ABSTRACT
Cysteine cathepsins are responsible for driving proteolytic degradation within the lysosome and in the extralysosomal milieu. They also have an integral role in autophagy, antigen presentation, cellular stress signaling, metabolism and lysosome-dependent cell death. Here, we discuss our findings on the role of CTSB (cathepsin B), a member of the cysteine cathepsin family, in regulating the bioavailability of lysosomes and autophagosomes and consider how this regulatory response influences host susceptibility to infectious agents. Our study demonstrates that under homeostatic conditions CTSB cleaves the calcium channel MCOLN1/TRPML1 in the lysosomes, maintaining suppression of the transcription factor TFEB and reducing expression of lysosomal and autophagy-related proteins. This response controls the number of lysosomes and autophagosomes in the cell. However, the activity of CTSB is exploited by the cytosolic bacterium Francisella novicida, leading to enhanced survival of the pathogen and increased susceptibility of the host to infection.
KEYWORDS: autophagosomes, autophagy, bacteria, cathepsins, hydrolases, infection, lysosomes, MTOR, phagosomes, TFEB
Eleven members of the cysteine cathepsin family are found in humans (CTSB, C, F, H, K, L, O, S, V, W, and Z); many of these are endopeptidases, which can cleave peptide bonds of their substrates. We found via a genome-wide expression analysis that mouse bone marrow-derived macrophages (BMDMs) lacking CTSB express increased levels of the gene encoding TFEB, a central transcription factor controlling expression of lysosomal and autophagy-related genes. Consistent with this observation, we found increased expression of genes encoding molecules within the Coordinated Lysosomal Expression and Regulation/CLEAR network and those encoding lysosomal and autophagy-related proteins in CTSB-deficient BMDMs. In addition, we observed an increased propensity of TFEB to translocate into the nucleus in CTSB-deficient BMDMs compared with wild-type BMDMs. These responses are specific to CTSB because BMDMs lacking other proteinases, including CTSG, ELANE/elastase, or PRTN3/neutrophil proteinase 3 have normal levels of expression of lysosomal and autophagy-related genes.
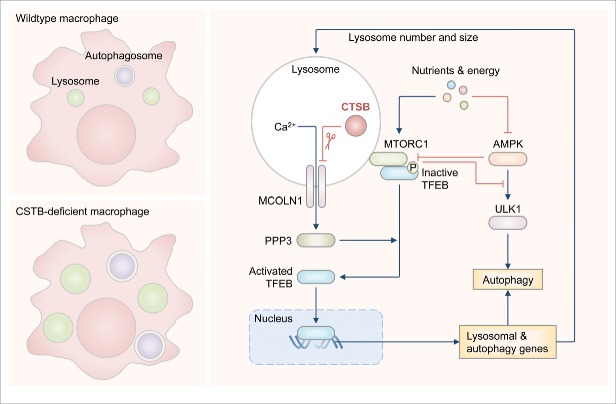
We then used transmission electron microscopy techniques to visualize the biological consequence of CTSB deficiency in BMDMs. The lack of CTSB results in an increase in the number and size of single-membrane lysosomes and double-membrane autophagosomes. These findings are further supported by confocal imaging and immunoblotting data showing an increased quantity of lysosomal and autophagy-related markers in CTSB-deficient BMDMs. These results suggest that CTSB might have a role in suppressing lysosomal biogenesis and autophagy under homeostatic conditions (Fig. 1).
Figure 1.
Cathepsin B controls lysosome and autophagosome populations. Top left panel: lysosome and autophagosome populations are kept in check in wild-type macrophages. Bottom left panel: the number and size of lysosomes and autophagosomes are expanded in CTSB-deficient macrophages. Right panel: under homeostatic conditions, CTSB directly cleaves the lysosomal calcium channel MCOLN1/TRPML1, and negatively regulates efflux of calcium and activation of the serine/threonine phosphatase PPP3/calcineurin. Inhibition of PPP3/calcineurin prevents its ability to dephosphorylate and activate the transcription factor TFEB. The phosphorylated form of TFEB is inactive and unable to induce transcription of lysosomal and autophagy-related genes. The kinase MTORC1 can phosphorylate TFEB to maintain its inactivity. Activated AMPK can inactivate MTORC1, unleashing TFEB and initiating autophagy. Overall, CTSB provides an apical signal controlling the expression of lysosomal and autophagy-related proteins as a way of maintaining the population and size of lysosomes and autophagosomes within a cell.
A previous study has found that pharmacological inhibition of CTSB impairs cleavage of the calcium channel MCOLN1/TRPML1. We proposed that CTSB cleaves MCOLN1 in the lysosome, which would result in a reduction of calcium efflux from the lysosomal lumen to the cytoplasm. Indeed, we found that cleavage of MCOLN1 is abolished in the lysosomes of CTSB-deficient BMDMs. Furthermore, we detected a relative increase in lysosomal calcium efflux in CTSB-deficient BMDMs stimulated with a MCOLN1 agonist compared with stimulated wild-type BMDMs. Enhanced calcium release into the cytoplasm in CTSB-deficient BMDMs can activate the serine/threonine phosphatase PPP3/calcineurin, allowing PPP3/calcineurin to dephosphorylate and activate TFEB (Fig. 1).
Inactivity of TFEB is maintained via its phosphorylation by the kinase MTORC1. Our data show that the activity of MTOR is reduced in the absence of CTSB, resulting in overt activation of TFEB. Because the kinase AMPK can inactivate MTORC1, we investigated whether CTSB deficiency directly leads to enhanced activation of AMPK. To address this question, we analyzed the kinetics of the activity of MTOR, TFEB and AMPK in wild-type BMDMs treated with a CTSB inhibitor. We found that inhibition of CTSB activity leads to rapid deregulation of MTOR and TFEB activity within 30 min, followed by increased activity of AMPK at 2 h. These data suggest that deregulation of AMPK is only secondary to inhibition of MTOR or activation of TFEB when the activity of CTSB is blocked.
The ability of CTSB to reduce the bioavailability of lysosomes in a cell might have a physiological consequence in certain disease pathogenesis. CTSE and CTSL provide immunity to Porphyromonas gingivalis and Mycoplasma pulmonis infection in mice, respectively. Given that the role of CTSB in the host defense against pathogens is unclear, we infected wild-type and CTSB-deficient mice with the cytosolic bacterium Francisella novicida or the vacuole-dwelling bacterium Salmonella enterica serovar Typhimurium and monitored the susceptibility of these mice to infection. We found that mice lacking CTSB are more resistant to infection by F. novicida, but not to infection by S. enterica serovar Typhimurium, compared with wild-type mice. The liver tissues of uninfected or F. novicida-infected CTSB-deficient mice express higher levels of lysosomal and autophagy-related proteins, but not the bacteriostatic factor LCN2, compared with the corresponding wild-type control mice. CTSB-deficient BMDMs are also more capable of controlling intracellular bacterial growth of F. novicida compared with infected wild-type BMDMs, a finding recapitulated in wild-type BMDMs treated with a CTSB inhibitor. Our observation of increased clearance of F. novicida in CTSB-deficient BMDMs is further supported by confocal imaging data showing F. novicida being sequestered within autophagosomes for proteolytic degradation. Because F. novicida bacteria are found both free in the cytoplasm and within vacuolar compartments, depending on the stage of the infection, it is probable that both populations are targeted and killed rapidly. Therefore, the activity of CTSB is exploited by F. novicida, resulting in permissiveness to bacterial replication and increasing host susceptibility to infection.
From an evolutionary perspective, the requirement for CTSB in negatively regulating lysosomal biogenesis and autophagy leading to increased susceptibility to a lethal infection by F. novicida might seem counterintuitive. However, we hypothesize that although the activity of CTSB is exploited by F. novicida, this cysteine protease might be important in controlling other infectious agents. The ability of CTSB to keep the biomass of lysosomes in check under homeostasis might thus provide a biological advantage overall.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Work from our laboratory is supported by the US National Institutes of Health (AI101935, AI124346, AR056296 and CA163507 to T.D.K.), the American Lebanese Syrian Associated Charities (to T.D.K.), and the R.G. Menzies Early Career Fellowship from the National Health and Medical Research Council of Australia (to S.M.M.).