Abstract
Lysyl oxidase-like 2 (LOXL2) is associated with invasiveness and metastasis in cancer. We analyzed the prognostic impact of LOXL2 in pancreatic cancer patients and investigated the role of LOXL2 in pancreatic cancer cell lines. Immunohistochemical analysis was performed in samples from 80 patients and showed LOXL2 expression in 81.2% of patients with pancreatic cancer. Regarding recurrence patterns, LOXL2-positive tumors showed a significantly higher rate of distant recurrence. The 1-year and 3-year disease-free survival rates were 84.6% and 0.0%, respectively, for LOXL2-negative patients, and 27.8 % and 0.0 %, respectively, for LOXL2-positive patients. On univariate analysis, combined resection of major vessels, depth of invasion, tumor stage, and LOXL2- positive status were significant factors for poor prognosis. After identification of LOXL2 expression in pancreatic cancer cell lines, LOXL2-silenced and LOXL2-overexpressed cell lines were used to perform transwell invasion and transendothelial migration assays.
In vitro studies indicated that LOXL2 silencing in MIA PaCa-2 and PANC-1 cells induced a mesenchymal–epithelial transition (MET)-like process associated with decreased invasive and migratory properties. LOXL2 overexpression in AsPC-1 and BxPC-3 cells enhanced the epithelial-mesenchymal transition (EMT)-like process and increased migratory and invasive activity. These clinical and preclinical data confirm that higher LOXL2 expression is associated with the invasiveness of pancreatic cancer cells and the low survival rate of pancreatic cancer patients. Our results suggest the clinical value of LOXL2 as a therapeutic target in pancreatic cancer.
Keywords: pancreas cancer, metastasis, EMT, LOXL2, prognosis
INTRODUCTION
Pancreatic cancer is one of the most aggressive and lethal malignancies. Although pancreatic cancer is a relatively rare disease, it is the fourth leading cause of cancer-related mortality in Korea. Despite the availability of several treatment modalities, the 5-year survival rate is reported to be lower than 5% [1]. This poor clinical prognosis is mainly associated with early local invasion and a high incidence of recurrence, both of which are characteristic of the disease. To improve the outcomes of pancreatic cancer, the underlying metastatic mechanisms should be better understood.
LOX-like 2 (LOXL2) is a member of the lysyl oxidase (LOX) family, which contains five homologs, LOX and four LOX-like proteins (LOXL1-4), which are secreted, copper-dependent amine oxidases [2–5]. LOXL2 catalyzes the covalent cross-linking of collagen and elastin component side chains through its lysyl oxidase activity, thereby stabilizing the proteins in the extracellular matrix (ECM). Additionally, several studies have shown that LOXL2 down-regulates E-cadherin expression and promotes epithelial-mesenchymal transition (EMT) [6–8]. Due to these mechanisms, LOXL2 is associated with aggressive cancer characteristics and poor patient prognosis. Several studies have reported that LOXL2 is associated with poor prognosis in cancer patients [9–13], and that overexpression of LOXL2 promotes invasion activity among cancer cells [10–12, 14, 15]. LOXL2 is up-regulated in human pancreatic cancer, and might be correlated with the regulation of different transcription factors associated with invasion and metastasis [16]. The activity of lysyl oxidase (e.g., LOX and LOXL2) also, correlates with oncogenic stress response and tumorigenesis in pancreatic ductal adenocarcinoma [17]. However, there is a paucity of clinical evidence regarding the role of LOXL2 and its functions in pancreatic cancer.
In this study, we aimed to confirm the role of LOXL2 in the increased invasiveness of pancreatic cancer cells and to evaluate the clinical impact of LOXL2 in patients with pancreatic cancer. To accomplish this, we examined pancreatic cancer tissue for LOXL2 expression via immunohistochemical (IHC) staining and evaluated the prognostic significance of LOXL2 for pancreatic cancer patients. Moreover, we performed in vitro studies that showed an association between an aggressive cancer prognosis and LOXL2 expression.
RESULTS
Clinicopathologic characteristics
The study involved patients of a mean age of 63.3 (± 9.7) years including 39 men and 41 women. Of the 80 patients, eight underwent pancreaticoduodenectomy, 41 underwent pylorus-preserving pancreaticoduodenectomy, 26 underwent distal pancreatectomy with splenectomy and five underwent total pancreatectomy. According to the 7th AJCC classification, one patient was stage IA, four were stage IB, 22 were stage IIA, 50 were stage IIB, and three were stage III. All patients received adjuvant chemotherapy with six cycles of gemcitabine every 4 weeks. Each chemotherapy cycle consisted of three weekly infusions of gemcitabine 1000mg/m2 administered via intravenous infusion over a 30-min period, followed by a 1-week pause.
Analysis of clinicopathologic features and LOXL2 status
Among the 80 patients, 65 (81.2%) were positive for LOXL2. The clinicopathologic features and LOXL2 status of patients in the study group are summarized in Table 1. There was no statistical difference in clinicopathologic characteristics according to LOXL2 status.
Table 1. Patients characteristics based on LOXL2 status.
| LOXL2 negative (N=15) | LOXL2 positive (N=65) | p-value | |
|---|---|---|---|
| Age (mean ± SD), yr | 63.1 ± 10.9 | 63.3 ± 9.4 | 0.961 |
| Sex | 0.858 | ||
| Male | 7 | 32 | |
| Female | 8 | 33 | |
| Operative Procedure | 0.186 | ||
| PD/PPPD | 7 | 42 | |
| Distal pancreatectomy | 8 | 18 | |
| Total pancreatectomy | 0 | 5 | |
| Combine resection | 0.551 | ||
| No | 13 | 52 | |
| Yes | 2 | 13 | |
| Tumor size (mean ± SD), cm | 3.5 ± 1.6 | 3.4 ± 1.8 | 0.782 |
| Histologic grade | 0.138 | ||
| Well differentiation | 2 | 12 | |
| Moderate differentiation | 8 | 45 | |
| Poor differentiation | 5 | 8 | |
| Depth of invasion | 0.713 | ||
| T1 | 0 | 1 | |
| T2 | 2 | 5 | |
| T3 | 13 | 56 | |
| T4 | 0 | 3 | |
| Lymph node metastasis | 0.881 | ||
| Negative | 5 | 23 | |
| Positive | 10 | 42 | |
| Perineural Invasion | 0.671 | ||
| No | 4 | 21 | |
| Yes | 11 | 44 | |
| Lymphovascular invasion | 0.775 | ||
| No | 7 | 33 | |
| Yes | 8 | 32 | |
| Tumor Stage | 0.461 | ||
| IA | 0 | 1 | |
| IB | 1 | 3 | |
| IIA | 4 | 18 | |
| IIB | 10 | 40 | |
| III | 0 | 3 |
Patterns of recurrence according to LOXL2 status
Among the 80 patients, 67 (83.8%) had tumor recurrence confirmed via pathologic or radiologic examination. Regarding recurrence patterns, LOXL2-positive tumors showed a significantly higher rate of distant recurrence (Table 2).
Table 2. Patterns of recurrence according to LOXL2 status.
| LOXL2 negative | LOXL2 positive | P-value | |
|---|---|---|---|
| Recurrence patterns | 0.044 | ||
| Local recurrence | 8 | 17 | |
| Distant recurrence | 5 | 37 |
Prognostic impact of LOXL2 status
In these 80 patients, the 1- and 3- year disease-free survival (DFS) rates were 38.8 % and 0.0%, respectively. On univariate and multivariate analyses, tumor stage and LOXL2-positive status were identified as independent prognostic factors for DFS (Table 3, 4). The 1 and 3-year DFS rates were 84.6% and 0.0%, respectively, for patients with LOXL2-negative tumors and 27.8% and 0.0%, respectively, for patients with LOXL2-positive tumors (Figure 1).
Table 3. Univariate prognostic factors of disease free survival rate in pancreatic cancer.
| 1-yr DFS | 3-yr DFS | p-value | |
|---|---|---|---|
| Age (yr) | 0.956 | ||
| ≥ 60 | 40.4 | 0.0 | |
| < 60 | 35.0 | 0.0 | |
| Sex | 0.152 | ||
| Male | 51.5 | 0.0 | |
| Female | 26.5 | 0.0 | |
| Operative Procedure | 0.190 | ||
| PD/PPPD | 26.2 | 0.0 | |
| Distal pancreatectomy | 60.0 | 0.0 | |
| Total pancreatectomy | 60.0 | 0.0 | |
| Combine resection | 0.876 | ||
| No | 37.5 | 0.0 | |
| Yes | 45.5 | 0.0 | |
| Tumor size (cm) | 0.892 | ||
| ≥ 2cm | 39.3 | 0.0 | |
| < 2cm | 40.0 | 0.0 | |
| Histologic grade | 0.373 | ||
| Well differentiation | 54.5 | 0.0 | |
| mod differentiation | 31.8 | 0.0 | |
| poor differentiation | 50.0 | 0.0 | |
| Depth of invasion | 0.717 | ||
| T1/T2 | 40.0 | 0.0 | |
| T3/T4 | 38.7 | 0.0 | |
| Lymph node metastasis | 0.240 | ||
| Negative | 52.2 | 0.0 | |
| Positive | 31.8 | 0.0 | |
| Perineural Invasion | 0.318 | ||
| No | 34.8 | 0.0 | |
| Yes | 40.9 | 0.0 | |
| Lymphovascular invasion | 0.4111 | ||
| No | 33.3 | 0.0 | |
| Yes | 44.1 | 0.0 | |
| Tumor Stage | 0.033 | ||
| IA/IB | 33.3 | 0.0 | |
| IIA | 61.1 | 0.0 | |
| IIB | 32.6 | 0.0 | |
| III | 0.0 | 0.0 | |
| LOXL2 status | 0.002 | ||
| Negative | 84.6 | 0.0 | |
| Positive | 27.8 | 0.0 |
Table 4. Univariate analysis of disease free survival rate in pancreatic cancer.
| Variables | P-values | Odds ratio | Confidence interval (95%) | |
|---|---|---|---|---|
| Lower | Upper | |||
| LOXL2 positive | 0.002 | 2.810 | 1.474 | 5.357 |
| Tumor stage | 0.047 | 1.623 | 1.007 | 2.618 |
Figure 1. Disease free survival rates according to LOXL2 status.
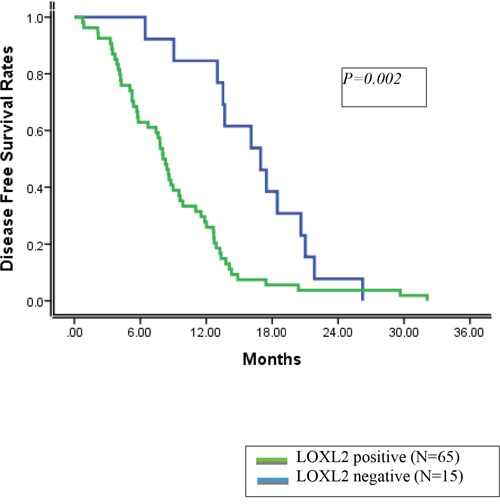
The overall 3-and 5-year survival rates were 20.0% and 6.1%, respectively. On univariate analysis, combined resection of major vessels, depth of invasion, tumor stage and LOXL2-positive status were significant factors for poor prognosis. In the multivariate analysis, combined resection of major vessels was identified as an independent prognostic factor for overall survival.
LOXL2 expression and invasiveness in pancreatic cancer cell lines
Expression of LOXL2 was analyzed in a series of pancreatic cancer cell lines: MIA PaCa-2, PANC-1, AsPC-1, and BxPC-3. LOXL2 was detected in MIA PaCa-2 and PANC-1 yet, not in AsPC-1, and BxPC-3 (Figure 2A). LOXL2 expression correlated with the expression of Snail and L1CAM and was inversely proportional to the expression of epithelial cell markers such as CDH1 (Figure 2A). An analysis of transwell invasion (Figure 2B) and transendothelial migration (Figure 2C) indicated that expressions of these genes were not correlated with the properties of migration and invasiveness of each pancreatic cancer cell, suggesting that each pancreatic cancer cell has LOXL2- independent mechanisms to control the expression of LOXL2, Snail, CDH1, and L1CAM, which is related to EMT and invasiveness.
Figure 2. LOXL2 expression and properties of migration and invasiveness were analyzed in pancreatic cancer cell lines.
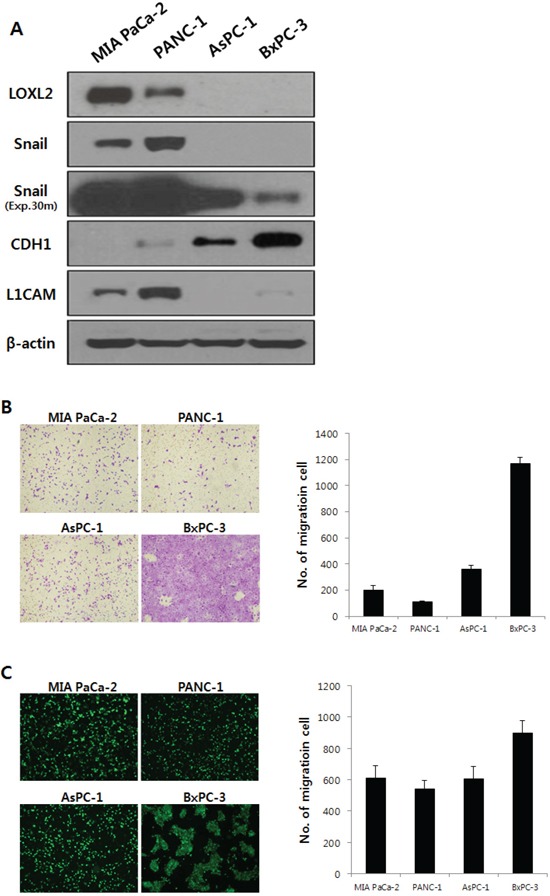
A. on Western blot analysis, LOXL2 was only detected in MIA PaCa-2 and PANC-1. Exp.30m, Snail was analyzed after 30 minutes of exposure. Each cell showed independent properties of migration and invasiveness (B. transwell invasion assay; C. transendothelial migration assay).
The effects of LOXL2 expression on invasiveness
We generated stable pancreatic carcinoma cells in which LOXL2 was silenced by siLOXL2, (MIA PaCa-2 and PANC-1) or ectopically expressed by pc3.1- LOXL2 (AsPC-1 and BxPC-3). Selected clones from MIA-PaCa-siLOXL2 and PANC1-siLOXL2 showed significantly reduced LOXL2 protein (Figure 3A and 3B) and transcript levels (Figure 3C and 3D). These cells exhibited significant changes in the expression of molecules related to invasiveness and EMT. LOXL2 silencing induced significant correlation between reduced Snail expression and increased CDH1 expression in PANC-1-siLOXL2 cells, though, not in MIA PaCa-2 cells. Considering that CDH1 protein was not detected in MIA PaCa-2 due to its transcriptional silencing (Figure 3A and 3B) and the low levels of CDH1 expression (MIA PaCa-2) and downregulation (MIA PaCa-2-siLOXL2), LOXL2 silencing and reduced Snail might not affect the re-expression of CDH1. The expressions of vimentin and N-cadherin, which are related to EMT, were not affected significantly by LOXL2, as demonstrated by slight up-regulation of these proteins on densitometric analysis of NIH images (Figure 3B). Downregulation of phospho-SRC and phospho-FAK was detected on Western blot analyses and reduced expressions of Snail and L1CAM were detected at both mRNA and protein levels (Figure 3A-3D). Contrary to a previous report by Rückert et al. [16], our experimental set for LOXL2 knockdown by siLOXL2 influenced the cell morphology, resulting in cells that were less spindle-shaped, less atypical, and smaller, though only in MIA-PaCa-2-siLOXL2; however, silencing or ectopic overexpression of LOXL2 did not affect the morphology of the other cell lines (Figure 3E). Importantly, silencing of LOXL2 resulted in a marked decrease in motility and the invasiveness capacity of MIA PaCa-2 (p < 0.001) and PANC-1 cells, as determined from transwell invasion and transendothelial migration assays, respectively (Figure 4A and 5).
Figure 3. Analysis of LOXL2 expression in pancreatic cancer cell lines with LOXL2-silencing and LOXL2-overexpression.
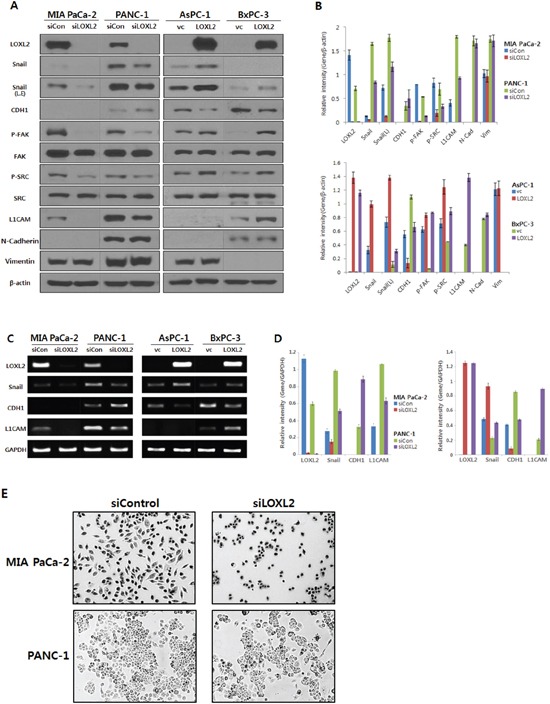
A. Western analysis and B. densitometric analysis using NIH image; LOXL2 silencing (siLOXL2) reduced LOXL2, Snail, p-FAK, p-SRC and L1CAM, and increased CDH1 in MIA PaCa-2 and PANC-1. LOXL2 overexpression (LOXL2) showed increased LOXL2, Snail, p-FAK, p-SRC and L1CAM, and reduced CDH1 in AsPC-1 and BxPC-3. C. RT-PCR analysis and D. quantitation of RT PCR; LOXL2 silencing has an effect on the reduction of LOXL2, Snail, and L1CAM, and on the increase of CDH1. LOXL2 overexpression has an effect on the increase of LOXL2, Snail, and L1CAM, and on the reduction of CDH1. E. MIA-PaCa-2-siLOXL2 cells exhibited a more epithelial phenotype than si-control. siCon, siRNA control using siRNA scramble; siLOXL2, siRNA for LOXL2; vc, vector(pc3.1) control; LOXL2, pc3.1-LOXL2; L.E, Snail was exposed for more 3 min more than other proteins.
Figure 4. Effect of LOXL2 on pancreatic cancer cell invasion based on transwell invasion assay.
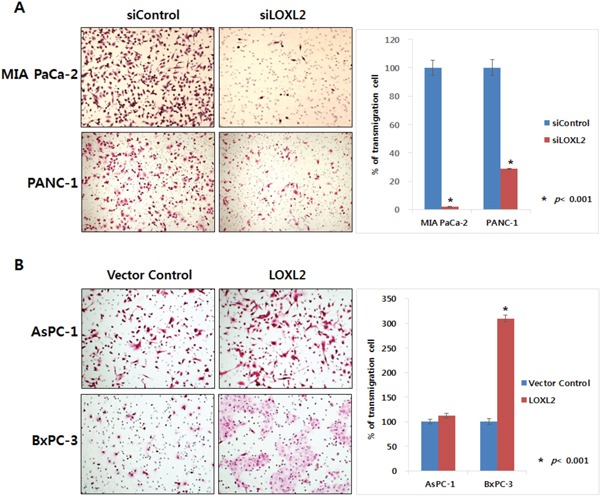
LOXL2-silencing A. and LOXL2-overexpression B. have an effect on the capacity of motility and invasiveness of pancreatic cancer cell lines.
Figure 5. Effect of LOXL2 on pancreatic cancer cell invasion based on transendothelial migration assay.
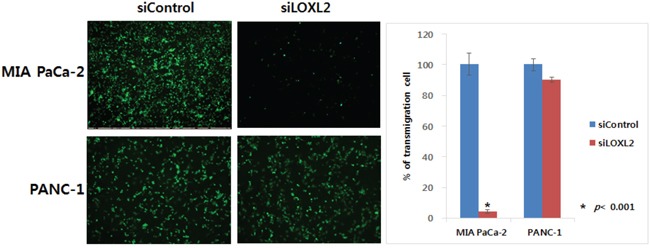
LOXL2-silencing has an effect on the capacity of motility and invasiveness of MIA PaCa-2 and PANC-1.
We also observed a significant influence of ectopic overexpression of LOXL2 in AsPC-1-LOXL2 and BxPC-3-LOXL2 cells. Decreased expression of CDH1 and increasing phospho-FAK / phospho-SRC were observed in AsPC-1-LOXL2 and BxPC-3-LOXL2 cells (Figure 3A-3D). Upregulation of L1CAM was detected only in BxPC-3-LOXL2. Upreulation of Snail expression was also detected in both AsPC1-LOXL2 and BxPC3-LOXL2. (Figure 3A-3D). Conversely, LOXL2 overe-xpression enhanced the migration potential of BxPC-3-LOXL2 (p < 0.001), but not AsPC-1-LOXL2. (Figure 4B).
DISCUSSION
LOXL2, a member of the LOX family, is responsible for the stabilization of collagen and elastin fibers in ECM through covalent oxidative deamination. Tissue fibrosis is associated with cancer progression through direct promotion of cellular transformation and metastasis [18]. Lu et al. reported that the increased collagen cross-linking in mouse mammary stroma induced by LOXL2 activity is associated with ECM stiffness and tumor invasion and progression [14]. These studies are in agreement with the notion that tumor fibrosis increases the invasive behavior of tumors by activating LOXL2 signals and suggest that LOXL2 is involved in cell adhesion, cell migration and invasion, and EMT transformation [6, 9].
Although many studies have shown that increased LOXL2 expression is associated with lymph node metastasis, lymphatic invasion, and advanced tumor stage [10, 11], IHC staining of LOXL2 was not associated with clinicopathologic variables in this study. This disparity might be explained by the unusual characteristics of pancreatic cancer, which exhibits a high expression of EMT properties even in its early stage. In fact, the ratio of LOXL2 positivity in early-stage pancreatic cancer was higher than that in breast cancer, renal cell carcinoma, and stomach cancer [10, 11, 19]. This disparity necessitates further investigation of LOXL2 in pancreatic cancer. Moreover, investigation of recurrence patterns showed that LOXL2 expression was significantly associated with distant metastasis, including the liver and lung, as described in a previous report [10]. This result suggests that LOXL2 expression might be associated with the EMT activity of pancreatic cancer. On univariate and multivariate analyses of DFS, the prognosis of LOXL2-positive patients was significantly worse than that of LOXL2-negative patients. These findings suggest that LOXL2 over-expression might be a poor prognostic factor in pancreatic cancer, based on evidence that LOXL2 expression in pancreatic cancer cells might contribute to metastasis in a clinical setting, and further suggest that inhibition of LOXL2 might provide a survival benefit to patients with pancreatic cancer.
To identify whether the clinical significance of LOXL2 expression in pancreas cancer patients is associated with metastasis and EMT properties, we conducted an in vitro study of LOXL2 in pancreatic cancer cells.
In previous studies, intracellular functions of LOXL2 have been postulated relating to the promotion of EMT and the invasiveness of cancer cells. LOXL2 induces EMT by stabilizing Snail, a suppressor of CDH1 [8, 20, 21] in carcinoma progression [6]. LOXL2 even controls CDH1 by regulating H3K4me3 deamination [22], and LOXL2-E47 EMT factor plays a role in the repression of CDH1 in early metastasis colonization of breast cancer cells [23]. Additionally, positive modulation of the FAK and SRC pathways by LOXL2 has been reported, which can contribute to cell migratory behavior [8, 11, 20, 24, 25]. More recently, Holly E. Baker et al. showed that LOXL2 activated fibroblasts via integrin-mediated FAK activation in tumor cell invasion and metastasis [26]. The expression of L1CAM, an adhesion molecule, is associated with poor prognosis and spontaneous metastasis of cancer cells to the lung [27–29]. Additionally, EMT and NF-κB activation is associated with the up-regulation of L1CAM, which enhances cell invasion and motility and tumor metastasis formation [30]. Gregg et al. suggested that hypoxia inducible factor dependent on expression of L1CAM and LOX family members (LOX, LOXL2, and LOXL4), which promotes cancer cell extravasation and metastatic niche formation in breast cancer cells [31].
We found that LOXL2 plays an integral role in the promotion of EMT and the invasiveness of pancreatic cancer cells. Our in vitro study indicated that LOXL2 activates EMT-like processes in pancreatic cell lines that are associated with invasive and migratory properties. Moreover, LOXL2 contributes positively to the activation of FAK/SRC and influences the expressions of CDH1, Snail and L1CAM, which are all related to EMT and the invasiveness of pancreatic tumor cells [12, 13, 15, 29, 32–35]. The present results support the notion that LOXL2 is involved in the maintenance of the mesenchymal phenotype in pancreatic cancer cells and are consistent with previous results in other studies [25, 36–39].
It has been reported that LOXL2 is one of the most highly and specifically upregulated genes in pancreatic cancer, compared to normal pancreatic tissues [40]. Proteomic profiling detected the gene product of LOXL2 in pancreatic cancer cells as well as in the secretions of pancreatic cancers [41, 42]. In addition, LOXL2 has been associated with pancreatic cancer pathology and tumorigenicity. For instance, inhibition of LOXL2 has been shown to result in the reduced viability of pancreatic cancer cells and their increased sensitivity to chemotherapy [16]. Moreover, in light of the promising role of LOXL2 in promoting the activation of EMT-related molecules that increase the aggressiveness of pancreatic cancer, it is noteworthy that LOXL2 was prominently expressed in the majority of pancreatic cancer tissues in our study.
Our finding has clinical importance in that provides a novel potential therapeutic target for pancreatic cancer. Based on experimental evidence, LOXL2 has previously been proposed as a therapeutic target in the treatment of pancreatic cancer [23, 43, 44]. The current targeting strategy for LOXL2 focuses on inhibiting its enzymatic activity. LOXL2 seems to be effectively targeted at the protein level either through the use of small-molecule inhibitors that may act both intracellularly and extracellularly, or through the use of antibodies [44]. Therefore, our data also support a therapeutic approach in which intracellular LOXL2 expression could be an effective target molecule for the improved survival of pancreatic cancer patients.
Although there were limitations to our study, including the retrospective study design and small sample size, our findings provide preclinical and clinical evidence that the contribution of LOXL2 enzymatic activity to metastasis in an experimental setting can be translated into poor survival outcomes in patients with pancreatic cancer.
In conclusion, results from an IHC analysis of tumors demonstrated that LOXL2 is an independent marker for metastatic disease and death in patients with pancreatic cancer. Additionally, our in vitro study demonstrated that LOXL2 expression promotes EMT and the invasiveness of pancreatic cancer cell lines, a finding compatible with the results of previous in vitro studies. These findings suggest that LOXL2 could potentially be a valuable target for the improvement of survival rates in patients with pancreatic cancer.
MATERIALS AND METHODS
Patients
Between June 2002 and December 2012, 84 patients underwent radical curative resection for pancreatic cancer at Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. Among these patients, four were excluded due to poorly preserved tissue samples, incomplete clinicopathologic data, or loss to follow-up. As a result 80 patients who had undergone curative resection were retrospectively reviewed. All patients were followed up for more than 6 months and the mean duration of follow-up was 33.2 ± 9.7 months. Patients were followed every 3 months during the first 12 months, and then every 6 months beyond the first year. This study was approved by the Institutional Review Board of Gangnam Severance Hospital, Yonsei University, Seoul, Korea (3-2014-0153).
Immunohistochemical (IHC) Staining
Serial sections (5 μm) of each block were adhered to poly-L-lysine covered slides and incubated at 62°C for 60 minutes. After the elimination of paraffin with xylene and staged ethyl alcohol dehydration, the sections were heated in a microwave containing a 10-mM citrate buffer (pH 6.0) solution for 15 minutes. The primary antibody against LOXL2 (Abcam, Cambridge, UK) was used at a 1:1,000 dilution according to the manufacturer's instructions. The procedure has been described in detail elsewhere [21]. Normal pancreas tissue present within the block and appropriate control tissues were used as positive controls.
IHC staining for LOXL2 was categorized as negative, “1+”,“2+”, or “3+” in high-power fields (200x magnification) according to the intensity of cytoplasmic staining (Figure 6). LOXL2-positive status was assigned for scores “2+”and “3+”. The interpretation of IHC was evaluated by two pathologists who had no information regarding the clinical outcomes.
Figure 6. Immunohistochemical analysis of LOXL2. LOXL2 expression was evaluated at a high-power field (x200 magnification).
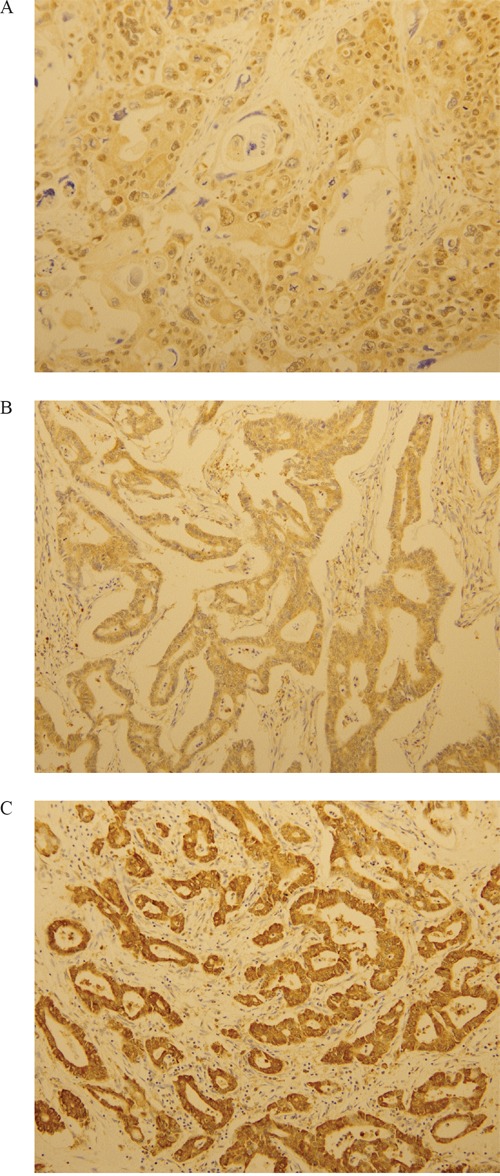
A. One positive for LOXL2. B. Two positives for LOXL2. C. Three positives for LOXL2.
Cell culture
Pancreatic cancer cell lines MIA PaCa-2, PANC-1, AsPC-1, and BxPC-3 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and grown in accordance with ATCC recommendations. MIA PaCa-2, PANC-1 and AsPC-1 were poor differentiation cell type, and BxPC3 is moderate to poor differentiation cell type [45]. MIA PaCa-2 and PANC-1 were characterized as LOXL2-positive whereas AsPC-1 and BxPC3 were characterized as LOXL2-negative pancreatic cancer cells.
Construction of LOXL2-siRNA and LOXL2 expression vector
For knockdown of LOXL2 mRNA, we used the following sequences: siLOXL2, 5′-CAGUCUAUUAUAGUCACAU-3′. As a negative control, we used siRNA targeting green fluorescence protein: 5′-GGUGUGCUGUUUGGAGGUCTT-3′. The human LOXL2 cDNA clone was provided from the Korea Human Gene Bank, Medical Genomics Research Center, KRIBB, Korea. The LOXL2 clone was inserted into the mammalian expression vector pcDNA3.1(+) (Invitrogen, San Diego, CA) by PCR using the following primers: (F: 5′- CTA GCT AGC ATG GAG AGG CCT CTG-3′ and R: 5′- CGC GGA TCC TTA CTG CGG GGA CAG -3′). The construct was verified by sequencing. Cells were transfected with LOXL2-siRNA or pc3.1-LOXL2 at 50 % confluence using the transfection reagent Oligofectamine (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions.
Reverse transcription polymerase chain reaction (RT-PCR) and quantitative RT-PCR
RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA), and cDNA was synthesized using the MMLV enzyme (Invitrogen). PCR was performed at 95°C for 10 min and in 25 cycles at 95°C for 15 s, 62°C for 30 s, and 72°C for 30 s on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). Each PCR product was analyzed in 20% agarose gel. Quantitative RT-PCR was performed on LightCycler®480 Real-Time PCR System (Roche Diagnostics, Mannheim, Germany) using SYBR Green I Matermix (Roche Diagnostics, Mannheim, Germany). Primers used for RT-PCR were LOXL2 (F: 5′-AACGAGGCGACCCTTGCAGC-3′ and R: 5′- GGGTGCGCTTGCGGTAGGTT-3′); Snail, (F: 5′-AATCGGAAGCCTAACTACAGCGAG-3′ and R: 5′-CTTTCCCACTGTCCTCATCTGACA-3′); CDH1, (F: 5′-GACGCGGACGATGATGTGAAC-3′and R: 5′-TGTACGTGGTGGGATTGAAGA-3′); L1CAM, (F: 5′-GCCACCTGTCATCACGGAAC-3′ and R: 5′-GTCCAGCGGAACTGCACTTC-3′) and GAPDH, (F: 5′-CGGGAAGCTTGTGATCAATGG-3′ and R: 5′-GGCAGTGATGGCATGGACTG-3′). The experiments were performed in triplicate and normalized to GAPDH.
Western blot analysis
Cell lysates were prepared in RIPA buffer (Sigma, St Louis, MO, USA) supplemented with protease inhibitors. Protein samples were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked and probed with primary antibody against LOXL2 (Origene; Rockville, MD, USA), Snail (Cell Signaling; Danvers, MA, USA), pFAK and FAK (Santa Cruz, CA, USA), pSRC (Millipore, Billerica, MA, USA), SRC (Santa Cruz, CA, USA), CDH1 (BD Biosciences; Sparks, MD), L1CAM (Novus Biologicals, Littleton, CO, USA), N-cadherin (Invitrogen, CA, USA), Vimentin (Dako, Glostrup, Denmark) and β-actin (Sigma, St Louis, MO, USA). The intensity of the western blotss was quantified by densitometric scanning with ImageJ (NIH) and normalized to β-actin.
Tranwell invasion assay
The invasive potential of pancreatic cancer cells was assessed in vitro in matrigel-coated invasion chambers (Corning; NY, USA) in accordance with the manufacturer's instructions. Briefly, cells in the log growth phase were serum starved for 24 h prior to seeding, detached by brief trypsinization and resuspended in medium containing the appropriate treatment. The matrigel invasion inserts were rehydrated and prepared as described in the manufacturer's instructions. Cells in serum-free medium at a density of 1 × 105 cells/ml/well were placed on the top of matrigel-coated polycarbonate filters (8 μm pore size) suspended in a membrane invasion culture system chamber; the chamber underneath the membrane contained complete medium. The cells were incubated in a CO2 incubator at 37°C for 5 h, after which the non-invasive cells were removed from the upper surface of the membrane and the invasive cells on the undersurface of the membrane were fixed and stained with hematoxylin–eosin (H&E; Sigma-Aldrich). These experiments were performed in triplicate at least three times. The invading cells were counted under a fluorescence microscope in five random high-power fields.
Transendothelial migration assay
HUVEs and EGMTM-2 media (Lonza, Walkersville, MD, USA) was purchased. HUVECs (3×104 in 200 μl of EGM-2 medium) were seeded onto 0.1% gelatin-coated inserts in the transwell chamber (8-μm pore size and 6.5-mm diameter) and allowed to form a monolayer for 48 h. The endothelial culture medium was removed from each insert and CFSE-labeled cancer cells (1×105 cells in 200μl of serum-free medium) were added on top of the HUVEC monolayer. Cancer cell culture medium containing 10% FBS was added to the lower chamber. After incubation for 48 or 72 h, non-migratory cells were wiped off with cotton swabs and the membrane was washed with PBS. Finally, the membranes were removed from the transwell insert, mounted onto slides and observed under a fluorescence microscope at ×10 magnification.
Statistical analysis
All clinicopathologic variables except age and tumor size were used as categorical variables. Differences in continuous variables between the two groups were evaluated using Student's t-test, and differences in categorical variables were evaluated by the chi-square test. The Kaplan-Meier method was used to calculate and display survival curves, and the log-rank test was performed to determine differences among all groups. Overall survival was defined as the time interval between the date of surgery and the date of death from cancer or the last follow-up. Recurrence-free survival was defined as the time interval between the date of surgery and the date of recurrence or last follow-up. The Cox proportional hazards regression method was used to determine independent prognostic factors. A P-value of less than 0.05 was considered to be statistically significant.
Footnotes
CONFLICTS OF INTEREST
All authors declare that we have no conflicts of interest in connection with this paper.
GRANT SUPPORT
This study was supported by a faculty research grant of Yonsei University College of Medicine (6-2014-0104) and a research grant from the National Cancer Center (1410311).
REFERENCES
- 1.Ministry of Health and Welfare Republic of Korea 2012 annual report of the Korea Central Cancer Registry. 2013 [Google Scholar]
- 2.Asuncion L, Fogelgren B, Fong KS, Fong SF, Kim Y, Csiszar K. A novel human lysyl oxidase-like gene (LOXL4) on chromosome 10q24 has an altered scavenger receptor cysteine rich domain. Matrix Biol. 2001;20:487–49. doi: 10.1016/s0945-053x(01)00161-5. [DOI] [PubMed] [Google Scholar]
- 3.Jourdan-Le Saux C, Tomsche A, Ujfalusi A, Jia L, Csiszar K. Central nervous system uterus heart and leukocyte expression of the LOXL3 gene encoding a novel lysyl oxidase- like protein. Genomics. 2001;74:211–218. doi: 10.1006/geno.2001.6545. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y, Boyd CD, Csiszar K. A new gene with sequence and structural similarity to the gene encoding human lysyl oxi- dase. J Biol Chem. 1995;270:7176–7182. doi: 10.1074/jbc.270.13.7176. [DOI] [PubMed] [Google Scholar]
- 5.Saito H, Papaconstantinou J, Sato H, Goldstein S. Regulation of a novel gene encoding a lysyl oxidase-related protein in cellular adhesion and senescence. J Biol Chem. 1997;272:8157–8160. doi: 10.1074/jbc.272.13.8157. [DOI] [PubMed] [Google Scholar]
- 6.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csuszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in Snail regulation and tumor progression. The EMBO Journal. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schietke R, Warnecke C, Wacker I, Schodel J, Mole DR, Campean V, Amann K, Goppelt-Struebe M, Behrens J, Eckardt KU, Wiesener MS. The Lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia: insights into cellular transformation processes mediated by HIF-1. J Biol Chem. 2010;26:6658–6669. doi: 10.1074/jbc.M109.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano A, Santamaría PG, Moreno-Bueno G. LOXL2 in epithelial cell plasticity and tumor progression. Future Oncol. 2012;8:1095–1108. doi: 10.2217/fon.12.105. [DOI] [PubMed] [Google Scholar]
- 9.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasahima H, Yashiro M, Kinoshita H, Fukuoka T, Morisaji T, Masuda G, Sakurai K, Kubo N, Ohira M, Hirakawa K. Lysyl oxidase-like 2(LOXL2) from stromal fibroblasts stimulates the progression of gastric cancer. Cancer Letters. 2014;354:438–446. doi: 10.1016/j.canlet.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Hase H, Jingushi K, Ueda Y, Kitae K, Egawa H, Ohshio I, Kawakami R, Kashiwagi Y, Tsukada Y, Kobayashi T, Nakata W, Fujita K, Uemura M, et al. LOXL2 status correlates with tumor stage and regulates intergrin levels to promote tumor progression in ccRCC. Mol Cancer Res. 2014;12:1807–1817. doi: 10.1158/1541-7786.MCR-14-0233. [DOI] [PubMed] [Google Scholar]
- 12.Galván JA, Zlobec I, Wartenberg M, Lugli A, Gloor B, Perren A, Karamitopoulou E. Expression of E-cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour-budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Br J Cancer. 2015;112:1944–1950. doi: 10.1038/bjc.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Ben Q, An W, Fei J, Xu M, Li G, Li Z, Yuan Y. Downregulation of L1CAM inhibits proliferation invasion and arrests cell cycle progression in pancreatic cancer cells in vitro. Exp Ther Med. 2014;7:785–790. doi: 10.3892/etm.2014.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, Messer M, von Werder A, Schmidt A, Mages J, Pagel P, Schnieke A, Schmid RM, Schneider G, et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology. 2009;137:361–371. doi: 10.1053/j.gastro.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Rückert F, Joensson P, Saeger HD, Grützmann R, Pilarsky C. Functional analysis of LOXL2 in pancreatic carcinoma. Int J Colorectal Dis. 2010;25:303–311. doi: 10.1007/s00384-009-0853-5. [DOI] [PubMed] [Google Scholar]
- 17.Wiel C, Augert A, Vincent DF, Gitenay D, Vindrieux D, Le Calvé B, Arfi V, Lallet-Daher H, Reynaud C, Treilleux I, Bartholin L, Lelievre E, Bernard D. Lysyl oxidase activity regulates oncogenic stress response and tumorigenesis. Cell Death Dis. 2013;4:e855. doi: 10.1038/cddis.2013.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn SG, Dong SM, Oshima A, Kim WH, Lee HM, Lee SA, Kwon SH, Lee JH, Lee JM, Jeong J, Lee HD, Green JE. LOXL2 expression is associated with invasiveness and negatively influences survival in breast cancer patients. Breast Cancer Res Treat. 2013;141:89–99. doi: 10.1007/s10549-013-2662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peinado H, Portillo F, Cano A. Switching on-off Snail: LOXL2 versus GSK3beta. Cell Cycle. 2005;4:1749–1752. doi: 10.4161/cc.4.12.2224. [DOI] [PubMed] [Google Scholar]
- 21.Cuevas EP, Moreno-Bueno G, Canesin G, Santos V, Portillo F, Cano A. LOXL2 catalytically inactive mutants mediate epithelial-to-mesenchymal transition. Biol Open. 2014;3:129–137. doi: 10.1242/bio.20146841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herranz N, Dave N, Millanes-Romero A, Morey L, Díaz VM, Lórenz-Fonfría V, Gutierrez-Gallego R, Jerónimo C, Di Croce L, García de Herreros A, Peiró S. Lysyl oxidase-like 2 deaminates lysine 4 in histone H3. Mol Cell. 2012;46:369–376. doi: 10.1016/j.molcel.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Canesin G, Cuevas EP, Santos V, López-Menéndez C, Moreno-Bueno G, Huang Y, Csiszar K, Portillo F, Peinado H, Lyden D, Cano A. Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: novel partners in E-cadherin repression and early metastasis colonization. Oncogene. 2015;34:951–964. doi: 10.1038/onc.2014.23. [DOI] [PubMed] [Google Scholar]
- 24.Peng L, Ran YL, Hu H, Yu L, Liu Q, Zhou Z, Sun YM, Sun LC, Pan J, Sun LX, Zhao P, Yang ZH. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30:1660–1669. doi: 10.1093/carcin/bgp178. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Bueno G, Salvador F, Martin A, Floristan A, Cuevas EP, Santos V, Montes A, Morales S, Castilla MA, Rojo-Sebastian A, Martinez A, Hardisson D, Csiszar K, et al. Lysyl oxidase-like 2 (LOXL2) a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med. 2011;3:528–544. doi: 10.1002/emmm.201100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker HE, Bird D, Lang G, Erler JT. Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol Cancer Res. 2013;11:1425–1436. doi: 10.1158/1541-7786.MCR-13-0033-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Wong CC, Wei H, Gilkes DM, Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B, Winnard PT, Jr, Raman V, Zhen L, Mitzner WA, Sukumar S, Semenza GL. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Weinspach D, Seubert B, Schaten S, Honert K, Sebens S, Altevogt P, Krüger A. Role of L1 cell adhesion molecule (L1CAM) in the metastatic cascade: promotion of dissemination colonization and metastatic growth. Clin Exp Metastasis. 2014;31:87–100. doi: 10.1007/s10585-013-9613-6. [DOI] [PubMed] [Google Scholar]
- 29.Tsutsumi S, Morohashi S, Kudo Y, Akasaka H, Ogasawara H, Ono M, Takasugi K, Ishido K, Hakamada K, Kijima H. L1 Cell adhesion molecule (L1CAM) expression at the cancer invasive front is a novel prognostic marker of pancreatic ductal adenocarcinoma. J Surg Oncol. 2011;103:669–673. doi: 10.1002/jso.21880. [DOI] [PubMed] [Google Scholar]
- 30.Kiefel H, Bondong S, Pfeifer M, Schirmer U, Erbe-Hoffmann N, Schäfer H, Sebens S, Altevogt P. EMT-associated up-regulation of L1CAM provides insights into L1CAM-mediated integrin signalling and NF-κB activation. Carcinogenesis. 2012;33:1919–1929. doi: 10.1093/carcin/bgs220. [DOI] [PubMed] [Google Scholar]
- 31.Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18:534–543. doi: 10.1016/j.molmed.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ischenko I, Guba M, Yezhelyev M, Papyan A, Schmid G, Green T, Fennell M, Jauch KW, Bruns CJ. Effect of Src kinase inhibition on metastasis and tumor angiogenesis in human pancreatic cancer. Angiogenesis. 2007;10:167–182. doi: 10.1007/s10456-007-9071-3. [DOI] [PubMed] [Google Scholar]
- 33.Je DW, O YM, Ji YG, Cho Y, Lee DH. The inhibition of SRC family kinase suppresses pancreatic cancer cell proliferation migration and invasion. Pancreas. 2014;43:768–776. doi: 10.1097/MPA.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 34.Nishioka R, Itoh S, Gui T, Gai Z, Oikawa K, Kawai M, Tani M, Yamaue H, Muragaki Y. SNAIL induces epithelial-to-mesenchymal transition in a human pancreatic cancer cell line (BxPC3) and promotes distant metastasis and invasiveness in vivo. Exp Mol Pathol. 2010;89:149–157. doi: 10.1016/j.yexmp.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail slug and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 36.Brekhman V, Neufeld G. A novel asymmetric 3D in-vitro assay for the study of tumor cell invasion. BMC Cancer. 2009;9:415. doi: 10.1186/1471-2407-9-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brekhman V, Lugassie J, Zaffryar-Eilot S, Sabo E, Kessler O, Smith V, Golding H, Neufeld G. Receptor activity modifying protein-3 mediates the protumorigenic activity of lysyl oxidase-like protein-2. FASEB J. 2011;25:55–65. doi: 10.1096/fj.10-162677. [DOI] [PubMed] [Google Scholar]
- 38.Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, Neufeld G. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666. [PubMed] [Google Scholar]
- 39.Hollosi P, Yakushiji JK, Fong KS, Csiszar K, Fong SF. Lysyl oxidase-like 2 promotes migration in noninvasive breast cancer cells but not in normal breast epithelial cells. Int J Cancer. 2009;125:318–327. doi: 10.1002/ijc.24308. [DOI] [PubMed] [Google Scholar]
- 40.Grutzmann R, Foerder M, Alldinger I, Staub E, Brummendorf T, Ropcke S, Li X, Kristiansen G, Jesnowski R, Sipos B, Lohr M, Luttges J, Ockert D, et al. Gene expression profiles of microdissected pancreatic ductal adenocarcinoma. Virchows Arch. 2003;443:508–517. doi: 10.1007/s00428-003-0884-1. [DOI] [PubMed] [Google Scholar]
- 41.Makawita S, Smith C, Batruch I, Zheng Y, Rückert F, Grützmann R, Pilarsky C, Gallinger S, Diamandis EP. Integrated proteomic profiling of cell line conditioned media and pancreatic juice for the identification of pancreatic cancer biomarkers. Mol Cell Proteomics. 2011;10:M111. doi: 10.1074/mcp.M111.008599. 008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gronborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, Molina H, Jensen ON, Hruban RH, Goggins MG, Maitra A, Pandey A. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5:157–171. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, Evans HR, Gartland A, Erler JT. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71:1561–1572. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer. 2012;2:540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 45.Deer EL, González-Hernández J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39:425–435. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]


