ABSTRACT
Ivermectin is a broad-spectrum antiparasitic drug that has recently been demonstrated to exhibit potent anticancer activity against colon cancer, ovarian cancer, melanoma and leukemia. However, the molecular mechanism underlying this anticancer effect remains poorly understood. We recently found that ivermectin markedly inhibits the growth of breast cancer cells by stimulating cytostatic macroautophagy/autophagy in vitro and in vivo. Ivermectin inhibits the AKT-MTOR signaling pathway by promoting ubiquitination-mediated degradation of PAK1 (p21 [RAC1] activated kinase 1), leading to increased autophagic flux. Together, our work unravels the molecular mechanism underpinning ivermectin-induced cytostatic autophagy in breast cancer, and characterizes ivermectin as a potential therapeutic option for breast cancer treatment.
KEYWORDS: AKT, breast cancer, cytostatic autophagy, ivermectin, PAK1
Avermectin is a potent broad-spectrum parasiticidal drug, the discovery of which led to the award of the 2015 Nobel Prize in physiology or medicine. Its derivative, ivermectin, has recently been reported to be a potential anticancer agent against colon cancer, ovarian cancer, melanoma, and leukemia. Our recent study demonstrates that ivermectin also exerts a promising anticancer effect for breast cancer as evidenced by marked growth inhibition after 24-h treatment with ivermectin in a range of breast cancer cell lines, with no obvious effects on nontumorigenic human breast cells. The anticancer activity of ivermectin was further confirmed in both mouse subcutaneous xenografts and orthotopic breast cancer models.
To determine the mechanism involved in ivermectin-induced growth inhibition of breast cancer cells, we examined whether ivermectin promotes apoptosis, which is a major form of cell death caused by chemotherapeutic agents. Interestingly, no significant apoptosis could be observed with the prolonged treatment of ivermectin until 48 h. Since emerging evidence demonstrates the important role of autophagy in response to drug treatment in cancer therapy, we presumed that ivermectin may inhibit the growth of breast cancer cells by inducing autophagy. Indeed, our unequivocal evidence indicates that ivermectin promotes autophagic flux in various breast cancer cell lines and in mouse xenografts, whereas autophagy cannot be significantly stimulated in ivermectin-treated nontumorigenic human breast cells. Moreover, inhibition of autophagy (either by autophagy inhibitors or siRNA interference) restores ivermectin-induced cell growth inhibition. Thus, our data indicate cytostatic autophagy contributes to the anticancer activity of ivermectin in breast cancer cells.
Four different functional forms of autophagy, which are context specific, have been characterized in anticancer treatment, including cytoprotective, cytotoxic, cytostatic and nonprotective autophagy. Cytoprotective autophagy acts as a drug-resistant mechanism that endows cancer cells with the ability to compromise anticancer effects in chemotherapy. Cytotoxic autophagy results in cell death alone or by apoptosis induction, whereas cytostatic autophagy represses cancer cell growth independent of apoptosis. Since current clinical efforts focus on perturbing cytoprotective autophagy to enhance drug sensitivity, it is of great importance to interrogate the bona fide role of drug-induced autophagy to enable more rational manipulation of autophagy for cancer treatment. Our study indicates that ivermectin mediates growth inhibition of breast cancer cells by inducing cytostatic autophagy, suggesting that drug-induced cytostatic autophagy may represent a feasible strategy for the treatment of apoptosis-deficient tumors. However, the complicated intrinsic link between autophagy and apoptosis requires further investigation.
We then dissected the molecular mechanisms underpinning ivermectin-induced autophagy in breast cancer. Ivermectin inhibits the expression of PAK1 (p21 [RAC1] activated kinase 1) in breast cancer cells, resulting in the inactivation of the AKT-MTOR signaling pathway, a key negative modulator of autophagy induction. Notably, ivermectin has no obvious effect on PAK1 and phosphorylated AKT levels in nontumorigenic human breast cells. PAK1 has been reported as an AKT-binding protein that facilitates AKT phosphorylation and activation. We found that ivermectin disrupts the interaction of PAK1 with AKT, leading to AKT inactivation. It has been reported that ivermectin inactivates PAK1 in ovarian cancer cells, but the mechanisms involved remain unknown. Our findings demonstrate that ivermectin inhibits PAK1 expression by ubiquitination-mediated degradation in breast cancer cells. Further studies will be required to clarify the molecular mechanisms underlying PAK1 ubiquitination induced by ivermectin.
PAK1 is abnormally expressed in various types of cancer, including breast, pancreatic, ovarian, colon and prostate cancer, and is involved in cancer cell growth and development of drug resistance. Thus, targeting PAK1 offers excellent potential for the treatment of PAK1-overexpressing tumors. Because ivermectin is capable of targeting PAK1 degradation, the use of ivermectin in traditional anticancer treatment may be a promising strategy to tackle PAK1-mediated drug resistance. The anticancer effect of ivermectin in combination with conventional chemotherapeutic drugs merits further investigation.
In conclusion, our study has demonstrated that ivermectin is a promising anticancer drug against breast cancer. Ivermectin induces cytostatic autophagy through blocking the PAK1-AKT-MTOR axis, leading to the growth inhibition of breast cancer cells (Fig. 1). Our data provide a molecular basis for the anticancer effect of ivermectin in breast cancer cells, suggesting that the use of ivermectin as a cytostatic autophagy inducer may constitute a novel therapeutic option for breast cancer treatment.
Figure 1.
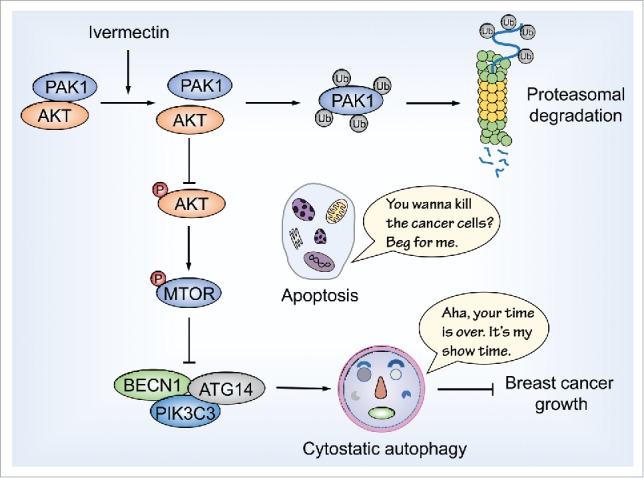
Ivermectin induces cytostatic autophagy by blocking the PAK1-AKT axis in breast cancer cells. Ivermectin treatment promotes ubiquitination-mediated degradation of PAK1, leading to inactivation of the AKT-MTOR signaling pathway. The inhibition of AKT-MTOR signaling subsequently induces the formation of the BECN1-containing PtdIns3 K complex and ultimately results in elevated autophagic flux, which represses the growth of breast cancer cells independent of apoptosis. Ub, ubiquitin.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the National 973 Basic Research Program of China (2013CB911300), the Chinese NSFC (81225015 and 81430071), and Sichuan Science-Technology Innovative Research Team for Young Scientist (2013TD0001).


