Abstract
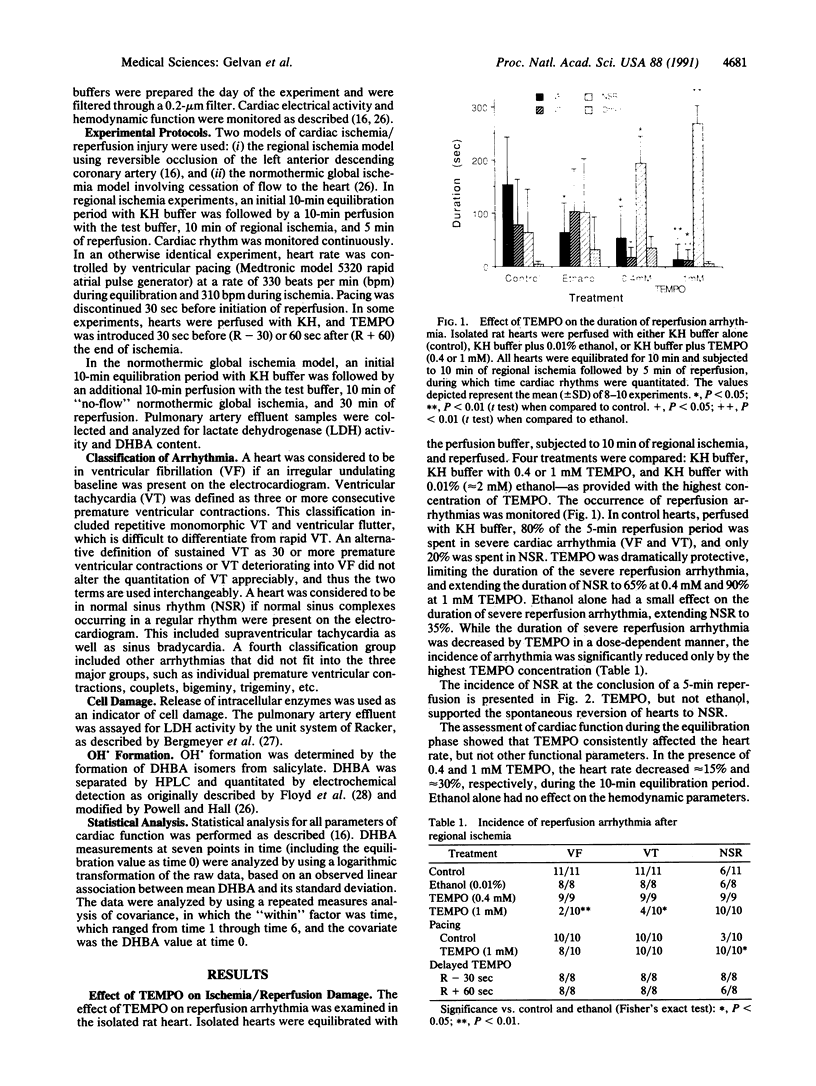
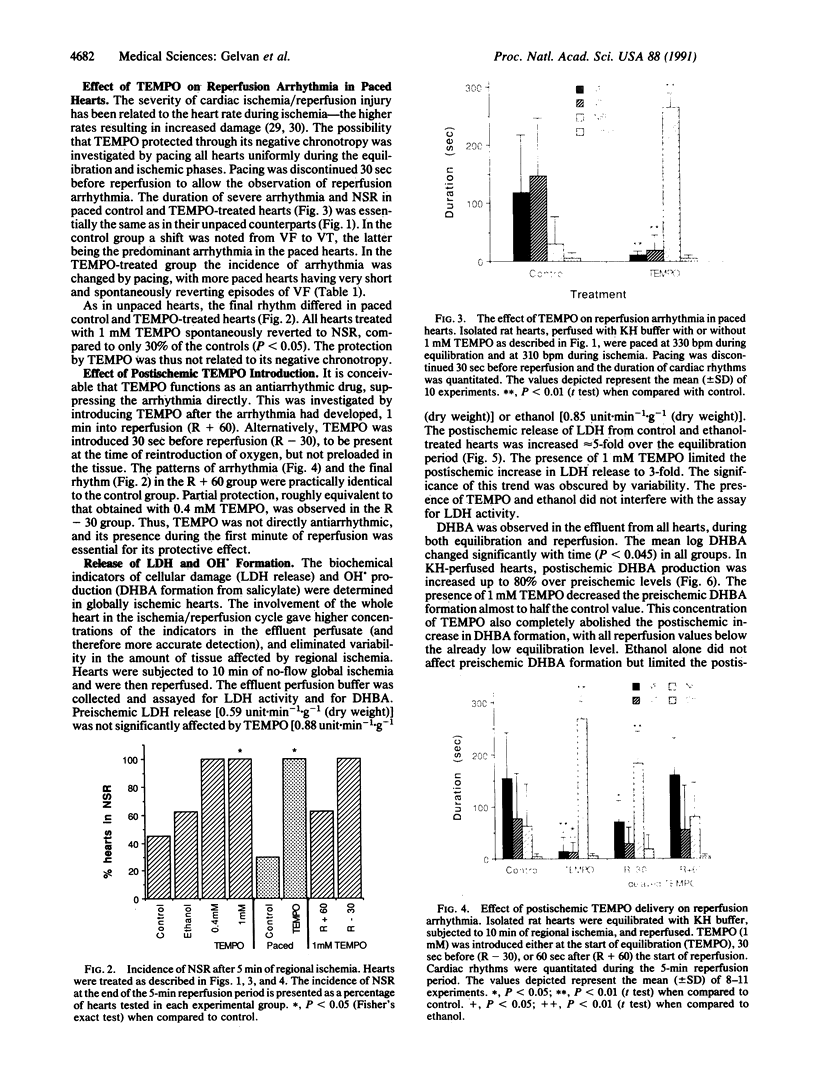
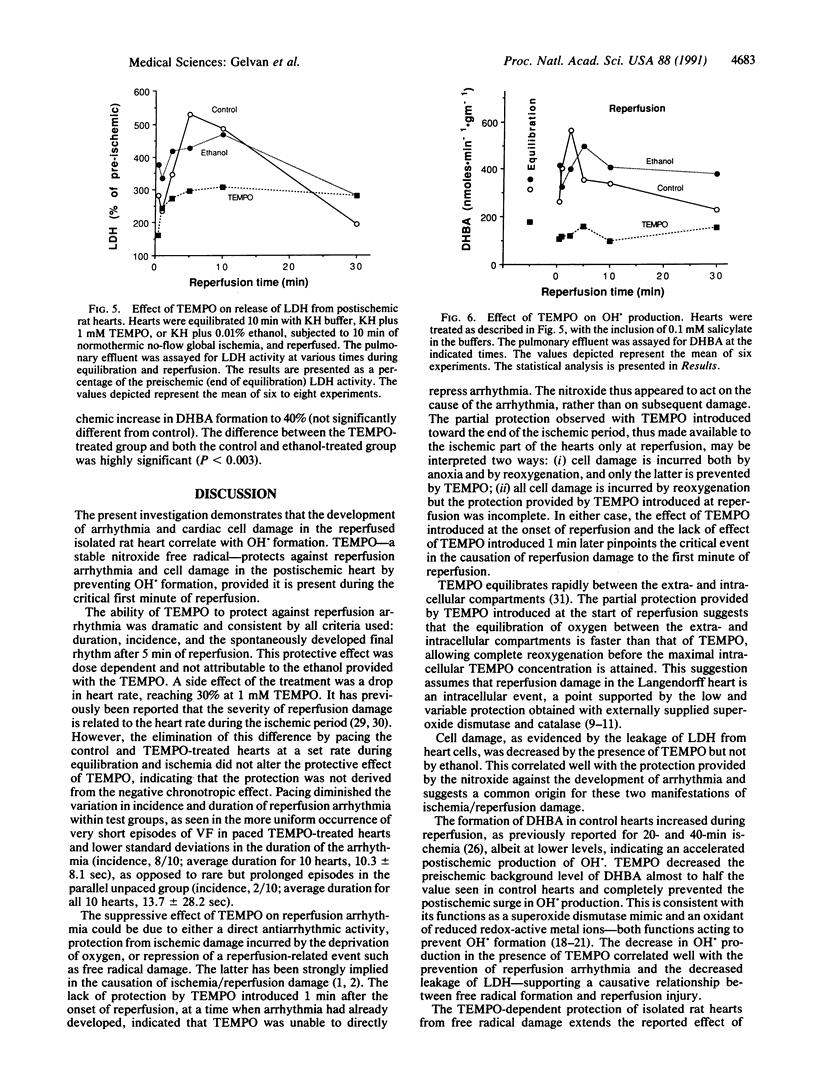
Experimental evidence is presented that directly links ischemia/reperfusion injury to the formation of oxygen-derived free radicals. 2,2,6,6-Tetramethylpiperidine-N-oxyl (TEMPO)--a stable nitroxide radical that disproportionates superoxide radicals and oxidizes reduced metal ions required for OH. formation--was tested for its ability to prevent reperfusion damage in the isolated rat heart subjected to regional ischemia. Severe reperfusion arrhythmia--ventricular fibrillation and ventricular tachycardia--were prominent in control hearts, and their duration was significantly reduced by the presence of 0.4 or 1 mM TEMPO. TEMPO also repressed both postischemic release of lactate dehydrogenase and OH. formation. TEMPO slowed the heart rate, but compensatory pacing did not alter the dramatic effect of the nitroxide on reperfusion arrhythmia. TEMPO was partially protective when introduced at the end of ischemia but had no effect when added 1 min into reperfusion. It was concluded that both reperfusion arrhythmia and cell damage were directly related to oxidative damage incurred during the critical first minute of reperfusion. TEMPO strongly protected against reperfusion injury by preventing the formation of OH. and not by decreasing heart rate or by direct suppression of arrhythmia.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosio G., Zweier J. L., Jacobus W. E., Weisfeldt M. L., Flaherty J. T. Improvement of postischemic myocardial function and metabolism induced by administration of deferoxamine at the time of reflow: the role of iron in the pathogenesis of reperfusion injury. Circulation. 1987 Oct;76(4):906–915. doi: 10.1161/01.cir.76.4.906. [DOI] [PubMed] [Google Scholar]
- Ankel E. G., Lai C. S., Hopwood L. E., Zivkovic Z. Cytotoxicity of commonly used nitroxide radical spin probes. Life Sci. 1987 Feb 2;40(5):495–498. doi: 10.1016/0024-3205(87)90116-0. [DOI] [PubMed] [Google Scholar]
- Applebaum Y. J., Kuvin J., Borman J. B., Uretzky G., Chevion M. The protective role of neocuproine against cardiac damage in isolated perfused rat hearts. Free Radic Biol Med. 1990;8(2):133–143. doi: 10.1016/0891-5849(90)90086-x. [DOI] [PubMed] [Google Scholar]
- Belkin S., Mehlhorn R. J., Hideg K., Hankovsky O., Packer L. Reduction and destruction rates of nitroxide spin probes. Arch Biochem Biophys. 1987 Jul;256(1):232–243. doi: 10.1016/0003-9861(87)90441-3. [DOI] [PubMed] [Google Scholar]
- Bernier M., Curtis M. J., Hearse D. J. Ischemia-induced and reperfusion-induced arrhythmias: importance of heart rate. Am J Physiol. 1989 Jan;256(1 Pt 2):H21–H31. doi: 10.1152/ajpheart.1989.256.1.H21. [DOI] [PubMed] [Google Scholar]
- Bernier M., Hearse D. J., Manning A. S. Reperfusion-induced arrhythmias and oxygen-derived free radicals. Studies with "anti-free radical" interventions and a free radical-generating system in the isolated perfused rat heart. Circ Res. 1986 Mar;58(3):331–340. doi: 10.1161/01.res.58.3.331. [DOI] [PubMed] [Google Scholar]
- Chevion M. A site-specific mechanism for free radical induced biological damage: the essential role of redox-active transition metals. Free Radic Biol Med. 1988;5(1):27–37. doi: 10.1016/0891-5849(88)90059-7. [DOI] [PubMed] [Google Scholar]
- Czapski G., Goldstein S., Meyerstein D. What is unique about superoxide toxicity as compared to other biological reductants? A hypothesis. Free Radic Res Commun. 1988;4(4):231–236. doi: 10.3109/10715768809055147. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K. Sensitive assay of hydroxyl free radical formation utilizing high pressure liquid chromatography with electrochemical detection of phenol and salicylate hydroxylation products. J Biochem Biophys Methods. 1984 Dec;10(3-4):221–235. doi: 10.1016/0165-022x(84)90042-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B. Iron toxicity and oxygen radicals. Baillieres Clin Haematol. 1989 Apr;2(2):195–256. doi: 10.1016/s0950-3536(89)80017-4. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Tosaki A. Free radicals and reperfusion-induced arrhythmias: protection by spin trap agent PBN in the rat heart. Circ Res. 1987 Mar;60(3):375–383. doi: 10.1161/01.res.60.3.375. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Tosaki A. Reperfusion-induced arrhythmias and free radicals: studies in the rat heart with DMPO. J Cardiovasc Pharmacol. 1987 Jun;9(6):641–650. doi: 10.1097/00005344-198706000-00002. [DOI] [PubMed] [Google Scholar]
- Hsia J. C., Boggs J. M. Pressure effect on the membrane action of a nerve-blocking spin label. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3179–3183. doi: 10.1073/pnas.70.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning A., Bernier M., Crome R., Little S., Hearse D. Reperfusion-induced arrhythmias: a study of the role of xanthine oxidase-derived free radicals in the rat heart. J Mol Cell Cardiol. 1988 Jan;20(1):35–45. doi: 10.1016/s0022-2828(88)80177-9. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Miller D. M., Buettner G. R., Aust S. D. Transition metals as catalysts of "autoxidation" reactions. Free Radic Biol Med. 1990;8(1):95–108. doi: 10.1016/0891-5849(90)90148-c. [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Samuni A., Krishna M. C., DeGraff W. G., Ahn M. S., Samuni U., Russo A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry. 1990 Mar 20;29(11):2802–2807. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- Powell S. R., Hall D. Use of salicylate as a probe for .OH formation in isolated ischemic rat hearts. Free Radic Biol Med. 1990;9(2):133–141. doi: 10.1016/0891-5849(90)90116-z. [DOI] [PubMed] [Google Scholar]
- Powell S., Saltman P., Uretzky G., Chevion M. The effect of zinc on reperfusion arrhythmias in the isolated perfused rat heart. Free Radic Biol Med. 1990;8(1):33–46. doi: 10.1016/0891-5849(90)90142-6. [DOI] [PubMed] [Google Scholar]
- Saltman P. Oxidative stress: a radical view. Semin Hematol. 1989 Oct;26(4):249–256. [PubMed] [Google Scholar]
- Samuni A., Godinger D., Aronovitch J., Russo A., Mitchell J. B. Nitroxides block DNA scission and protect cells from oxidative damage. Biochemistry. 1991 Jan 15;30(2):555–561. doi: 10.1021/bi00216a033. [DOI] [PubMed] [Google Scholar]
- Samuni A., Krishna C. M., Mitchell J. B., Collins C. R., Russo A. Superoxide reaction with nitroxides. Free Radic Res Commun. 1990;9(3-6):241–249. doi: 10.3109/10715769009145682. [DOI] [PubMed] [Google Scholar]
- Samuni A., Krishna C. M., Riesz P., Finkelstein E., Russo A. A novel metal-free low molecular weight superoxide dismutase mimic. J Biol Chem. 1988 Dec 5;263(34):17921–17924. [PubMed] [Google Scholar]
- Samuni A., Min A., Krishna C. M., Mitchell J. B., Russo A. SOD-like activity of 5-membered ring nitroxide spin labels. Adv Exp Med Biol. 1990;264:85–92. doi: 10.1007/978-1-4684-5730-8_12. [DOI] [PubMed] [Google Scholar]
- Swartz H. M., Sentjurc M., Morse P. D., 2nd Cellular metabolism of water-soluble nitroxides: effect on rate of reduction of cell/nitroxide ratio, oxygen concentrations and permeability of nitroxides. Biochim Biophys Acta. 1986 Aug 29;888(1):82–90. doi: 10.1016/0167-4889(86)90073-x. [DOI] [PubMed] [Google Scholar]
- Tosaki A., Balint S., Szekeres L. Pacing and reperfusion induced arrhythmias: protection by slow heart rate in the rat heart. Cardiovasc Res. 1988 Nov;22(11):818–825. doi: 10.1093/cvr/22.11.818. [DOI] [PubMed] [Google Scholar]
- Woodward B., Zakaria M. N. Effect of some free radical scavengers on reperfusion induced arrhythmias in the isolated rat heart. J Mol Cell Cardiol. 1985 May;17(5):485–493. doi: 10.1016/s0022-2828(85)80053-5. [DOI] [PubMed] [Google Scholar]