ABSTRACT
One of the main unanswered questions regarding the early steps of macroautophagy/autophagy is the mechanism of membrane-modeling events required for autophagosome formation. Three independent studies have recently revealed an actin cytoskeleton involvement in this process, providing significant details regarding the role of actin in nucleation events both inside and outside the phagophore membrane during its expansion and assembly.
KEYWORDS: actin, autophagosome shape, autophagy, CAPZ, JMY, nucleation-promoting factor, WASL, WHAMM
The participation of the actin cytoskeleton in the early events of autophagosome formation under starvation conditions has been noted for several years. For example, actin mutations in yeast, as well as treatment of yeast and mammalian cells with chemicals that block actin polymerization, affect recruitment of components of the core autophagy machinery as well as of cargo components, in several selective and nonselective autophagy pathways.1 For example, in a selective autophagic process in yeast termed the cytoplasm-to-vacuole targeting (Cvt) pathway, Atg11, Atg8 and precursor Ape1 recruitment from the cytosol, and of Atg9 from peripheral membranous compartments, to the phagophore assembly site (PAS) is affected by such perturbations of the actin cytoskeleton. Also the uptake of organelle cargo, such as the endoplasmic reticulum (ER) and peroxisomes, into phagophores (the precursors to autophagosomes) during starvation is actin microfilament-dependent in yeast (for more detail see reference 1). Similarly in mammals, autophagosome formation is impaired by affecting actin polymerization/depolymerization cycles,2-4 and ATG9 trafficking is myosin II-dependent.5 Finally, in mammals there is colocalization of actin filaments with 2 members of the class III phosphatidylinositol 3-kinase (PtdIns3K) complex, ATG14 and BECN1/Beclin 1,3 which are required at the very initial step of autophagosome formation. Actin filaments also colocalize with ZFYVE1/DFCP1 (zinc finger FYVE-type containing 1/double FYVE-containing protein 1) present at the omegasome, a transient phosphatidylinositol 3-phosphate (PtdIns3P)-rich structure from which some autophagosomes originate.3 However, despite strong evidence of actin cytoskeleton participation in some early steps of autophagy, the precise role of actin in autophagosome formation remained unclear, leaving open questions regarding whether actin filaments serve only as motors transporting required components to the autophagosome formation site or whether actin filaments are also involved in membrane invagination, as demonstrated for the formation of endocytic vesicles.6 Three studies, by Mi et al., Kast et al., and Coutts and Thangue,7-9 have now answered these questions by proving that actin nucleation actually shapes autophagosomes.
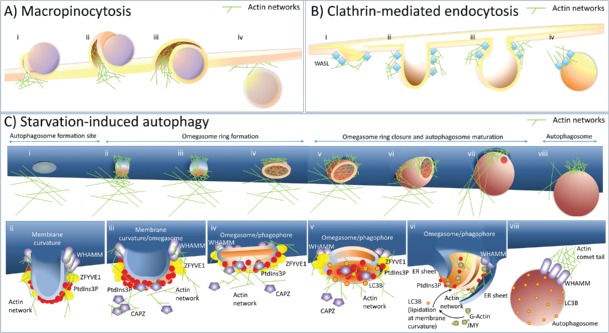
It is widely accepted that actin, with its polymerization and branching abilities, creates highly dynamic networks that allow a cell to interact with and react to the environment, for example by modulating membranes. The assistance of actin in various vesicle formation processes (for example during endocytosis, macropinocytosis and phagocytosis) was already confirmed.10 Macropinocytosis, for instance, involves actin cytoskeleton rearrangement (polymerization, depolymerization and contraction) to enforce outward bulging of the plasma membrane (Fig. 1Ai).11 Further, the rearrangement of actin networks leads to the cup-shaped extensions of the cell surface and formation of membrane ruffles, each of which, by folding back onto itself (Fig. 1Aii and Aiii) and by fusion at the base of the plasma membrane, forms macropinosomes (Fig. 1Aiv). In contrast, during clathrin-dependent endocytosis, actin filaments are involved in membrane invagination into the cytoplasm (Fig. 1Bi). During this event, actin association begins by the activation of the ARP2/3 complex, via nucleation-promoting factors such as WASL/N-WASP (Wiskott-Aldrich syndrome like), which is recruited to the periphery of the clathrin-coated vesicle formation site (Fig. 1Bi).12,13 Further, actin polymerization and branching not only expand and form endocytic cups (Fig. 1 Bii), but also promote elongation and contraction of the neck of the deeply-invaginated clathrin-coated structure (Fig. 1Biii) to allow endocytic vesicle budding from the plasma membrane (Fig. 1Biv).14 At the final step of clathrin-coated vesicle formation, the actin network reorganizes into a comet tail and drives the newly-formed vesicle away from the plasma membrane (Fig. 1Biv).
Figure 1.
A model explaining the role of actin in membrane modulation and shaping of autophagosomes. (A) Actin networks are involved in the plasma membrane protrusion(s) during macropinocytosis. (i) To form a macropinosome, actin networks force the plasma membrane to engulf extracellular cargo, for instance, as a cup-shaped extension of the cell membrane. (ii) These plasma membrane extrusions may fold back on the plasma membrane and form a cave-like invagination. (iii) A membrane fusion/fission event separates the lumen of the macropinosome from the extracellular space. (iv) After cargo engulfment, the newly formed macropinosome is moved further into the cytoplasm. (B) During clathrin-mediated endocytosis, actin polarization/depolarization events promote clathrin-coated vesicle formation. (i) Clathrin-mediated endocytosis is initiated at PtdIns(4,5)P2-rich sites in the plasma membrane enriched also for subunits of clathrin coat proteins (not shown) which bind the actin nucleation factor, WASL/N-WASP. (ii) WASL initiates actin nucleation, which causes plasma membrane deformation into a bulb. (iii) Further actin polymerization and branching promote elongation and contraction of the neck required for vesicle closure. (iv) After vesicle scission, the newly formed endosome is moved further into the cytoplasm by a comet-tail mechanism, which involves actin filaments. (C) During starvation-induced autophagy, actin networks shape autophagosomes from inside and outside the phagophore. (i) Autophagosome formation starts at ER membrane curvature sites rich in ATG14. At this stage actin is not detected at the ER sites specific to autophagosome formation. (ii) ATG14 recruits the PIK3C3/VPS34 kinase complex (not shown) required for PtdIns3P generation. PtdIns3P is then recognized by effectors, such as ZFYVE1. The actin nucleation-promoting factor, WHAMM, is also recruited to ER sites specific to autophagosome formation, via its N-terminal segment. Actin nucleation stabilizes and promotes membrane curvature, which can stimulate further PtdIns3P and ZFYVE1 recruitment. (iii) High levels of PtdIns3P remove from the actin filaments a negative regulator of actin polymerization and branching, the actin-capping protein, CAPZ. (iv) This removal of CAPZ stimulates actin polymerization and may cause flattening of the structure and omegasome formation with a highly-curved membrane existing at the limiting edge that is saturated with ZFYVE1. (v) PtdIns3P produced by the membrane-bound PIK3C3/VPS34 kinase complex further enhances curvature of the double-membrane structure facilitating phagophore formation. Simultaneously, the edge exhibiting local lipid-packing defects serves as a platform for the recruitment of WIPI2 (not shown), followed by recruitment of the ATG12–ATG5-ATG16L1 complex (not shown) and LC3/GABARAP lipidation. (vi) LC3 recruits another nucleation-promoting factor, JMY, and this attracts more actin and expands the actin network to allow phagophore extension. The growing phagophore is also surrounded by 2 sheets of ER, which hypothetically might be used by the actin network to create the autophagosome shape, as well as being used as a force required to stabilize a highly-curved limiting edge required for phagophore expansion. (vii) Further formation of actin networks affects autophagosome shape and size preceded by phagophore closure and omegasome dissociation. (viii) When an autophagosome is formed, WHAMM drives it into the cytoplasm using an actin comet-tail mechanism.
Similarities to the clathrin-coated vesicle formation events are reflected in a recent model8 to explain autophagosome membrane shaping and biogenesis during starvation. The authors show that during starvation-induced autophagy, the nucleation-promoting factor, WHAMM (WAS protein homolog associated with actin, Golgi membranes and microtubules), regulates autophagosome formation through a similar actin-comet tail mechanism (Fig. 1C), which is analogous to the action of WASL during clathrin-dependent endocytosis (Fig. 1B). Although WHAMM was reported previously to bind microtubules and to be involved in ER-to-Golgi transport,15 Kast et al.,8 demonstrate that WHAMM, through activation of the ARP2/3 complex, can also positively stimulate autophagosome biogenesis upon starvation. In starved cells, WHAMM was observed not to colocalize with general ER markers but to accumulate at the interface between neighboring autophagosomes, whose number and size increase with enhanced WHAMM expression. Additionally, knocking WHAMM down or blocking its interaction with the ARP2/3 complex not only inhibits comet tail formation at autophagosomes but also reduces the autophagosome size and number. Analogously, formation of clathrin-coated vesicles is abrogated in wasl knockout mice or by WASL knockdown in HeLa cells, which suggests that WASL and WHAMM might work in a similar manner, but for the formation of different types of vesicles.
WHAMM-positive puncta associate and comigrate with autophagosomal markers, LC3 and SQSTM1/p62, as well as with ZFYVE1, which is widely used as a marker of omegasomes (transient structures existing only during autophagosome formation [Fig. 1Cii-vii]). But they do not colocalize with ATG14, an early autophagy marker and an essential regulator of the PtdIns3K complex that functions prior to omegasome formation (Fig. 1Ci). WHAMM does not colocalize either with the lysosomal marker LAMP1, which is used in this study to include the population of late autophagosomes fused to lysosomes (autolysosomes). Thus, WHAMM is neither present during very early stages of autophagosome formation, nor at the late stages, but acts somewhere between these stages (Fig. 1Cii-viii). WHAMM recruits the ARP2/3 complex to the autophagosome formation sites at the ER and stimulates actin network assembly (Fig. 1Cii). The recruitment of the ARP2/3 complex by WHAMM also promotes formation and/or elongation of the phagophore (Fig. 1Ciii-vii) and, consequently, further autophagosome detachment and movement (Fig. 1Cviii).8
The localization of WHAMM to ER sites specific to autophagosome formation is controlled by its N-terminal segment. This segment is the most variable region between WHAMM and other nucleation-promoting factors including JMY (junction mediating and regulatory protein, p53 cofactor), with similar domain architecture. Nevertheless, JMY colocalizes with LC3-positive vesicles too, but does not associate with the actin comet tail as WHAMM does.8 This observation indicates that the 2 nucleation-promoting factors play either related or complementary, but not identical, roles in autophagosome membrane shaping. Consistent with this assumption, Coutts and Thangue7 demonstrate that JMY is indeed involved in early stages of autophagosome biogenesis and maturation. JMY accumulates at autophagosomes but neither colocalizes with lysosomal markers nor is it degraded during autophagy. Moreover, knocking JMY down causes a reduction in the number and size of LC3 puncta, even after a short time period, but does not affect autophagic flux. An important observation is that JMY acts to promote autophagosome formation through a functional LC3-interacting region motif, which might be involved in directly binding JMY to the LC3B isoform (Fig. 1Cvi). Such a motif is not conserved in WHAMM, supporting the hypothesis that the roles of these 2 proteins in autophagosome shaping may be complementary, but considerably different. The authors demonstrated that the presence of JMY at the autophagosome attracts actin and affects autophagosome shape and size. JMY expression, as well as its WH2 (Wiskott-Aldrich syndrome homology region 2) domain fusion to LC3, induce formation of LC3-positive, elongated filamentous structures and, consistently, the expression of a JMY mutant lacking the WH2 domain (JMYΔWH2) causes the formation of enlarged globular autophagosomes. This means that a fully functional JMY protein is a rate-limiting factor for actin recruitment and nucleation at the phagophore structure, thereby influencing autophagosome formation (Fig. 1Cvi).
In completely formed autophagosomes, because LC3 is present primarily on the inner, but not the outer, membrane of the autophagosome, it naturally raises the question whether actin nucleation can also be stimulated on the inner membrane of the phagophore. Certainly this can happen. As Mi et al.,9 demonstrate by using super resolution and electron microscopy, actin is present inside the central cavity of newly-formed autophagosomes (Fig. 1Cvi). Actin colocalizes with, or is surrounded by, autophagy proteins such as LC3 and ZFYVE1 (Fig. 1Cv-vi). The puncta detected by the authors are also positive for several branched actin network markers, namely: actin-dynamizing protein, CFL (cofilin), actin-capping protein, CAPZ, or the aforementioned ARP2/3 complex. Due to the CAPZ role as a negative regulator of actin polymerization and branching, the knockdown of the CAPZ protein gives a similar result9 as the expression of the positive actin nucleation regulator, JMY.7 Depletion of CAPZ causes formation of LC3- and ZFYVE1-positive elongated filamentous structures, providing evidence that CAPZ also controls the actin network from the inside of the phagophore. Interestingly, the actin-capping function of CAPZ was previously shown to be controlled by phosphoinositides, such as PtdIns(4,5)P2 and PtdIns4P.16 These phosphoinositides can cause rapid disassociation of CAPZ from the barbed ends of actin filaments. Because of that, the ability of PtdIns(4,5)P2 to remove CAPZ has been proposed as a mechanism that stimulates actin nucleation around PtdIns(4,5)P2-enriched membranes. The omegasome is a structure rich in another phosphoinositide, PtdIns3P.17 The authors9 found that CAPZ binds to PtdIns3P and that it stimulates uncapping of actin and promotes actin network construction (see description for Fig. 1Ciii-v). It is tempting to ask if the level of PtdIns3P might then be another regulator of the actin network formation at the phagophore membrane structures. It is worth noting here that during clathrin-dependent endocytosis, the auto-inhibitory conformation of WASL can be altered upon interaction with both CDC42 and PtdIns(4,5)P2, which cause actin assembly at the surface of the plasma membrane that has high PtdIns(4,5)P2 levels.18 Unfortunately it is unknown if WHAMM and JMY are activated by PtdIns3P, just as WASL is activated by PtdIns(4,5)P2.
It is still unknown what exactly actin does inside phagophores. Could it surround portions of the cytoplasm designed for degradation and serve as a scaffold for growing the phagophore membrane and/or could it be a factor that forces phagophore expansion? Both roles are highly probable. It is known that one of the E2-like enzymes engaged in ubiquitination-type conjugation reactions, ATG3, facilitates LC3/GABARAP lipidation only on membranes exhibiting local lipid-packing defects,19 for example at highly-curved membranes existing at the limiting edge of the growing phagophore (Fig. 1Cvi). Therefore, the highly curved rim seems to be functionally required for transient association of the early acting autophagic machinery and other proteins/components required for phagophore expansion. During autophagy induced by starvation, the phagophore structure is observed to be surrounded by 2 sheets of ER 20,21 which hypothetically might be used by actin networks to create the autophagosome shape, as well as being used as a force required to obtain a highly-curved limiting edge (Fig. 1Cvi). All 3 studies discussed here were focused mainly on the actin role during starvation-induced autophagy, when a portion of the cytoplasm is captured for autophagic clearance mostly in a nonselective way. Therefore, it remains to be seen if actin nucleation will still have such a substantial role in autophagosome formation during selective types of autophagy, wherein different cargos are used as scaffolds and as drivers for phagophore expansion.
In summary, actin networks are engaged in autophagosome formation via their involvement in vesicle shaping from both inside and outside of the autophagosome. This role of actin does not preclude other roles in autophagosome formation (such as actin-dependent ATG9A trafficking for phagophore expansion) or at later stages of autophagy (for instance in cargo selection and trafficking) and in autophagosome-lysosome fusion (for more detail see reference22), but these are not the subject of this paper, which focuses on the shaping of autophagosomes. The studies described above characterized previously unknown players of the autophagosome formation machinery and finally created a model explaining actin involvement in autophagosomal membrane shaping (Fig. 1C). We are left with a better understanding of the involvement of actin networks in autophagosome formation upon starvation. Nonetheless, this model still needs to be expanded as these studies pose a number of unresolved questions. First, it is unknown how actin is recruited to the autophagosome formation sites at the ER. Mi et al.,9 demonstrated partial disassembly of polymerized actin in combination with the appearance of actin puncta at omegasomes upon starvation, but it remains unclear how starvation induces actin depolymerization and its recruitment to the autophagosome formation sites. Second, a PtdIns3P role in this process might be relevant since genetic and pharmacological inhibition of PtdIns3P synthesis prevents actin puncta occurrence at omegasomes. Third, it is unknown if the actin network inside autophagosomes is disrupted at the last point of autophagosome formation or if it is degraded along with the cargo. Finally, the role of actin, if any, in autophagosome scission still remains enigmatic.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Chancellor's Associates Chair and NIH grant GM069373 to SS and by an European Molecular Biology Organization (EMBO long term fellowship) and the UC San Diego Frontiers of Innovation Scholars Program (FISP) to K.Z-R.
References
- [1].Monastyrska I, Rieter E, Klionsky DJ, Reggiori F. Multiple roles of the cytoskeleton in autophagy. Biol Rev Camb Philos Soc 2009; 84(3):431-48; PMID:19659885; http://dx.doi.org/ 10.1111/j.1469-185X.2009.00082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aplin A, Jasionowski T, Tuttle DL, Lenk SE, Dunn WA Jr. Cytoskeletal elements are required for the formation and maturation of autophagic vacuoles. J Cell Physiol 1992; 152(3):458-66; PMID:1506410; http://dx.doi.org/ 10.1002/jcp.1041520304 [DOI] [PubMed] [Google Scholar]
- [3].Aguilera MO, Beron W, Colombo MI. The actin cytoskeleton participates in the early events of autophagosome formation upon starvation induced autophagy. Autophagy 2012; 8(11):1590-603; PMID:22863730; http://dx.doi.org/ 10.4161/auto.21459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zavodszky E, Seaman MN., Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC. Mutation in VPS35 associated with Parkinson disease impairs WASH complex association and inhibits autophagy. Nat Commun 2014; 5:3828; PMID:24819384; http://dx.doi.org/ 10.1038/ncomms4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tang HW, Wang YB, Wang SL, Wu MH, Lin SY, Chen GC. Atg1-mediated myosin II activation regulates autophagosome formation during starvation-induced autophagy. EMBO J 2011; 30(4):636-51; PMID:21169990; http://dx.doi.org/ 10.1038/emboj.2010.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boettner DR, Chi RJ, Lemmon SK. Lessons from yeast for clathrin-mediated endocytosis. Nat Cell Biol 2012; 14(1):2-10; http://dx.doi.org/ 10.1038/ncb2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Coutts AS, La Thangue NB. Actin nucleation by WH2 domains at the autophagosome. Nat Commun 2015; 6:7888; PMID:26223951; http://dx.doi.org/ 10.1038/ncomms8888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kast DJ, Zajac AL, Holzbaur EL, Ostap EM, Dominguez R. WHAMM directs the Arp2/3 complex to the ER for autophagosome biogenesis through an actin comet tail mechanism. Curr Biol 2015; 25(13):1791-1797; PMID:26096974; http://dx.doi.org/ 10.1016/j.cub.2015.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mi N, Chen Y, Wang S, Chen M, Zhao M, Yang G, Ma M, Su Q, Luo S, Shi J, et al.. CapZ regulates autophagosomal membrane shaping by promoting actin assembly inside the isolation membrane. Nat Cell Biol 2015; 17(9):1112-23; PMID:26237647; http://dx.doi.org/ 10.1038/ncb3215 [DOI] [PubMed] [Google Scholar]
- [10].Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol 2012; 12(7):492-502; PMID:22699831; http://dx.doi.org/ 10.1038/nri3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol 2008; 9(8):639-49; PMID:18612320; http://dx.doi.org/ 10.1038/nrm2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Smythe E, Ayscough KR. Actin regulation in endocytosis. J Cell Sci 2006; 119(Pt 22):4589-98; PMID:17093263; http://dx.doi.org/ 10.1242/jcs.03247 [DOI] [PubMed] [Google Scholar]
- [13].Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 2006; 7(6):404-14; PMID:16723976; http://dx.doi.org/ 10.1038/nrm1940 [DOI] [PubMed] [Google Scholar]
- [14].Collins A, Warrington A, Taylor KA, Svitkina T. Structural organization of the actin cytoskeleton at sites of clathrin-mediated endocytosis. Curr Biol 2011; 21(14):1167-75; PMID:21723126; http://dx.doi.org/ 10.1016/j.cub.2011.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Campellone KG, Webb NJ, Znameroski EA, Welch MD. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell 2008; 134(1):148-61; PMID:18614018; http://dx.doi.org/ 10.1016/j.cell.2008.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schafer DA, Jennings PB, Cooper JA. Dynamics of capping protein and actin assembly in vitro: uncapping barbed ends by polyphosphoinositides. J Cell Biol 1996; 135(1):169-79; PMID:8858171; http://dx.doi.org/ 10.1083/jcb.135.1.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 2008; 182(4):685-701; PMID:18725538; http://dx.doi.org/ 10.1083/jcb.200803137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Posor Y, Eichhorn-Grunig M, Haucke V. Phosphoinositides in endocytosis. Biochim Biophys Acta 2015; 1851(6):794-804; PMID:25264171; http://dx.doi.org/ 10.1016/j.bbalip.2014.09.014 [DOI] [PubMed] [Google Scholar]
- [19].Nath S, Dancourt J, Shteyn V, Puente G, Fong WM, Nag S, Bewersdorf J, Yamamoto A, Antonny B, Melia TJ. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat Cell Biol 2014; 16(5):415-24; PMID:24747438; http://dx.doi.org/ 10.1038/ncb2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol 2009; 11(12):1433-7; PMID:19898463; http://dx.doi.org/ 10.1038/ncb1991 [DOI] [PubMed] [Google Scholar]
- [21].Yla-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy 2009; 5(8):1180-5; PMID:19855179; http://dx.doi.org/ 10.4161/auto.5.8.10274 [DOI] [PubMed] [Google Scholar]
- [22].Kruppa AJ, Kendrick-Jones J, Buss F. Myosins, Actin and Autophagy. Traffic 2016; 17(8):878-90; PMID:27146966; http://dx.doi.org/ 10.1111/tra.12410 [DOI] [PMC free article] [PubMed] [Google Scholar]