK samples was required to include a participant in the area under the concentration–time curve (AUC) analysis. GraphPad Prism 5.0c Software, Inc. (La Jolla, CA) was used to prepare curve-fitting analysis and to calculate the median cytotoxicity concentration (CC50) and the half-maximal effective concentration values (EC50, based on CVL dilution or drug concentrations) for all CVLs. Correlations between levels of MIV-150, Zn+2, and CG in CVL, between MIV-150 in CVL and cervical tissue, and between MIV-150 and CG and their respective antiviral activity, were calculated.
RESULTS
Disposition and Demographics
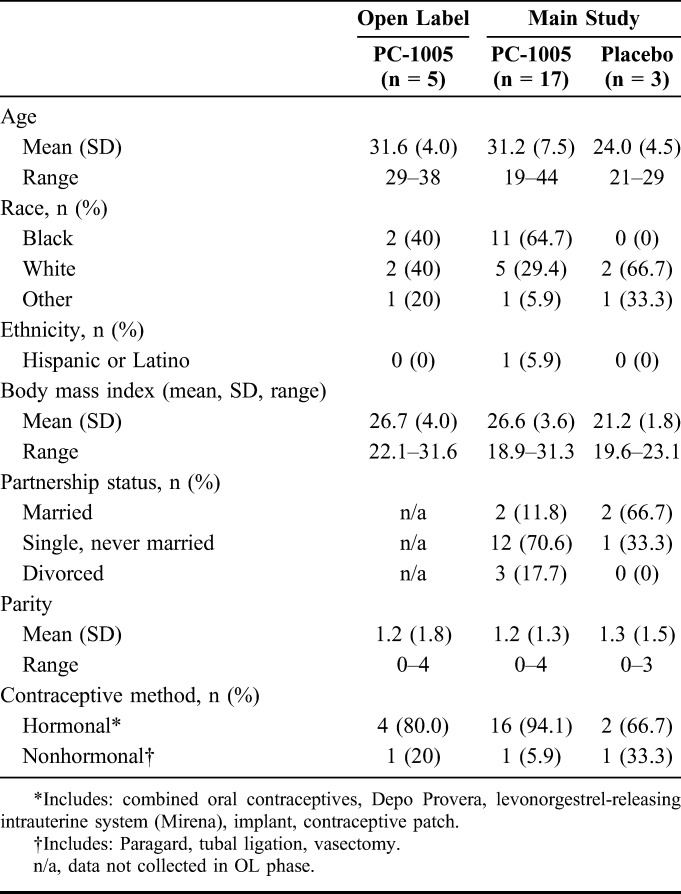
Between June 2014 and March 2015, 25 women were enrolled in the PC-558 trial. All 5 participants in the OL period and 17/20 participants (14/17, PC-1005; 3/3, placebo) in the randomized period completed follow-up. One PC-1005 participant withdrew consent before dosing, and 2 were lost to follow-up (one before dosing, one after day 1/dose 1). Most participants were single, black, or African American, and averaged 29.9 years of age (Table 1).
TABLE 1.
Demographics and Other Background Characteristics of Participants in PC-558 (n = 25)
Exposure and Adherence
The 5 participants in the OL period received all 3 in-clinic doses of PC-1005. In the randomized period, the average number of used applicators per participant was 12.8 for PC-1005 and 14.0 for placebo. Adherence to home use (based on self-report and applicator count) was 97.9%; 11/14 participants and 3/3 participants in the PC-1005 and placebo groups, respectively, reported inserting all home doses. Among women in the PC-1005 group, 2 reported missing 1 home dose, 1 reported missing 2 home doses, and 2 missed an in-clinic dose because of inclement weather.
Safety
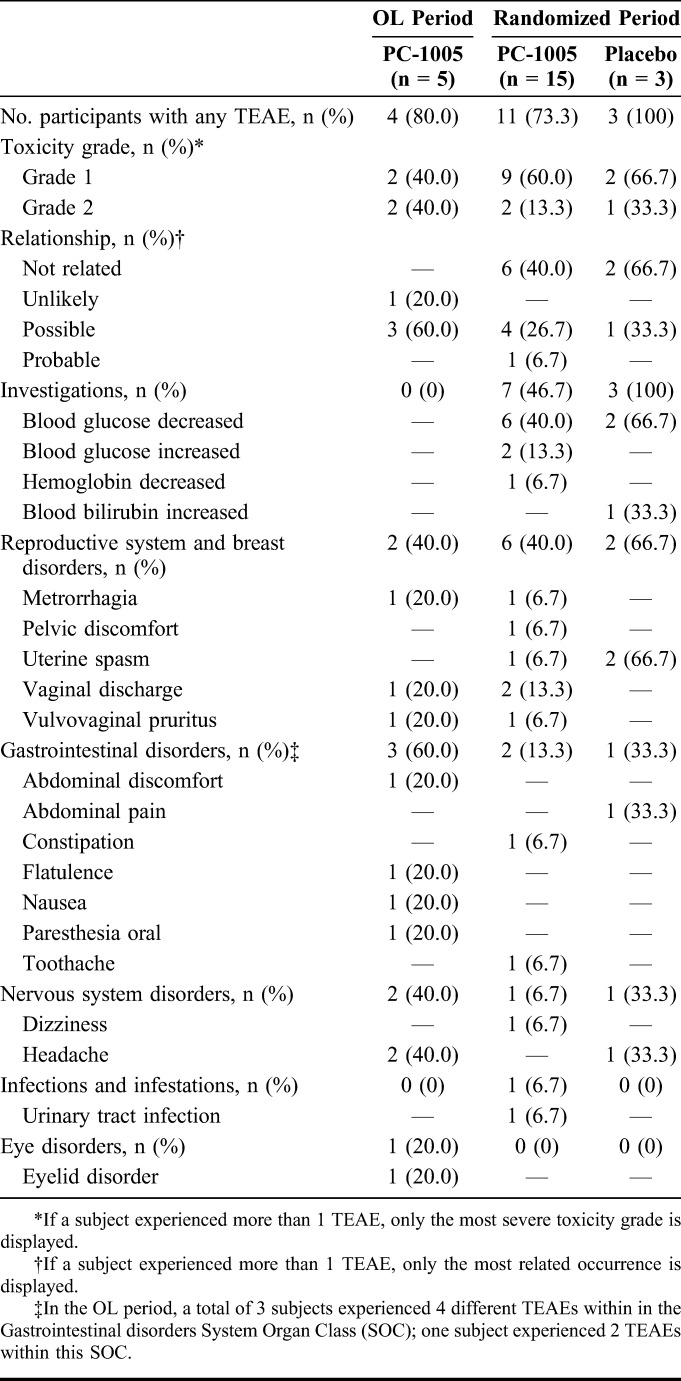
There were no safety concerns in the OL period, enabling the randomized period to proceed. TEAEs in the randomized period were recorded for 11/15 participants in the PC-1005 group and 3/3 participants in the placebo group (Table 2), the majority of which were Division of AIDS grade 1. No grade 3 or 4 TEAEs were recorded. There were no deaths, serious adverse events, or AEs leading to discontinuation. Related events (as determined by the investigator based on the temporal relationship) included metrorrhagia, vaginal discharge, vulvovaginal pruritus, abdominal discomfort, constipation, and urinary tract infection in the PC-1005 group; and uterine spasm (cramps) in both groups. No significant histopathologic changes in cervical or vaginal biopsies were observed in any of the 17 women who completed the randomized period (data not shown).
TABLE 2.
Adverse Events Reported for Participants in PC-558, Regardless of Causality (n = 23)
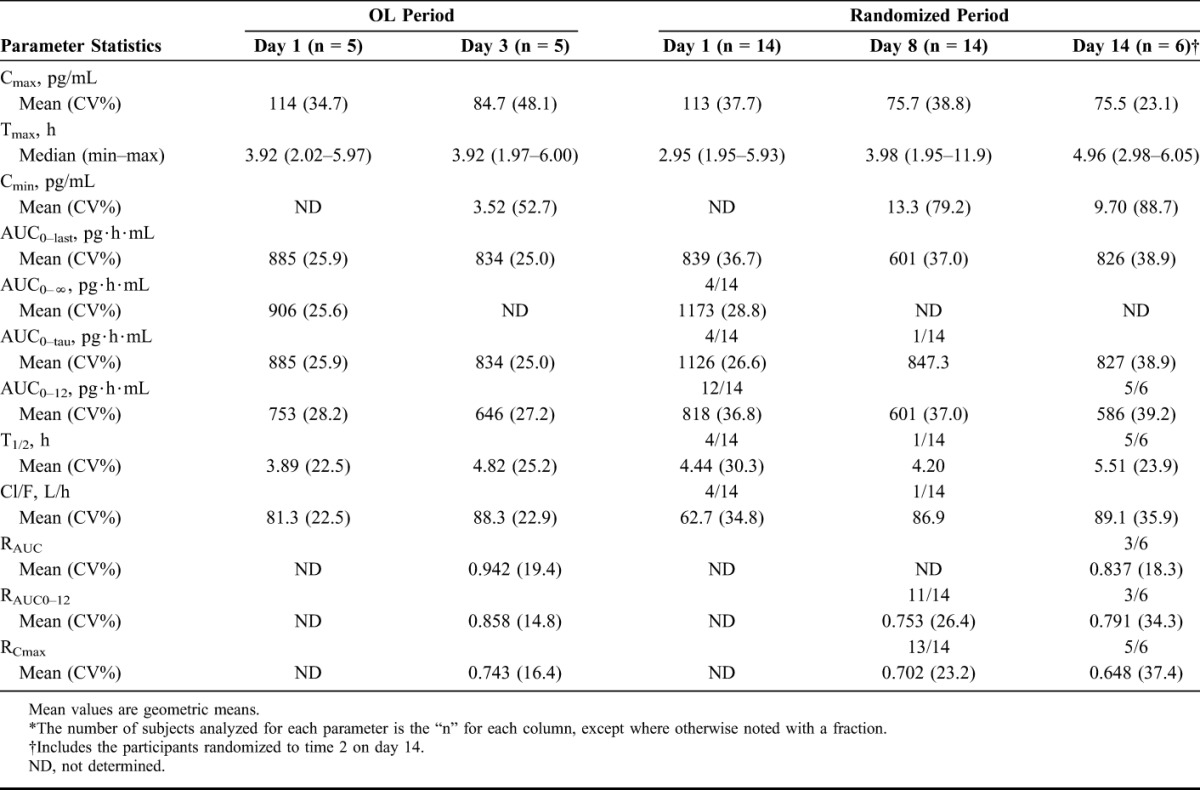
Pharmacokinetics
Figure 1 illustrates the mean MIV-150 concentration at each time point for the OL and randomized periods of the study. As shown in Table 3, the mean maximum concentration (Cmax) of MIV-150 in plasma was virtually identical on day 1 in the OL and randomized periods 113 and 114 pg/mL, respectively. The mean Cmax was lower on day 3 (OL period) and days 8 and 14 (randomized period) than the corresponding day 1 values. The mean area under the concentration–time curve from time zero to 12 hours postdose (AUC0–12) on day 3 was lower than on day 1 (646 versus 753 pg·h·mL), and was lower on day 8 (601 pg·h·mL) and day 14 (586 pg·h·mL) than day 1 (818 pg·h·mL). A similar pattern was observed for AUC0–tau (AUC during a dosing interval). With once-daily dosing, the mean half-life on day 14 was 5.5 hours with no drug accumulation observed. All postdose Zn2+ concentrations in both periods and in all participants were unchanged from baseline and were within the normal range (60–130 μ/dL; data not shown).
FIGURE 1.
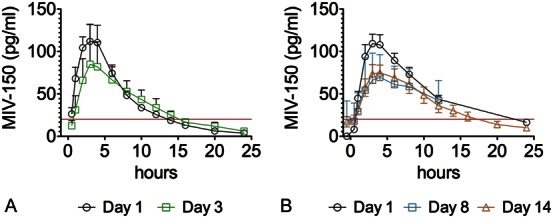
Mean MIV-150 plasma concentration–time profile, linear scale. Concentration of MIV-150 in plasma during OL (A) and randomized (B) periods. All plasma values assayed as nondetectable were assigned values of zero (0). All zero and nonzero plasma values were used in summaries and plasma PK parameter calculations. Line horizontal to x-axis is the lower limit of quantitation (20 pg/mL) for MIV-150 in plasma.
TABLE 3.
Summary of MIV-150 Plasma PK Parameters*
Acceptability
Of the 17 participants completing the randomized period, 11/17 (pooled across groups) reported liking the gel, overall, and all 17 said the gel was easy to use. Although most participants (14/17) were not bothered by gel leakage, 7/17 recommended reducing the gel volume. Almost all participants (13/14, PC-1005; 3/3, placebo) reported that they would be willing to use a similar gel if it effectively reduced the risk of HIV infection, and they were worried about getting HIV. Detailed acceptability data will be presented in a forthcoming publication.
Pharmacodynamics
Among participants randomized to PC-1005, CVLs collected at baseline and at 4 hours (n = 7) or 24 hours (n = 6) after dose 14 were available for evaluation. (One participant missed the 24-hour postdose CVL collection because severe weather shut down the clinic.) Anti-HIV activity was detected for 3 participants at baseline (mean EC50 of 0.096 dilution), 7/7 participants at 4 hours postdose (mean EC50 of 0.025 dilution, corresponding to a mean of 0.78 nM of MIV-150), and 1/6 participants at 24 hours postdose (EC50 of 0.377 dilution). Anti-HPV activity was detected for 4 participants at baseline (mean EC50 of 0.036 dilution), 7/7 participants at 4 hours postdose (mean EC50 of 0.0002 dilution, corresponding to a mean of 67.43 ng/mL of CG), and 4/6 participants at 24 hours postdose (mean EC50 of 0.029 dilution, corresponding to a mean of 212.87 ng/mL CG). In some cases, HIV or HPV infection was reduced when cells were infected in the presence of baseline CVLs compared with virus controls, which presumably reflects the endogenous activity of genital tract secretions. This endogenous activity of CVLs in microbicide trials has been described previously.43
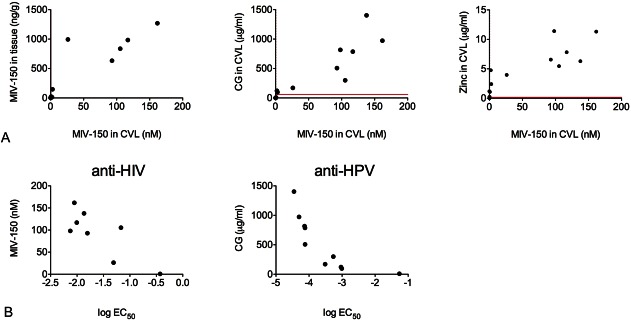
As shown in Figure 2, MIV-150 and CG concentrations were correlated with each other (r = 0.956; P < 0.0001), with zinc concentrations (r = 0.8487, MIV-150; r = 0.9172, CG; P < 0.0001 for both) and with their respective antiviral activity (r = −0.6190, MIV-150; not significant; r = −0.9879, CG; P < 0.0001). MIV-150 in tissue and CVL were also strongly correlated (r = 0.968; P < 0.0001). EC50 values based on drug concentration suggest that vaginal fluid components did not affect the antiviral properties of MIV-150 and CG. Anti-HSV-2 activity was not detected. As shown in Figure 2B, samples with high active pharmaceutical ingredient concentrations resulted in lower EC50 values based on CVL dilution because the greater the amount of active pharmaceutical ingredient in the sample, the more dilution required to reach the EC50 value.
FIGURE 2.
Correlation of active pharmaceutical ingredient levels with each other, in different compartments, and their respective antiviral activity. A, Concentrations of MIV-150 in plasma, CVL, and tissue were determined using LC-MS/MS, Zn2+ in plasma and CVL using ICP-MS, and CG in CVL using ELISA. Lines parallel to x-axis and y-axis are the lower limit of quantitation for each API/compartment. B, API concentrations were determined as described above; antiviral activity was determined using the TZM-bl and luciferase assays for anti-HIV-1 and anti-HPV16 PsV activity, respectively. The graphs show the log EC50 values for each sample versus API concentration.
DISCUSSION
A 4-g dose of PC-1005 gel inserted once daily for up to 14 days was well tolerated by sexually abstinent, HIV-seronegative women. CVLs obtained at 4 hours postdose demonstrated anti-HIV and anti-HPV activity in all 7 women assessed. Based on self-reports, most (14/17) participants completing the trial inserted all home doses and the majority (11/17) reported liking the gel, overall. No safety signals emerged in AEs, laboratory, or clinical parameters. As expected based on animal studies, MIV-150 was absorbed at low levels, with no drug accumulation observed in daily dosing. The lower Cmax and AUC values for MIV-150 on days 8 and 14 versus day 1 likely reflect the induction of drug-metabolizing enzymes by MIV-150. Human CYP3A4 has been implicated in metabolism of MIV-15044 and other NNRTIs and has been shown to be induced by the NNRTI rilpivirine.45 In addition, a similar decline of MIV-150 in plasma after MIV-150–sustained exposure has been seen in macaque preclinical studies.44 How this may affect tissue PK and efficacy is unknown and should be explored in future studies. In addition, Zn2+ concentrations in plasma were similar before and after PC-1005 exposure. Studies in macaques indicating a similar PK profile have shown that PC-1005 affords complete protection against simian-human immunodeficiency virus-reverse transcriptase in animals challenged 8 hours after single or repeated gel application.27,35,36 Eight hours of protection substantially exceeds the target product profile specifications for PC-1005, an on-demand gel used around the time of intercourse. Low systemic concentrations of MIV-150 also minimize the potential for adverse reactions.
MIV-150 and CG concentrations in CVLs collected 4 hours postdose were 300 and 10,000 times their respective EC50 values, and MIV-150 tissue concentrations were 100 times and 700 times the in vitro EC90 and EC50 values, respectively. An important advantage of this study is that antiviral activity was measured rather than estimated based on active pharmaceutical ingredient concentrations and presumed EC50 values. Anti-HIV and anti-HPV activity were detected in cell-based assays demonstrating that efficacy of MIV-150 and CG is not negated by vaginal fluids. Both MIV-150 and CG in CVL had the expected EC50 values (<2 nM for MIV-150, <100 ng/mL for CG).28 More MIV-150 and CG were detected in CVL samples collected 4 hours postdose than 24 hours postdose, corresponding to PC-1005's greater anti-HIV and anti-HPV activity at 4 hours. These findings mirror preclinical macaque data.35,36,46 Although Zn2+ concentrations also increased at 4 hours postdose (compared with baseline), it was not possible to demonstrate the impact of Zn2+ on anti-HSV-2 activity because viral infection was blocked in baseline CVLs in the assay used (data not shown). Explant studies have shown activity of PC-1005 against HIV and HSV-2.47
Safety findings from this study indicate that PC-1005 seems comparable with the extensive data available for tenofovir gel8 and early clinical data available for dapivirine (gel and IVR).48–51 Differences in the PK profiles (such as Cmax and T1/2) between tenofovir, dapivirine, and MIV-150 are likely due, in part, to the much higher concentrations of tenofovir and dapivirine loaded in the respective gels (due to the higher EC50 values, in the case of tenofovir, compared with MIV-150).8,46,48–50 Unlike dapivirine, which is only active against HIV, and tenofovir, which is active against HIV and has recently been shown to prevent HSV-2,52 PC-1005 is the only MPT that also has activity against HPV. The capacity of PC-1005 gel to block HPV is critical given that multiple strains of HPV—including low risk types that are not included in any of the currently available vaccines—have been shown to double HIV risk in certain settings.53
Data from this first-in-human trial provide the foundation for further exploration of PC-1005 gel's safety when used over longer periods of time, multiple times per day, by sexually active women, and when used rectally. Given that more than one-third of participants recommended reducing the amount of gel delivered, future studies will need to identify an acceptable volume when the gel is used as designed: on demand in the context of sexual activity. Despite overall low levels of adherence in recent microbicide trials, there are women who achieve high rates of adherence using an on-demand regimen.15
In conclusion, PC-1005 vaginal gel is safe and well tolerated. CVLs collected 4 hours after dosing had anti-HIV and anti-HPV activity in cell-based assays. These promising findings warrant the further development of PC-1005 as an MPT for prevention of HIV and other STIs.
ACKNOWLEDGMENTS
The authors wish to thank the women who volunteered to participate in the trial; the Alabama Microbicide Clinical Research Site team for study implementation; ICON Development Solutions for data management and analysis; Thierry Bonnaire, Radhika Menon, Arif Samad, and Samantha Seidor for manufacturing support; Keith Levendosky, Olga Mizenina, Larisa Kizima, and Aixa Rodriguez and the Teleshova Laboratory for evaluating clinical samples; Christine Mauck for her guidance on protocol development; Craig Hendrix for his advice on PK analysis; Polly Harrison, Salim Abdool Karim, Ian McGowan, Douglas Taylor, and Cynthia Woodsong for their thoughtful review of the manuscript; and Virginia Kallianes for assistance with manuscript preparation.
Footnotes
Supported by President's Emergency Plan for AIDS Relief through the U.S. Agency for International Development Award No. GPO-A-00-04-00019-00.
Presented in part at HIV Research for Prevention (HIV R4P), October 28–31, 2014, Cape Town, South Africa, Conference on Retroviruses and Opportunistic Infections (CROI), February 22–25, 2016, Boston, MA.
The authors have no conflicts of interest to disclose.
The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of the U.S. Agency for International Development.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). The Gap Report. Geneva, Switzerland: UNAIDS; 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf. Accessed October 18, 2015. [Google Scholar]
- 2.UNAIDS and African Union. Empower Young Women and Adolescent Girls: Fast-Track the End of the AIDS Epidemic in Africa. Geneva, Switzerland: UNAIDS; 2015. Available at: http://www.unaids.org/sites/default/files/media_asset/JC2746_en.pdf. Accessed October 18, 2015. [Google Scholar]
- 3.Bruni L, Diaz M, Castellsagué M, et al. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–1799. [DOI] [PubMed] [Google Scholar]
- 4.Looker KJ, Magaret AS, Turner KM, et al. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One. 2015;10:e114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Romero JA, Deal C, Herold BC, et al. Multipurpose prevention technologies: the future of HIV and STI protection. Trends Microbiol. 2015;23:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elias CJ, Heise LL. Challenges for the development of female-controlled vaginal microbicides. AIDS. 1994;8:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Tolley EE, Friedland BA, Gafos M, et al. Socioeconomic and behavioral factors influencing choice, adherence and success of microbicide formulations. In: Neves JD, Sarmento B, eds, Drug Delivery and Development of Anti-HIV Microbicides. Chapter 16 Singapore: Pan Stanford Publishing; 2014. [Google Scholar]
- 8.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. CAPRISA 004 Trial Group. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrazzo JM, Ramjee G, Richardson BA, et al. VOICE Study Team. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Follow-on African Consortium for Tenofovir Studies (FACTS). FACTS 001 Study. Available at: https://factsconsortium.wordpress.com/facts-001-study. Accessed October 18, 2015. [Google Scholar]
- 11.Muchomba FM, Gearing RE, Simoni JM, et al. State of the science of adherence in pre-exposure prophylaxis and microbicide trials. J Acquir Immune Defic Syndr. 2012;61:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Straten A, Van Damme L, Haberer JE, et al. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–F19. [DOI] [PubMed] [Google Scholar]
- 13.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016 Feb 22; 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Partnership for Microbicides (IPM). The Ring Study. Available at: http://www.ipmglobal.org/the-ring-study. Accessed October 15, 2015. [Google Scholar]
- 15.Friedland B, Gehret Plagianos M, Govender S, et al. Baseline predictors of high adherence to a coitally-dependent microbicide gel based on an objective marker of use: findings from the Carraguard phase 3 trial. AIDS Behav. 2015:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalichman S, Simbayi LC, Cain D, et al. Heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sex Trans Infect. 2009;85:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabir M, Iliyasu Z, Abubakar IS, et al. Sexual behavior among students in tertiary institutions in Kano, northern Nigera. J Comm Med Prim Health Care. 2004;16:17–22. [Google Scholar]
- 18.Matasha E, Ntembelea T, Payaud P, et al. Sexual and reproductive health among primary and secondary school pupils in Mwanza, Tanzania: need for intervention. AIDS Care. 1998;10:571–582. [DOI] [PubMed] [Google Scholar]
- 19.Medivir. Medivir Licenses MIV-150 to Population Council: Press release; 2003. Available at: http://globenewswire.com/news-release/2003/07/30/299151/43259/en/Medivir-licenses-MIV-150-to-Population-Council.html. Accessed October 18, 2015. [Google Scholar]
- 20.Joint FAO/WHO Expert Committee on Food Additives (JECFA). Safety Evaluation of Certain Food Additives. 1999. Available at: http://www.inchem.org/documents/jecfa/jecmono/v042je08.htm. Accessed October 18, 2015. [Google Scholar]
- 21.US Food and Drug Administration. Zicam Fact Sheet 2009. 2009. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm166927.htm#.ViVmtj9Bnzs. Accessed October 19, 2015. [Google Scholar]
- 22.Fenstermacher KJ, DeStefano JJ. Mechanism of HIV reverse transcriptase inhibition by zinc: formation of a highly stable enzyme-(primer-template) complex with profoundly diminished catalytic activity. J Biol Chem. 2011;286:40433–40442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Romero JA, Abraham CJ, Rodriguez A, et al. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob Agents Chemother. 2012;56:358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenney J, Rodríguez A, Kizima L, et al. A modified zinc acetate gel, a potential nonantiretroviral microbicide, is safe and effective against simian-human immunodeficiency virus and herpes simplex virus 2 infection in vivo. Antimicrob Agents Chemother. 2013;57:4001–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arens M, Travis S. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J Clin Microbiol. 2000;38:1758–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buck CB, Thompson CD, Roberts JN, et al. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodríguez A, Kleinbeck K, Mizenina O, et al. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antivir Res. 2014;108:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kizima L, Rodríguez A, Kenney J, et al. A potent combination microbicide that targets SHIV-RT, HSV-2 and HPV. PLoS One. 2014;9:e94547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias CJ, Coggins C, Alvarez F, et al. Colposcopic evaluation of a vaginal gel formulation of iota-carrageenan. Contraception. 1997;56:387–389. [DOI] [PubMed] [Google Scholar]
- 30.Coggins C, Blanchard K, Alvarez F, et al. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sex Transm Infect. 2000;76:480–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilmarx PH, van de Wijgert JHHM, Chaikummao S, et al. Safety and acceptability of the candidate microbicide Carraguard in Thai women: findings from a phase II clinical trial. J Acquir Immune Defic Syndr. 2006;43:327–334. [DOI] [PubMed] [Google Scholar]
- 32.van de Wijgert J, Braunstein S, Morar N, et al. Carraguard vaginal gel safety in HIV-positive women and men in South Africa. J Acquir Immune Defic Syndr. 2007;46:538–546. [DOI] [PubMed] [Google Scholar]
- 33.Kilmarx PH, Blanchard K, Chaikummao S, et al. A randomized, placebo-controlled trial to assess the safety and acceptability of use of Carraguard vaginal gel by heterosexual couples in Thailand. Sex Transm Dis. 2008;35:226–232. [DOI] [PubMed] [Google Scholar]
- 34.McLean CA, van de Wijgert JHHM, Jones HE, et al. HIV genital shedding and safety of Carraguard use by HIV-infected women: a crossover trial in Thailand. AIDS. 2010;24:717–722. [DOI] [PubMed] [Google Scholar]
- 35.Kenney J, Aravantinou M, Singer R, et al. An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques. PLoS One. 2011;6:e15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenney J, Singer R, Derby N, et al. A single dose of a MIV-150/zinc acetate gel provides 24 hours of protection against vaginal simian human immunodeficiency virus reverse transcriptase infection, with more limited protection rectally 8-24 h after gel use. AIDS Res Hum Retroviruses. 2012;28:1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnable P, Calenda G, Ouattara L, et al. A MIV-150/zinc acetate gel inhibits SHIV-RT infection in macaque vaginal explants. PLoS One. 2014;9:e108109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnable P, Calenda G, Bonnaire T, et al. MIV-150/zinc acetate gel inhibits cell-associated simian-human immunodeficiency virus reverse transcriptase infection in a macaque vaginal explant model. Antimicrob Agents Chemother. 2015;59:3829–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tien D, Schnaare RL, Kang F, et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21:845–853. [DOI] [PubMed] [Google Scholar]
- 40.Morrow K, Ruiz M. Assessing microbicide acceptability: a comprehensive and integrated approach. AIDS Behav. 2008;12:272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ugaonkar SR, Wesenberg A, Wilk J, et al. A novel intravaginal ring to prevent HIV-1, HSV-2, HPV, and unintended pregnancy. J Control Release. 2015;213:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Division of AIDS (DAIDS). Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events; and Addendum 1; Female Genital Grading Table for Use in Microbicides Studies. Available at: http://rsc.tech-res.com/Document/safetyandpharmacovigilance/DAIDS_AE_GRADING_TABLE_v2_NOV2014.pdf; http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Addendum_1_Female_Genital_Grading_Table_v1_Nov_2007.pdf. Accessed October 19, 2015. [Google Scholar]
- 43.Keller M, Mesquita PMM, Torres NM, et al. Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials. PLoS One. 2010;5:e8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu M, Keele BF, Aravantinou M, et al. Exposure to MIV-150 from a high-dose intravaginal ring results in limited emergence of drug resistance mutations in SHIV-RT infected rhesus macaques. PLoS One. 2014;9:e89300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss J, Haefeli WE. Potential of the novel antiretroviral drug rilpivirine to modulate the expression and function of drug transporters and drug-metabolising enzymes in vitro. Int J Antimicrob Agents. 2013;41:484–487. [DOI] [PubMed] [Google Scholar]
- 46.Hsu M, Aravantinou M, Menon R, et al. A combination microbicide gel protects macaques against vaginal simian human immunodeficiency virus-reverse transcriptase infection, but only partially reduces herpes simplex virus-2 infection after a single high-dose cochallenge. AIDS Res Hum Retroviruses. 2014;30:174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villegas G, Calenda G, Zhang S, et al. In vitro exposure to PC-1005 and cervicovaginal lavages from women vaginally administered PC-1005 inhibit HIV-1 and HSV-2 infection in human cervical mucosa. Antimicrob Agents Chemother. 2016 July 5; 10.1128/AAC.00392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nel A, Coplan P, Smythe SC, et al. Pharmacokinetic assessment of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS Res Hum Retroviruses. 2010;26:1181–1190. [DOI] [PubMed] [Google Scholar]
- 49.Nel A, Smythe SC, Habibi S, et al. Pharmacokinetics of 2 dapivirine vaginal microbicide gels and their safety vs. hydroxyethyl cellulose-based universal placebo gel. J Acquir Immune Defic Syndr. 2010;55:161–169. [DOI] [PubMed] [Google Scholar]
- 50.Nel A, Smythe S, Young K, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:416–423. [DOI] [PubMed] [Google Scholar]
- 51.Romano J, Variano B, Coplan P, et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses. 2009;25:483–488. [DOI] [PubMed] [Google Scholar]
- 52.Abdool Karim SS, Abdool Karim Q, Kharsany ABM, et al. Tenofovir gel for the prevention of herpes simplex virus type 2 infection. N Engl J Med. 2015;373:530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karim QA, Liebenberg L, Leask K, et al. HPV Infection Enhanced HIV Acquisition in CAPRISA 004 Trial Participants in Kwazulu-Natal, South Africa. 30th International Papillomavirus Conference & Clinical and Public Health Workshops; September 17–21, 2015; Lisbon, Portugal. Abstract HPV15-0372.